Analysis of the Efficacy of Two Treatment Protocols for Patients with Symptomatic Oral Lichen Planus: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
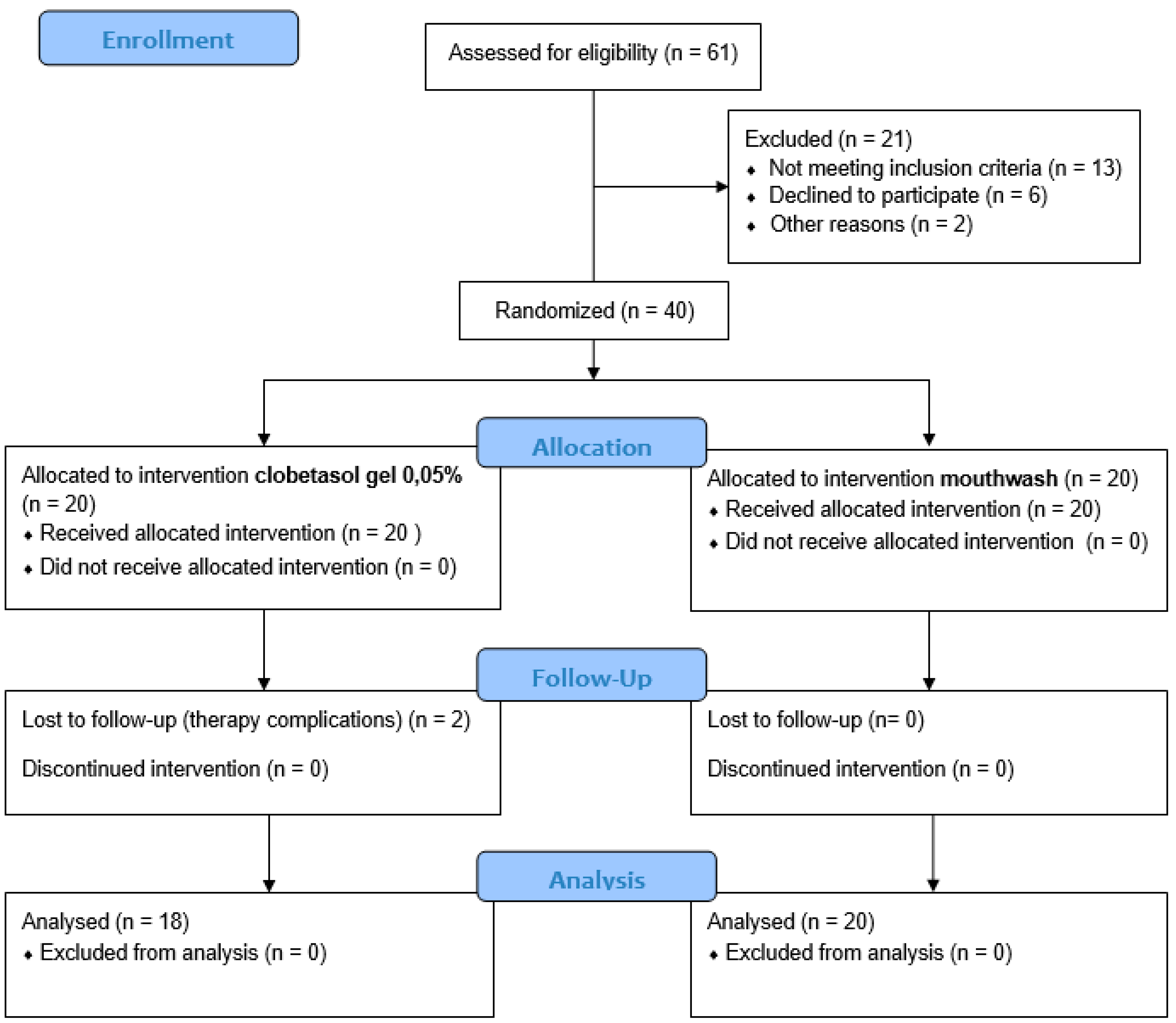
2.2. Power Sample Size and Randomization
2.3. Treatment Protocols
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Primary Endpoint
3.2. Secondary Endpoint
4. Discussion
5. Conclusions
- The anti-inflammatory (mouthwash) could be used in the treatment of symptomatic forms of OLP with a Thongprasom score < 2, as it resulted in good symptom control and significant activity in preventing lesion progression.
