The Immobilization Effect of Natural Mineral Materials on Cr(VI) Remediation in Water and Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Aqueous Solution Experiment
2.2.2. Adsorption Methods
2.2.3. Soil Experiment
3. Results and Discussion
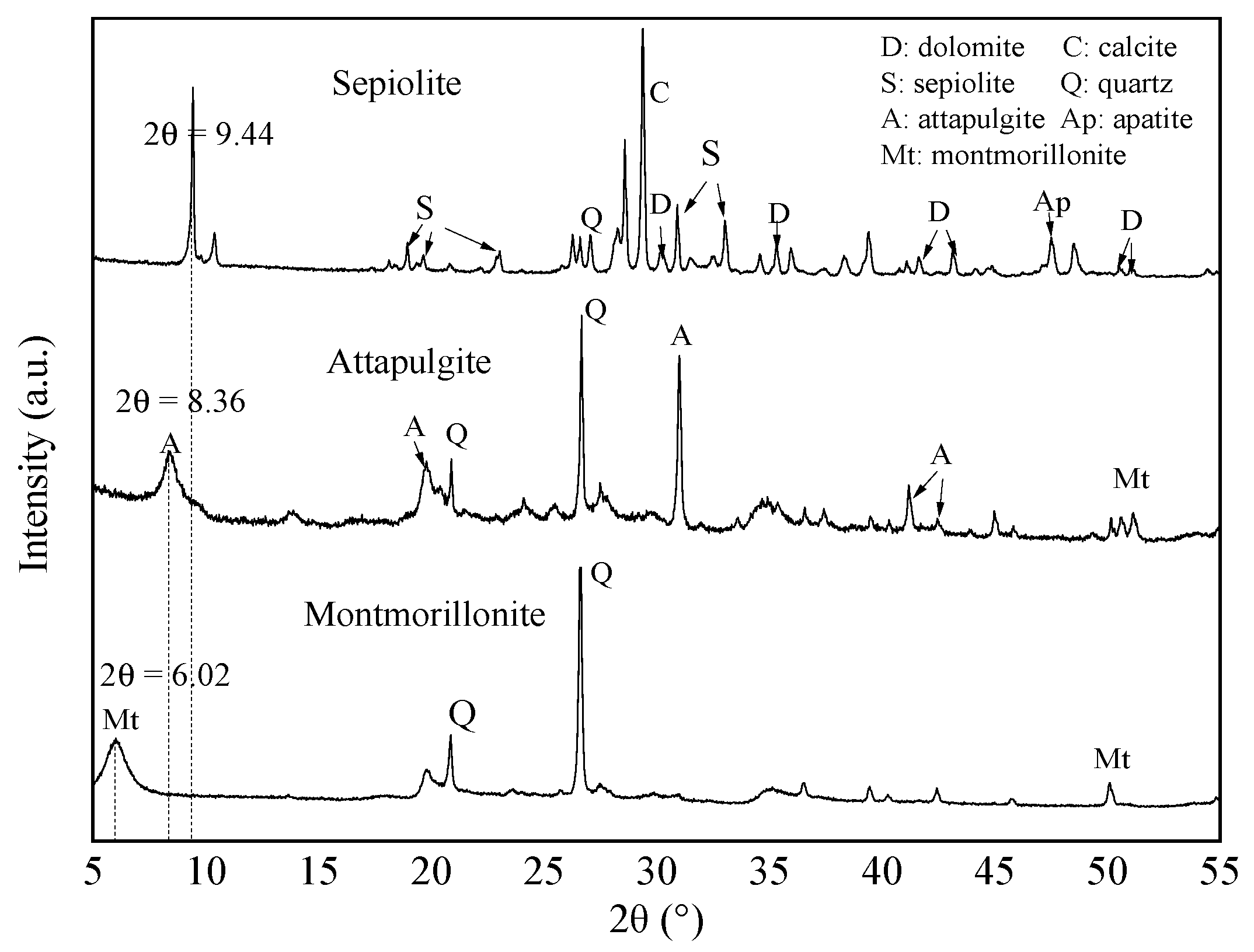
