French Public Familiarity and Attitudes toward Clinical Research during the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Sample
2.2. Data Collection
2.3. Statistical Analysis
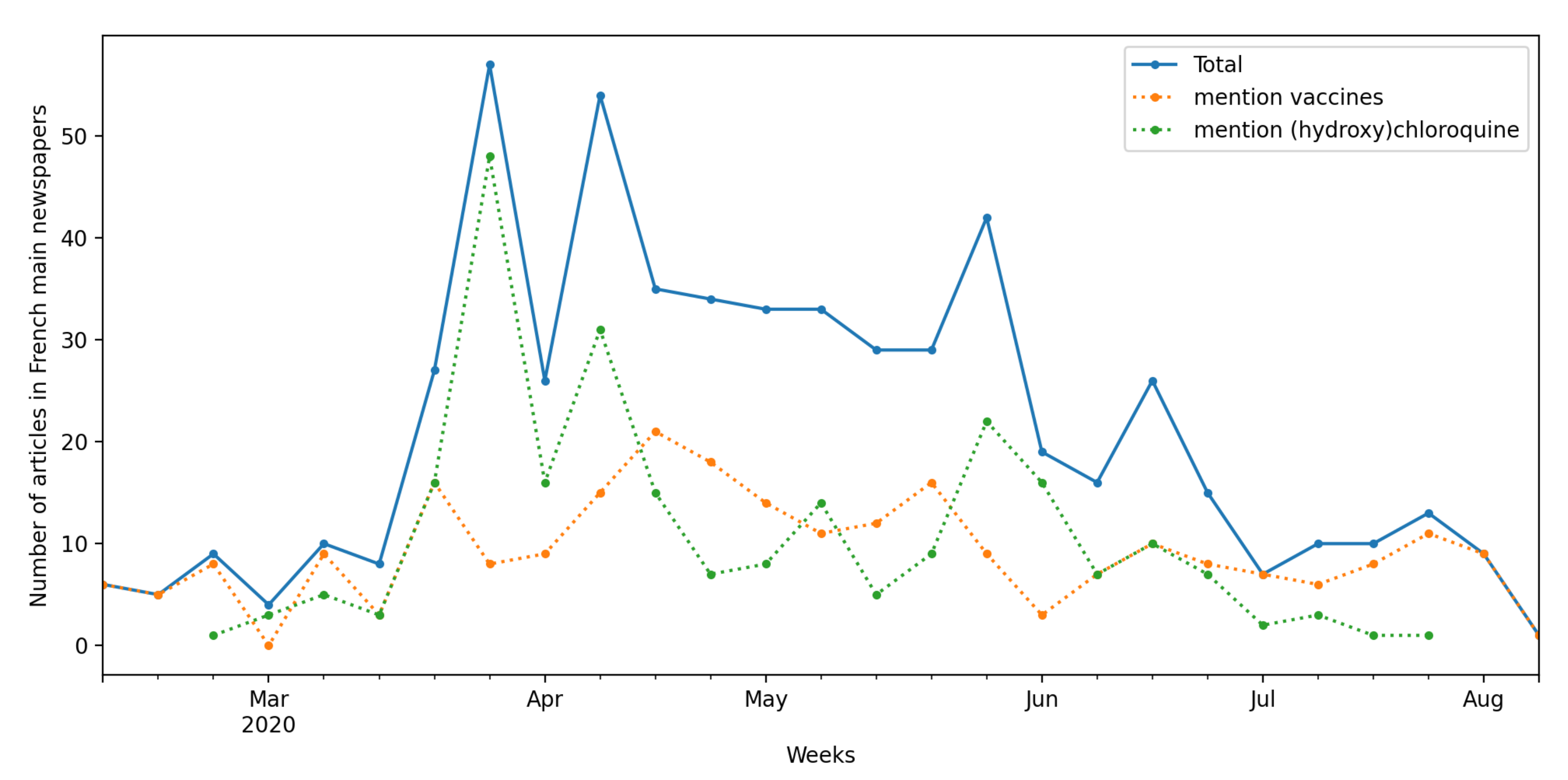
3. Context: The French Media Coverage of Clinical Research
4. Results
4.1. French Attitudes toward Clinical Research
4.2. Factors Associated with Familiarity with and Attitudes toward Clinical Trials
5. Discussion
Attitudes toward Clinical Research in a Context of Strong Mistrust of the Pharmaceutical Industry and Politicians
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruano, J.; Gómez-García, F.; Pieper, D.; Puljak, L. What evidence-based medicine researchers can do to help clinicians fighting COVID-19? J. Clin. Epidemiol. 2020, 124, 183–185. [Google Scholar] [CrossRef]
- Sattui, S.E.; Liew, J.W.; Graef, E.R.; Coler-Reilly, A.; Berenbaum, F.; Duarte-García, A.; Harrison, C.; Konig, M.F.; Korsten, P.; Putman, M.S.; et al. Swinging the pendulum: Lessons learned from public discourse concerning hydroxychloroquine and COVID-19. Expert Rev. Clin. Immunol. 2020, 16, 659–666. [Google Scholar] [CrossRef]
- Saag, M. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2020, 10–11. [Google Scholar] [CrossRef]
- Raulin, N. Le buzz sur la chloroquine freine l’essai clinique européen Discovery. Libération. 2020. Available online: https://www.liberation.fr/france/2020/03/26/le-buzz-sur-la-chloroquine-freine-l-essai-clinique-europeen-discovery_1783176/ (accessed on 2 February 2021).