- Clobetasol seems to be confirmed once again as the treatment of first choice in the most severe forms of OLP (Thongprasom score > 2), as the study showed that the anti-inflammatory has a limited ability to induce remission of signs in subjects with severe forms of OLP, compared with clobetasol.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ficarra, G. Autoimmune diseases of the mucosa. In Manuandbook of Pathology and Oral Medicine, 3rd ed.; McGraw-Hill: Milan, Italy, 2015; p. 145. [Google Scholar]
- Ebrahimi, M.; Nylander, E.; Bäcklund, B.; Wahlin, Y.B.; Coates, P.J.; Nylander, K. The use of a novel ELISA method for detection of antibodies against p63 in sera from patients diagnosed with oral and/or genital and skin lichen planus. J. Oral Pathol. Med. 2010, 39, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, I.; Schifter, M.; Lockhart, P.B.; Wray, D.; Brennan, M.; Migliorati, C.A.; Axéll, T.; Bruce, A.J.; Carpenter, W.; Eisenberg, E. Oral lichen planus and oral lichenoid lesions: Diagnostic and therapeutic considerations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, S25.e1–S25.e12. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhang, J.; Sun, W.; Du, G.; Zhou, G. Inflammation-related cytokines in oral lichen planus: An overview. J. Oral Pathol. Med. 2015, 44, 1–14. [Google Scholar] [CrossRef]
- Eisen, D.; Carrozzo, M.; Bagan Sebastian, J.V.; Thongprasom, K. Number V Oral lichen planus: Clinical features and management. Oral Dis. 2005, 11, 338–349. [Google Scholar] [CrossRef]
- Carbone, M.; Arduino, P.G.; Carrozzo, M.; Gandolfo, S.; Argiolas, M.; Bertolusso, G.; Conrotto, D.; Pentenero, M.; Broccoletti, R. Course of oral lichen planus: A retrospective study of 808 northern Italian patients. Oral Dis. 2009, 15, 235–243. [Google Scholar] [CrossRef]
- Eisen, D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J. Am. Acad. Dermatol. 2002, 46, 207–214. [Google Scholar] [CrossRef]
- Piboonniyom, S.-O.; Treister, N.; Pitiphat, W.; Woo, S.-B. Scoring system for monitoring oral lichenoid lesions: A preliminary study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 696–703. [Google Scholar] [CrossRef]
- Mutafchieva, M.Z.; Draganova-Filipova, M.N.; Zagorchev, P.I.; Tomov, G.T. Oral lichen planus–known and unknown: A review. Folia Med. 2018, 60, 528–535. [Google Scholar] [CrossRef]
- Bagan, J.-V.; Eisen, D.; Scully, C. The diagnosis and management of oral lichen planus: A consensus approach. Oral Biosci. Med. 2004, 1, 21–27. [Google Scholar]
- Scully, C.; Carrozzo, M. Oral mucosal disease: Lichen planus. Br. J. Oral Maxillofac. Surg. 2008, 46, 15–21. [Google Scholar] [CrossRef]
- Lodi, G.; Scully, C.; Carrozzo, M.; Griffiths, M.; Sugerman, P.B.; Thongprasom, K. Current controversies in oral lichen planus: Report of an international consensus meeting. Part 2. Clinical management and malignant transformation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 100, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Córdova, P.; Rubio, A.; Echeverría, P. Oral lichen planus: A look from diagnosis to treatment. J. Oral Res. 2014, 3, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Mattsson, U.; Magnusson, B.; Jontell, M. Squamous cell carcinoma in a patient with oral lichen planus treated with topical application of tacrolimus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, e19–e25. [Google Scholar] [CrossRef] [PubMed]
- Kaliakatsou, F.; Hodgson, T.; Lewsey, J.; Hegarty, A.; Murphy, A.; Porter, S. Management of recalcitrant ulcerative oral lichen planus with topical tacrolimus. J. Am. Acad. Dermatol. 2002, 46, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Shichinohe, R.; Shibaki, A.; Nishie, W.; Tateishi, Y.; Shimizu, H. Successful treatment of severe recalcitrant erosive oral lichen planus with topical tacrolimus. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chow, M. Pimecrolimus: A review. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Swift, J.C.; Rees, T.D.; Plemons, J.M.; Hallmon, W.W.; Wright, J.C. The effectiveness of 1% pimecrolimus cream in the treatment of oral erosive lichen planus. J. Periodontol. 2005, 76, 627–635. [Google Scholar] [CrossRef]