3.1. XRD Analysis
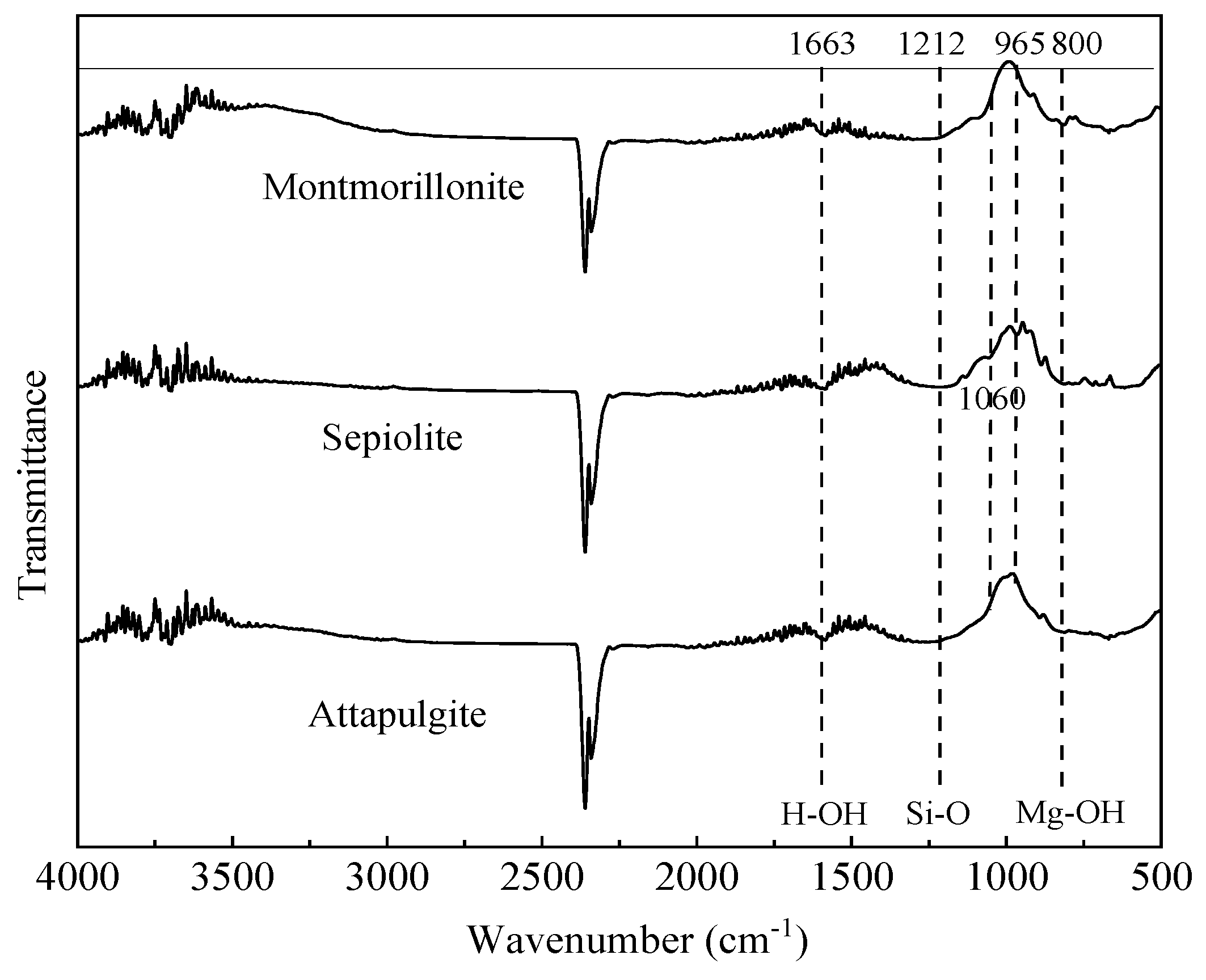
3.2. FTIR Spectral Analysis
3.3. BET Analysis