- Briggs, C.; Hallin, D. Making Health Public; Routledge: London, UK, 2016. [Google Scholar] [CrossRef]
- Levine, R.J. The impact of HIV infection on society’s perception of clinical trials. Kennedy Inst. Ethics J. 1994, 4, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Weinfurt, K.P.; Lin, L.; Sugarman, J. Public views regarding the responsibility of patients, clinicians, and institutions to participate in research in the United States. Clin. Trials 2019, 16, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; McKay, T. Clinical trials as treatment option: Bioethics and health care disparities in substance dependency. Soc. Sci. Med. 2009, 69, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Keating, P.; Cambrosio, A. Cancer clinical trials: The emergence and development of a new style of practice. Bull. Hist. Med. 2007, 81, 197–223. [Google Scholar] [CrossRef]
- Appelbaum, P.S.; Roth, L.H.; Lidz, C.W.; Benson, P.; Lidz, W.; Roth, H.; Appelbaum, P.S.; Benson, P. False Hopes and Best Data: Consent to Research and the Therapeutic Misconception. Hast. Cent. Rep. 1987, 17, 20–24. [Google Scholar] [CrossRef]
- Besle, S.; Schultz, E.; Hollebecque, A.; Varga, A.; Baldini, C.; Martin, P.; Postel-Vinay, S.; Bahleda, R.; Gazzah, A.; Michot, J.M.; et al. Organisational factors influencing early clinical trials enrollment: Gustave Roussy experience. Eur. J. Cancer 2018, 98, 17–22. [Google Scholar] [CrossRef]
- Naidoo, N.; Nguyen, V.T.; Ravaud, P.; Young, B.; Amiel, P.; Schanté, D.; Clarke, M.; Boutron, I. The research burden of randomized controlled trial participation: A systematic thematic synthesis of qualitative evidence. BMC Med. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- LEEM. Observatoire Sociétal du MéDicament. Technical Report. 2019. Available online: https://www.leem.org/publication/observatoire-societal-du-medicament-2019-ipsos-pour-le-leem (accessed on 2 February 2021).