- Sloberg, K.; Hersle, K.; Mobacken, H.; Thilander, H. Topical tretinoin therapy and oral lichen planus. Arch. Dermatol. 1979, 115, 716–718. [Google Scholar] [CrossRef]
- Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dent. J. 2018, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Thongprasom, K. Oral lichen planus: Challenge and management. Oral Dis. 2018, 24, 172–173. [Google Scholar] [CrossRef]
- Oberti, L.; Alberta, L.; Massimo, P.; Francesco, C.; Dorina, L. Clinical Management of Oral Lichen Planus: A Systematic Review. Mini Rev. Med. Chem. 2019, 19, 1049–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thongprasom, K.; Prapinjumrune, C.; Carrozzo, M. Novel therapies for oral lichen planus. J. Oral Pathol. Med. 2013, 42, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Goss, E.; Carrozzo, M.; Castellano, S.; Conrotto, D.; Broccoletti, R.; Gandolfo, S. Systemic and topical corticosteroid treatment of oral lichen planus: A comparative study with long-term follow-up. J. Oral Pathol. Med. 2003, 32, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Lo Muzio, L.; Della Valle, A.; Mignogna, M.D.; Pannone, G.; Bucci, P.; Bucci, E.; Sciubba, J. The treatment of oral aphthous ulceration or erosive lichen planus with topical clobetasol propionate in three preparations: A clinical and pilot study on 54 patients. J. Oral Pathol. Med. 2001, 30, 611–617. [Google Scholar] [CrossRef]
- Vincent, S.; Fotos, P.; Baker, K.; Williams, T. Oral lichen planus: The clinical, historical, and therapeutic features of 100 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1990, 70, 165–171. [Google Scholar] [CrossRef]
- Lo Muzio, L.; Campisi, G.; Farina, A.; Rubini, C.; Pastore, L.; Giannone, N.; Colella, G.; Leonardi, R. Effect of p63 expression on survival in oral squamous cell carcinoma. Cancer Investig. 2007, 25, 464–469. [Google Scholar] [CrossRef]
- Lehner, T.; Lyne, C. Adrenal function during topical oral corticosteroid treatment. Br. Med. J. 1969, 4, 138–141. [Google Scholar] [CrossRef] [Green Version]
- Plemons, J.M.; Rees, T.D.; Zachariah, N. Absorption of a topical steroid and evaluation of adrenal suppression in patients with erosive lichen planus. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 688–693. [Google Scholar] [CrossRef]
- Keshari, D.; Patil, K.; Mahima, V. Efficacy of topical curcumin in the management of oral lichen planus: A randomized controlled-trial. J. Adv. Clin. Res. Insights 2015, 2, 197–203. [Google Scholar] [CrossRef]
- Baccaglini, L.; Thongprasom, K.; Carrozzo, M.; Bigby, M. Urban legends series: Lichen planus. Oral Dis. 2013, 19, 128–143. [Google Scholar] [CrossRef]
- Shetty, R.R.; Burde, K.N.; Guttal, K.S. The efficacy of topical hyaluronic acid 0.2% in the management of symptomatic oral lichen planus. J. Clin. Diagn. Res. JCDR 2016, 10, ZC46. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Cantore, S.; Scacco, S.; Coletti, D.; Tatullo, M. Mesenchymal Stem Cells as Promoters, Enhancers, and Playmakers of the Translational Regenerative Medicine. Stem. Cells Int. 2018, 30, 6927401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashem, A.S.; Issrani, R.; Elsayed, T.E.; Prabhu, N. Topical hyaluronic acid in the management of oral lichen planus: A comparative study. J. Investig. Clin. Dent. 2019, 10, e12385. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, K.; Zhang, B.; Tu, Q.; Yao, Y.; Cui, B.; Ren, B.; He, J.; Shen, X.; Van Nostrand, J.D. Salivary mycobiome dysbiosis and its potential impact on bacteriome shifts and host immunity in oral lichen planus. Int. J. Oral Sci. 2019, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ballantyne, J.C.; Fishman, S.M.; Rathmell, J.P. Bonica’s Management of Pain; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Carrozzo, M.; Gandolfo, S. The management of oral lichen planus. Oral Dis. 1999, 5, 196–205. [Google Scholar] [CrossRef]