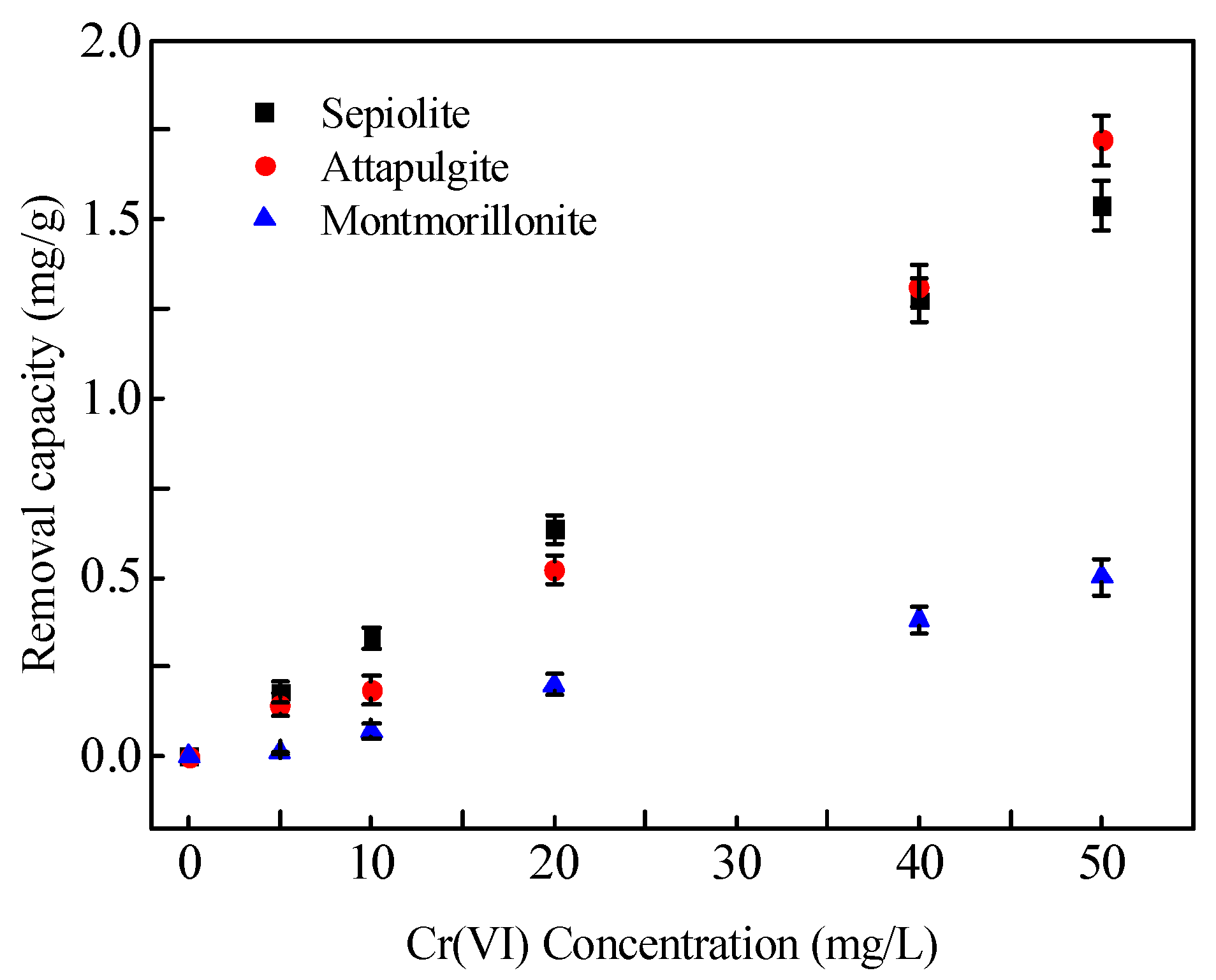
3.4. Removal of Cr(VI) from Aqueous Solution Using Mineral Materials
3.4.1. Removal of Cr(VI) from Aqueous