- Van den Bogaert, S.; Declercq, J.; Christiaens, T.; Jacobs, G.; Bracke, P. In the land of pharma: A qualitative analysis of the reputational discourse of the pharmaceutical industry. Public Relat. Inq. 2018, 7, 127–147. [Google Scholar] [CrossRef]
- Jureidini, J.; McHenry, L.B. The Illusion of Evidence-Based Medicine: Exposing the Crisis of Credibility in Clinical Research; Wakefield Press: Adelaide, SA, Australia, 2020. [Google Scholar]
- Klein, E.; Solomon, A.J.; Corboy, J.; Bernat, J. Physician compensation for industry-sponsored clinical trials in multiple sclerosis influences patient trust. Mult. Scler. Relat. Disord. 2016, 8, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Hwong, A.R.; Sah, S.; Lehmann, L.S. The Effects of Public Disclosure of Industry Payments to Physicians on Patient Trust: A Randomized Experiment. J. Gen. Intern. Med. 2017, 32, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Bauchner, H.; Fontanarosa, P.B. Restoring confidence in the pharmaceutical industry. JAMA J. Am. Med Assoc. 2013, 5, 1561–1570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Comis, R.L.; Miller, J.D.; Aldigé, C.R.; Krebs, L.; Stoval, E. Public attitudes toward participation in cancer clinical trials. J. Clin. Oncol. 2003, 21, 830–835. [Google Scholar] [CrossRef]
- Yang, Z.J.; Mccomas, K.; Gay, G.; Leonard, J.P.; Dannenberg, A.J.; Dillon, H. Motivation for health information seeking and processing about clinical trial enrollment. Health Commun. 2010, 25, 423–436. [Google Scholar] [CrossRef]
- Mancini, J.; Briggs, A.; Elkin, E.; Regan, J.; Hickey, C.; Targett, C.; Ager, R.; Masuda, S.; Bach, P.; Sabbatini, P. The impact of patient education on consideration of enrollment in clinical trials. J. Community Support. Oncol. 2018, 16, e81–e88. [Google Scholar] [CrossRef]
- Mills, E.; Wilson, K.; Rachlis, B.; Griffith, L.; Wu, P.; Guyatt, G.; Cooper, C. Barriers to participation in HIV drug trials: A systematic review. Lancet Infect. Dis. 2006, 6, 32–38. [Google Scholar] [CrossRef]
- Unger, J.M.; Cook, E.; Tai, E.; Bleyer, A. Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am. Soc. Clin. Oncol. 2017, 2860, 185–198. [Google Scholar] [CrossRef]
- Anderson, A.; Borfitz, D.; Getz, K. Differences in Clinical Research Perceptions and Experiences by Age Subgroup. Ther. Innov. Regul. Sci. 2020, 54, 93–102. [Google Scholar] [CrossRef]
- Anderson, A.; Borfitz, D.; Getz, K. Global Public Attitudes About Clinical Research and Patient Experiences With Clinical Trials. JAMA Netw. Open 2018, 1, e182969. [Google Scholar] [CrossRef]
- Nay, O.; Béjean, S.; Benamouzig, D.; Bergeron, H.; Castel, P.; Ventelou, B. Achieving universal health coverage in France: Policy reforms and the challenge of inequalities. Lancet 2016, 387, 2236–2249. [Google Scholar] [CrossRef]
- Ward, J.K.; Peretti-Watel, P.; Bocquier, A.; Seror, V.; Verger, P. Vaccine hesitancy and coercion: All eyes on France. Nat. Immunol. 2019, 7–9. [Google Scholar] [CrossRef]
- Gauchat, G. The cultural authority of science: Public trust and acceptance of organized science. Public Underst. Sci. 2011, 20, 751–770. [Google Scholar] [CrossRef]
- Berlivet, L.; Löwy, I. Hydroxychloroquine Controversies: Clinical Trials, Epistemology, and the Democratization of Science. Med. Anthropol. Q. 2020, 3, 1–17. [Google Scholar] [CrossRef]
- Bauer, M.W.; Pansegrau, P.; Shukla, R. The Cultural Authority of Science; Routledge: London, UK, 2018. [Google Scholar] [CrossRef]
- Metcalfe, J.; Riedlinger, M.; Bauer, M.W.; Chakraborty, A.; Gascoigne, T.; Guenther, L.; Joubert, M.; Kaseje, M.; Herrera-Lima, S.; Revuelta, G.; et al. The COVID-19 mirror: Reflecting science-society relationships across 11 countries. J. Sci. Commun. 2020, 19, A05. [Google Scholar] [CrossRef]
- Rouquette, A.; Nadot, T.; Labitrie, P.; Van den Broucke, S.; Mancini, J.; Rigal, L.; Ringa, V. Validity and measurement invariance across sex, age, and education level of the French short versions of the European Health Literacy Survey Questionnaire. PLoS ONE 2018, 13, e0208091. [Google Scholar] [CrossRef]
- Hosmer, D.; Lemeshow, S.; Sturdivant, R. Applied Logistic Regression Analysis, 3rd ed.; Wiley: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Ferney, J. Coronavirus, la recherche mondiale se mobilise. La Croix 2020. Available online: https://www.la-croix.com/Sciences-et-ethique/Sante/Coronavirus-recherche-mondiale-mobilisee-2020-02-01-1201075651 (accessed on 2 February 2021).