- Lener, E.V.; Brieva, J.; Schachter, M.; West, L.E.; West, D.P.; el-Azhary, R.A. Successful treatment of erosive lichen planus with topical tacrolimus. Arch. Derm. 2001, 137, 419–422. [Google Scholar]
- Schäcke, H.; Döcke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Carbone, M.; Conrotto, D.; Carrozzo, M.; Broccoletti, R.; Gandolfo, S.; Scully, C. Topical corticosteroids in association with miconazole and chlorhexidine in the long-term management of atrophic-erosive oral lichen planus: A placebo-controlled and comparative study between clobetasol and fluocinonide. Oral Dis. 1999, 5, 44–49. [Google Scholar] [CrossRef]
- Conrotto, D.; Carbone, M.; Carrozzo, M.; Arduino, P.; Broccoletti, R.; Pentenero, M.; Gandolfo, S. Ciclosporin vs. clobetasol in the topical management of atrophic and erosive oral lichen planus: A double-blind, randomized controlled trial. Br. J. Dermatol. 2016, 154, 139–145. [Google Scholar] [CrossRef]
- Andreasen, J. Oral lichen planus: I. A clinical evaluation of 115 cases. Oral Surg. Oral Med. Oral Pathol. 1968, 25, 31–42. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. In A Systematic Review; SAGE Publications: London, UK, 2016. [Google Scholar]
- Asparuhova, M.B.; Kiryak, D.; Eliezer, M.; Mihov, D.; Sculean, A. Activity of two hyaluronan preparations on primary human oral fibroblasts. J. Periodontal. Res. 2019, 54, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Gallelli, L.; Meacci, F.; Brugnolli, A.; Prosperi, L.; Roberta, S.; Eccher, C.; Mazzoli, S.; Lanzafame, P.; Caciagli, P. The efficacy of umbelliferone, arbutin, and N-acetylcysteine to prevent microbial colonization and biofilm development on urinary catheter surface: Results from a preliminary study. J. Pathog. 2016, 2016, 1590952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tümen, İ.; Akkol, E.K.; Taştan, H.; Süntar, I.; Kurtca, M. Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine (Pinus pinaster Ait). J. Ethnopharmacol. 2018, 211, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Goyal, R.; Sharma, L. Potential biological efficacy of Pinus plant species against oxidative, inflammatory and microbial disorders. BMC Complement. Altern. Med. 2015, 16, 35. [Google Scholar] [CrossRef] [Green Version]
- Hegarty, A.; Hodgson, T.; Lewsey, J.; Porter, S. Fluticasone propionate spray and betamethasone sodium phosphate mouthrinse: A randomized crossover study for the treatment of symptomatic oral lichen planus. J. Am. Acad. Dermatol. 2002, 47, 271–279. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Leonardi, R. Independent impact of periodontitis and cardiovascular disease on elevated soluble urokinase-type plasminogen activator receptor (suPAR) levels. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Amantea, M.; Paduano, F.; Santacroce, L.; Gentile, S.; Scacco, S. Bioimpedance Detection of Oral Lichen Planus Used as Preneoplastic Model. J. Cancer. 2015, 6, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Seta, R.; Mascitti, M.; Campagna, R.; Sartini, D.; Fumarola, S.; Santarelli, A.; Giuliani, M.; Cecati, M.; Muzio, L.L.; Emanuelli, M. Overexpression of nicotinamide N-methyltransferase in HSC-2 OSCC cell line: Effect on apoptosis and cell proliferation. Clin. Oral Investig. 2019, 23, 829–838. [Google Scholar] [CrossRef]