- Angell, M. Industry-Sponsored Clinical Research. A Broken system. JAMA 2008, 300, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C.; Benson, C.A.; del Rio, C.; Edwards, K.M.; Fowler, V.G.; Fredricks, D.N.; Limaye, A.P.; Murray, B.E.; Naggie, S.; Pappas, P.G.; et al. COVID-19—Lessons Learned and Questions Remaining. Clin. Infect. Dis. 2020, 1–16. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Rebeaud, M.E.; Guihur, A.; Mahamat-Saleh, Y.; Fiolet, T. Hydroxychloroquine and COVID-19: A tale of populism and obscurantism. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Mede, N.G.; Schäfer, M.S. Science-related populism: Conceptualizing populist demands toward science. Public Underst. Sci. 2020, 29, 473–491. [Google Scholar] [CrossRef]
- Coconel. Confinement, Masques, Chloroquine, Vaccin: Ce Qu’en pensent les Français; Technical Report; ORS PACA’: Marseille, France, 2020. [Google Scholar]
- Gauchat, G. Politicization of Science in the Public Sphere: A Study of Public Trust in the United States, 1974 to 2010. Am. Sociol. Rev. 2012, 77, 167–187. [Google Scholar] [CrossRef]
- Jenkins, V.; Fallowfield, L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br. J. Cancer 2000, 82, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Abraham, J. Unhealthy Pharmaceutical Regulation; Springer: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- Hauray, B. Une médecine détournée ? influences industrielles et crise de confiance dans le domaine du médicament. Mouvements 2019, 2, 53–66. [Google Scholar] [CrossRef]
- Musselin, C. New forms of competition in higher education. Socio-Econ. Rev. 2018, 16, 657–683. [Google Scholar] [CrossRef]
- Giry, J.; Schultz, E. L’ANR en ph(r)ase critique. Forme et origine de la critique engagée. Zilsel 2017, 2, 63–96. [Google Scholar] [CrossRef]
- Sharrocks, K.; Spicer, J.F.; Camidge, D.R.; Papa, S. The impact of socioeconomic status on access to cancer clinical trials. Br. J. Cancer 2014, 111, 1684–1687. [Google Scholar] [CrossRef]
- Easter, M.M.; Henderson, G.E.; Davis, A.M.; Churchill, L.R.; King, N.M.P. The many meanings of care in clinical research. Sociol. Health Illn. 2006, 6, 695–712. [Google Scholar] [CrossRef]
- Bateman-House, A.; Kimberly, L.; Redman, B.; Dubler, N.; Caplan, A. Right-to-try laws: Hope, hype, and unintended consequences. Ann. Intern. Med. 2015, 163, 796–797. [Google Scholar] [CrossRef]
- Wadmann, S. Physician-industry collaboration: Conflicts of interest and the imputation of motive. Soc. Stud. Sci. 2014, 44, 531–554. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Clarke, R.M.; Jarrett, C.; Eckersberger, E.; Levine, Z.; Schulz, W.S.; Paterson, P. Measuring trust in vaccination: A systematic review. Hum. Vaccines Immunother. 2018, 14, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Oreskes, N.; Conway, E.M. Merchants of Doubt: How a Handful of Scientists Obscured the Truth on Issues from Tobacco Smoke to Global Warming; Bloomsbury Publishing: New York, NY, USA, 2011. [Google Scholar]
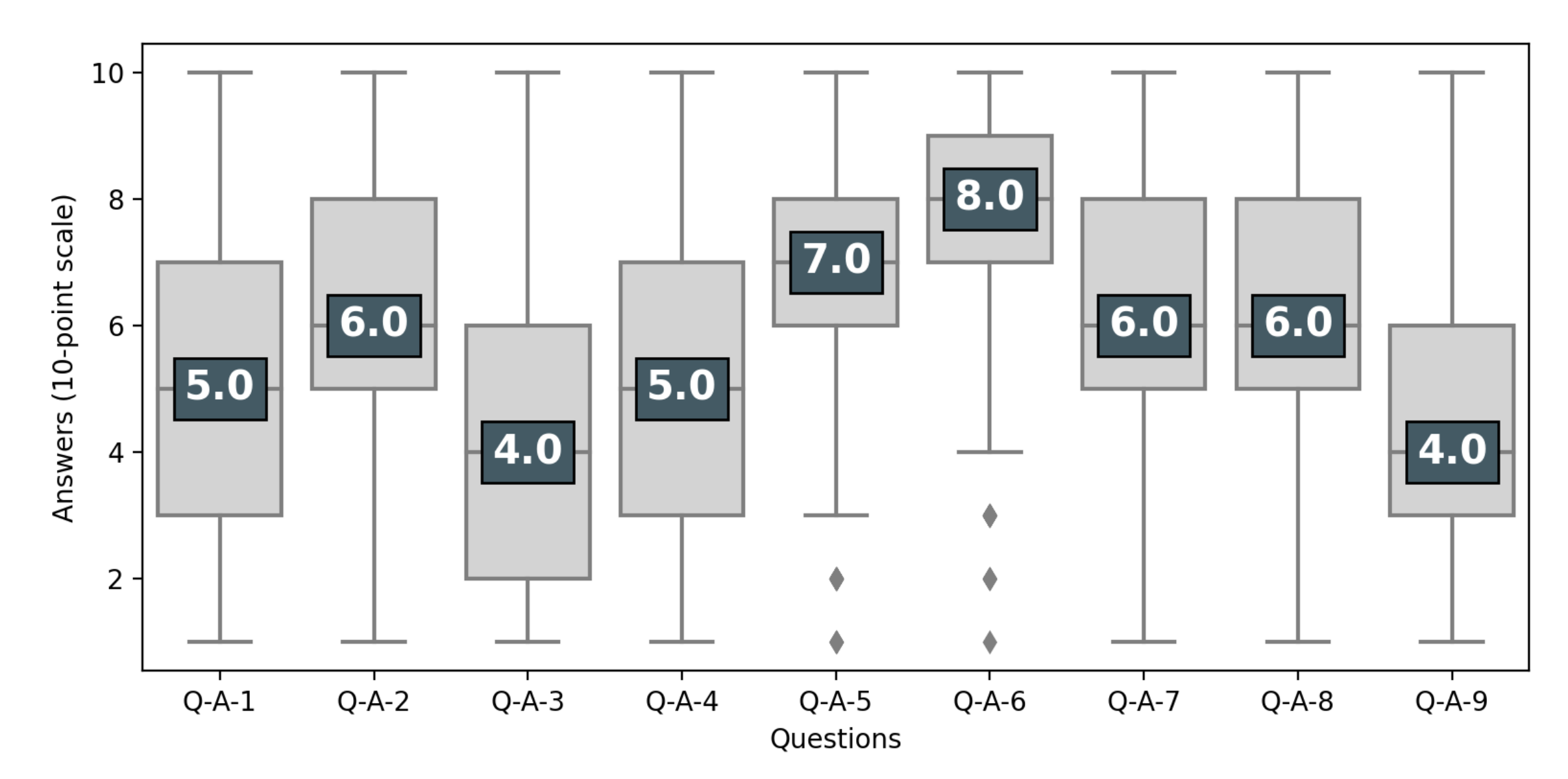

| Questions on Clinical Trials | 1 = Disagree Completely; 10 = Agree Completely | |
| Q-A-1 | Clinical trials are only useful as a last resort-after trying all available treatments | 10-point scale |
| Q-A-2 | Clinical trials offer an alternative to a treatment that you wish to avoid (invasive surgery, chemotherapy, etc.) | 10-point scale |
| Q-A-3 | Clinical trials are only suitable for people with a life-threatening condition | 10-point scale |
| Q-A-4 | Participants in clinical trials are only “guinea pigs” | 10-point scale |
| Q-A-5 | Clinical trials give people hope by giving them access to new treatments that they could not get otherwise | 10-point scale |
| Q-A-6 | Individuals who participate in research help advance medical knowledge and treatments for other sick people | 10-point scale |
| Q-A-7 | Sick people should have the right to test new drugs if they wish to do so, even if doctors disagree | 10-point scale |
| Q-A-8 | Receiving experimental treatment is a great opportunity | 10-point scale |
| Q-A-9 | Experimental treatments are like any other treatment | 10-point scale |
| Questions on Medical Research | 1 = Agree Completely; 4 = Disagree Completely | |
| Q-B-1 | Industry plays an important role in clinical research | 4-point scale |
| Q-B-2 | Medical research is done only by doctors | 4-point scale |
| Q-B-3 | Fighting unemployment is more important than funding medical research | 4-point scale |
| Q-B-4 | Citizens must be able to give their opinion on public research choices | 4-point scale |
| Q-B-5 | Priority should be given to new diseases | 4-point scale |
| Q-B-6 | The way research is done on cancer/covid is specific compared to other diseases | 4-point scale |
| Variables | Weighted Frequency | Proportion (%) | |
|---|---|---|---|
| Sex | female | 515.5 | 51.4 |
| male | 487.5 | 48.6 | |
| Age | [18–35] | 306.9 | 30.6 |
| [35–45] | 189.6 | 18.9 | |
| [45–55] | 195.6 | 19.5 | |
| [55–65] | 182.5 | 18.2 | |
| [65–75] | 128.4 | 12.8 | |