- Santarelli, A.; Mascitti, M.; Rubini, C.; Bambini, F.; Giannatempo, G.; Lo Russo, L.; Sartini, D.; Emanuelli, M.; Procaccini, M.; Lo Muzio, L. Nuclear Survivin as a Prognostic Factor in Squamous-Cell Carcinoma of the Oral Cavity. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 566–570. [Google Scholar] [CrossRef]
- Patini, R.; Gallenzi, P.; Spagnuolo, G.; Cordaro, M.; Cantiani, M.; Amalfitano, A.; Arcovito, A.; Callà, C.; Mingrone, G.; Nocca, G. Correlation Between Metabolic Syndrome, Periodontitis and Reactive Oxygen Species Production. A Pilot Study. Open Dent. J. 2017, 11, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Troiano, G.; Dioguardi, M.; Giannatempo, G.; Laino, L.; Testa, N.F.; Cocchi, R.; De Lillo, A.; Lo Muzio, L. Orofacial granulomatosis: Clinical signs of different pathologies. Med. Princ. Pract. 2015, 24, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, A.; Mascitti, M.; Lo Russo, L.; Sartini, D.; Troiano, G.; Emanuelli, M.; Lo Muzio, L. Survivin-Based Treatment Strategies for Squamous Cell Carcinoma. Int. J. Mol. Sci. 2018, 19, 971. [Google Scholar] [CrossRef] [Green Version]
- Dioguardi, M.; Caloro, G.A.; Troiano, G.; Giannatempo, G.; Laino, L.; Petruzzi, M.; Lo Muzio, L. Oral manifestations in chronic uremia patients. Ren. Fail. 2016, 38, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krifka, S.; Hiller, K.A.; Bolay, C.; Petzel, C.; Spagnuolo, G.; Reichl, F.X.; Schmalz, G.; Schweikl, H. Function of MAPK and downstream transcription factors in monomer-induced apoptosis. Biomaterials 2012, 33, 740–750. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Clobetasol (n = 18) | Anti-Inflammatory Mouthwash (n = 20) |
|---|---|---|
| Age (years), mean ± SD | 65.55 ± 9.61 | 62.5 ± 9.13 |
| Age (years), range | 48–80 | 32–79 |
| Gender (male/female) | 8 a 10 | 10 a 10 |
| Females/Total (%) | 44% | 50% |
| Males/Total (%) | 46% | 50% |
| Age males, mean ± SD | 63.08 ± 11.47 | 62 ± 13.67 |
| Age females, mean ± SD | 67.88 ± 6.92 | 62.4 ± 13.07 |
| Scheme | |||||
|---|---|---|---|---|---|
| Treatment | Baseline (T0) | After 3 Months (T1) | p-Value | ||
| Median | Min–Max | Median | Min–Max | ||
| Clobetasol | 3 | 1–5 | 2.5 | 0–3 | <0.001 |
| Anti-inflammatory | 1 | 1–4 | 1.5 | 1–3 | 0.02 |
| Symptoms Score (Numerical Pain Score (NRS) Score) | |||
|---|---|---|---|
| Treatment | Baseline (T0) | After 3 Months (T1) | p-Value |
| Mean ± SD | Mean ± SD | ||
| Clobetasol | 4.67 ± 2.25 | 2.33 ± 1.64 | <0.001 * |
| Anti-inflammatory | 3.05 ± 1.23 | 1.85 ± 1.23 | 0.02 ** |
| Downstaging Score | |||||
|---|---|---|---|---|---|
| Parameters | Clobetasol | Anti-Inflammatory | p-Value | ||
| Median | Min–Max | Median | Min–Max | ||
| Symptoms | 3 | 0–4 | 1 | 0–2 | 0.009 * |
| Signs | 1 | 0–3 | 1 | 0–3 | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santonocito, S.; Polizzi, A.; De Pasquale, R.; Ronsivalle, V.; Lo Giudice, A.; Isola, G. Analysis of the Efficacy of Two Treatment Protocols for Patients with Symptomatic Oral Lichen Planus: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 56. https://doi.org/10.3390/ijerph18010056
Santonocito S, Polizzi A, De Pasquale R, Ronsivalle V, Lo Giudice A, Isola G. Analysis of the Efficacy of Two Treatment Protocols for Patients with Symptomatic Oral Lichen Planus: A Randomized Clinical Trial. International Journal of Environmental Research and Public Health. 2021; 18(1):56. https://doi.org/10.3390/ijerph18010056
Chicago/Turabian StyleSantonocito, Simona, Alessandro Polizzi, Rocco De Pasquale, Vincenzo Ronsivalle, Antonino Lo Giudice, and Gaetano Isola. 2021. "Analysis of the Efficacy of Two Treatment Protocols for Patients with Symptomatic Oral Lichen Planus: A Randomized Clinical Trial" International Journal of Environmental Research and Public Health 18, no. 1: 56. https://doi.org/10.3390/ijerph18010056