| Education | 1-Below HSD | 169.8 | 16.9 |
| 2-HSD | 240.1 | 23.9 | |
| 3-Above HSD | 593.1 | 59.1 | |
| Financial difficulties | No | 643.0 | 64.1 |
| Yes | 360.0 | 35.9 | |
| Health condition | 1-Good | 649.2 | 64.7 |
| 2-Average | 281.3 | 28.0 | |
| 3-Bad | 72.5 | 7.2 | |
| HI seeking behaviour | No | 136.1 | 13.6 |
| Yes | 866.9 | 86.4 | |
| Concerns about COVID19 | No | 54.5 | 5.4 |
| Some | 683.6 | 68.2 | |
| Yes | 264.9 | 26.4 | |
| Job in health sector | No | 827.4 | 82.5 |
| Yes | 175.6 | 17.5 | |
| Health literacy | 1-Adequate | 604.8 | 60.3 |
| 2-Problematic | 263.6 | 26.3 | |
| 3-Inadequate | 134.6 | 13.4 | |
| Familiarity with CTs | 1-Never heard of CTs | 37.6 | 3.7 |
| 2-Only know the term, CTs | 315.1 | 31.4 | |
| 3-Somewhat familiar with what CTs are | 454.6 | 45.3 | |
| 4-Very familiar | 149.2 | 14.9 | |
| 5-Extremely familiar | 46.5 | 4.6 | |
| Know that CTs are divided in phases | NA | 37.6 | 3.7 |
| No | 479.4 | 47.8 | |
| Yes | 486.0 | 48.5 | |
| Attitudes toward CTs | Negative < 5 | 78.7 | 8.2 |
| Neutral = 5 | 191.4 | 19.8 | |
| Positive > 5 | 695.3 | 72.0 | |
| Trust in doctors | 1-Yes | 933.8 | 93.1 |
| 2-No | 69.2 | 6.9 | |
| Trust in researchers | 1-Yes | 896.6 | 89.4 |
| 2-No | 106.4 | 10.6 | |
| Trust in the industry | 1-Yes | 258.1 | 25.7 |
| 2-No | 744.9 | 74.3 | |
| Trust in politicans | 1-Yes | 178.4 | 17.8 |
| 2-No | 824.6 | 82.2 | |
| Q-B-1 (importance of industry) | Agree | 685.3 | 68.3 |
| Disagree | 317.7 | 31.7 | |
| Q-B-2 (only doctors do medical research) | Agree | 293.5 | 29.3 |
| Disagree | 709.5 | 70.7 | |
| Q-B-3 (priority of fighting unemployement) | Agree | 282.6 | 28.2 |
| Disagree | 720.4 | 71.8 | |
| Q-B-4 (importance of citizens’ opinion) | Agree | 747.1 | 74.5 |
| Disagree | 255.9 | 25.5 | |
| Q-B-5 (prioritize new diseases) | Agree | 416.1 | 41.5 |
| Disagree | 586.9 | 58.5 | |
| Q-B-6-cancer (cancer research is specific) | Agree | 200.6 | 40.0 |
| Disagree | 301.0 | 60.0 | |
| Q-B-6-corona (covid research is specific) | Agree | 253.7 | 50.6 |
| Disagree | 247.7 | 49.4 |
| Good Familiarity | p | Positive Attitude | p | ||
|---|---|---|---|---|---|
| Sex | female | 93.4 (18.1%) | 0.2897 | 342.1 (66.4%) | 0.0432 |
| male | 102.2 (21.0%) | 353.2 (72.5%) | |||
| Age | [0–25] | 25.4 (19.5%) | 0.783 | 85.1 (65.3%) | 0.115 |
| [25–45] | 69.2 (18.9%) | 249.9 (68.3%) | |||
| [45–65] | 79.2 (20.9%) | 260.0 (68.7%) | |||
| [65+] | 21.9 (17.1%) | 100.3 (78.1%) | |||
| Education | 1-Below HSD | 21.7 (12.8%) | 0.0025 | 102.3 (60.2%) | 0.0043 |
| 2-HSD | 37.3 (15.5%) | 160.5 (66.8%) | |||
| 3-Above HSD | 136.6 (23.0%) | 432.5 (72.9%) | |||
| Financial difficulties | No | 133.0 (20.7%) | 0.2422 | 476.1 (74.0%) | <0.0001 |
| Yes | 62.7 (17.4%) | 219.1 (60.9%) | |||
| Health litteracy | 1-Adequate | 139.8 (23.1%) | 0.0018 | 439.8 (72.7%) | 0.0151 |
| 2-Problematic | 37.2 (14.1%) | 171.1 (64.9%) | |||
| 3-Inadequate | 18.7 (13.9%) | 84.5 (62.8%) | |||
| HI seeking behaviour | No | 11.3 (8.3%) | 0.0006 | 76.3 (56.1%) | 0.0005 |
| Yes | 184.4 (21.3%) | 619.0 (71.4%) | |||
| Health condition | 1-Good | 126.4 (19.5%) | 0.768 | 452.6 (69.7%) | 0.4605 |
| 2-Average | 52.9 (18.8%) | 188.8 (67.1%) | |||
| 3-Bad | 16.4 (22.6%) | 53.9 (74.3%) | |||
| COVID19 concerns | No | 14.2 (26.1%) | 0.2999 | 34.2 (62.8%) | 0.5451 |
| Some | 125.8 (18.4%) | 477.8 (69.9%) | |||
| Yes | 55.7 (21.0%) | 183.3 (69.2%) | |||
| Trust in scientists | 1-Yes | 180.6 (20.1%) | 0.1818 | 641.0 (71.5%) | <0.0001 |
| 2-No | 15.1 (14.2%) | 54.3 (51.0%) | |||
| Trust in doctors | 1-Yes | 181.6 (19.4%) | 0.9754 | 662.5 (71.0%) | 0.0001 |
| 2-No | 14.1 (20.4%) | 32.7 (47.3%) | |||
| Trust in politicians | 1-Yes | 40.8 (22.9%) | 0.2525 | 138.7 (77.7%) | 0.0093 |
| 2-No | 154.9 (18.8%) | 556.6 (67.5%) | |||
| Trust in the industry | 1-Yes | 62.5 (24.2%) | 0.0339 | 185.2 (71.8%) | 0.3647 |
| 2-No | 133.2 (17.9%) | 510.0 (68.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultz, É.; Ward, J.K.; Atlani-Duault, L.; Holmes, S.M.; Mancini, J. French Public Familiarity and Attitudes toward Clinical Research during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 2611. https://doi.org/10.3390/ijerph18052611
Schultz É, Ward JK, Atlani-Duault L, Holmes SM, Mancini J. French Public Familiarity and Attitudes toward Clinical Research during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health. 2021; 18(5):2611. https://doi.org/10.3390/ijerph18052611
Chicago/Turabian StyleSchultz, Émilien, Jeremy K. Ward, Laëtitia Atlani-Duault, Seth M. Holmes, and Julien Mancini. 2021. "French Public Familiarity and Attitudes toward Clinical Research during the COVID-19 Pandemic" International Journal of Environmental Research and Public Health 18, no. 5: 2611. https://doi.org/10.3390/ijerph18052611
APA StyleSchultz, É., Ward, J. K., Atlani-Duault, L., Holmes, S. M., & Mancini, J. (2021). French Public Familiarity and Attitudes toward Clinical Research during the COVID-19 Pandemic. International Journal of Environmental Research and Public Health, 18(5), 2611. https://doi.org/10.3390/ijerph18052611