Solution by Mineral Materials
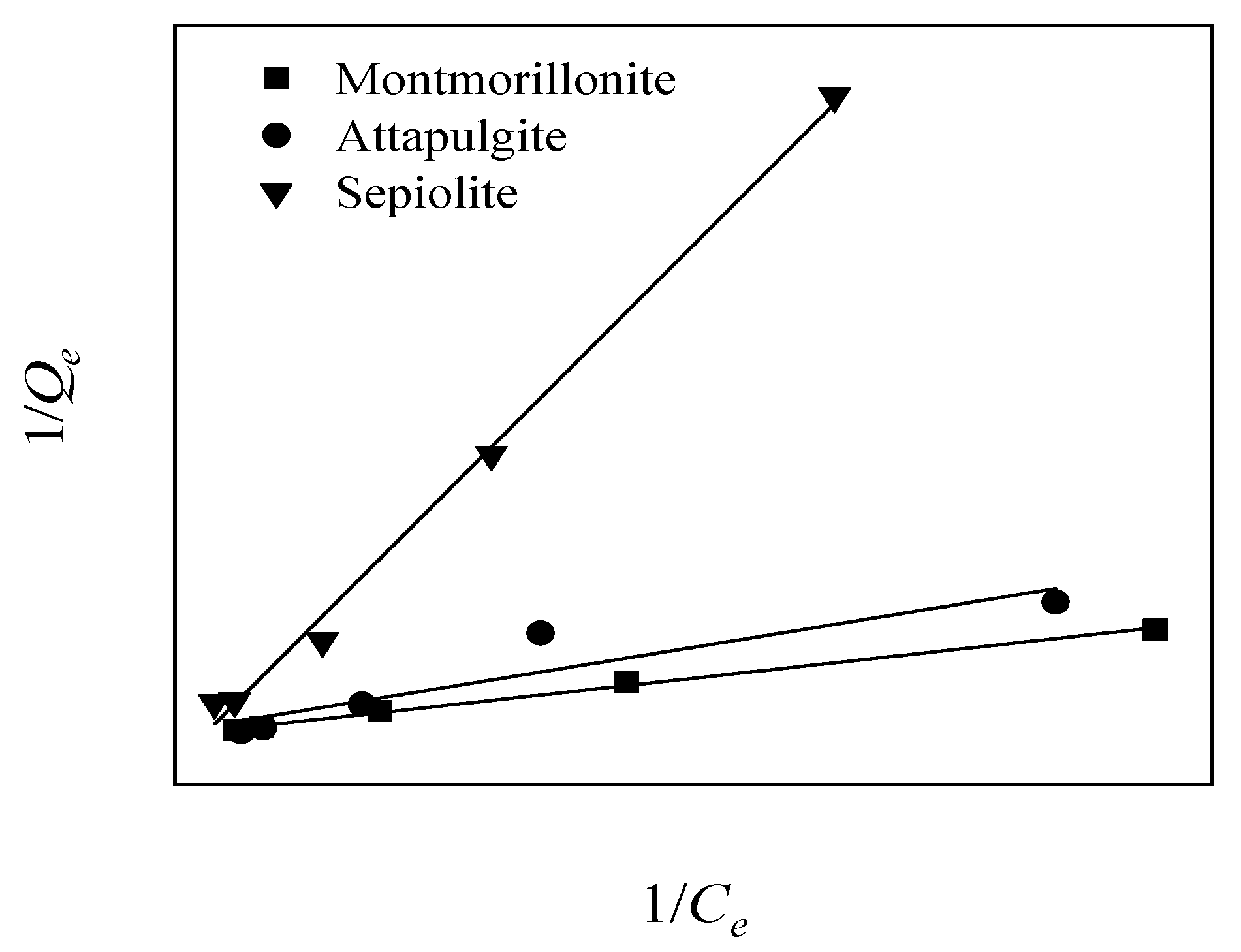
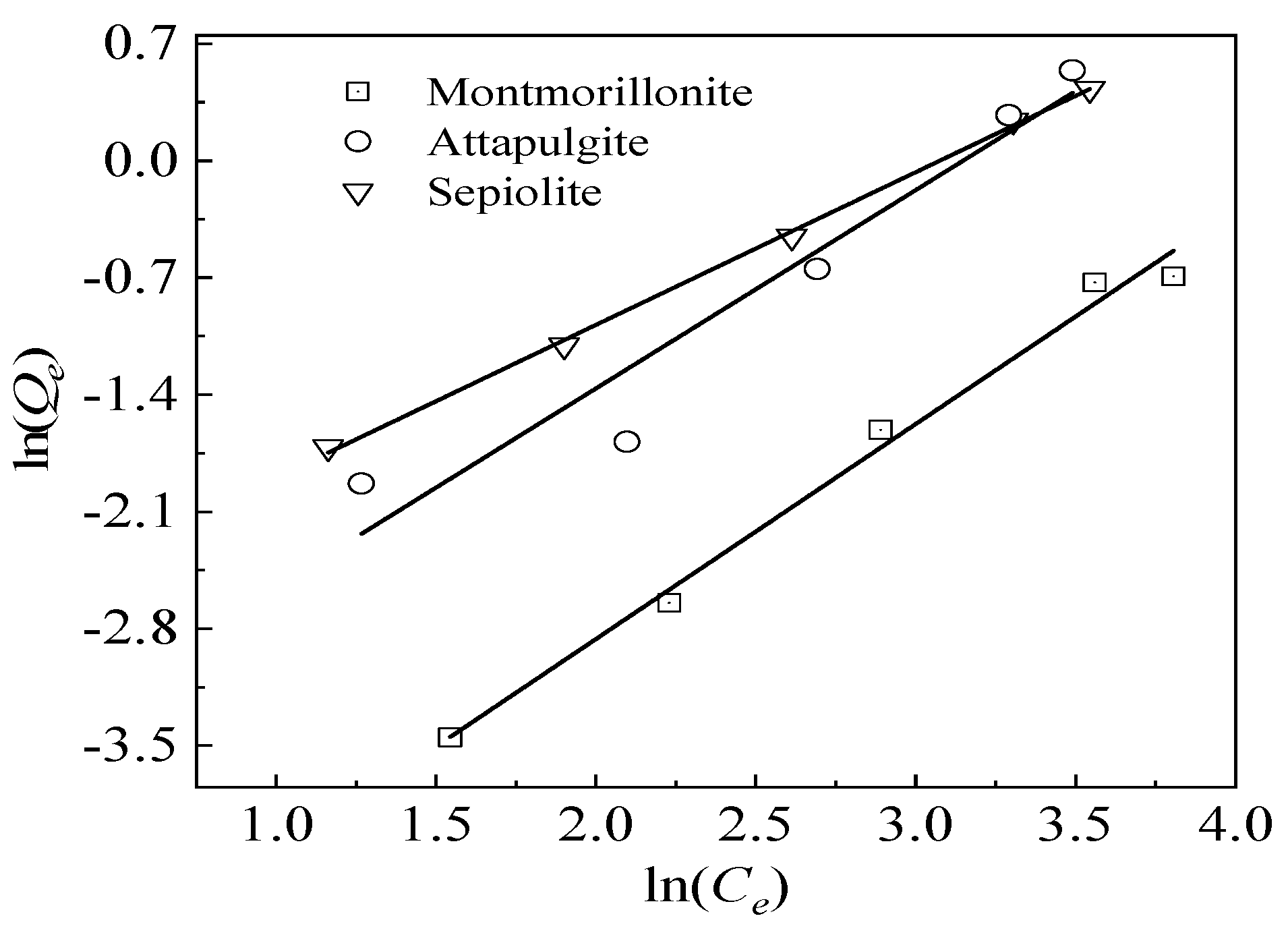
3.4.2. Adsorption Isotherms
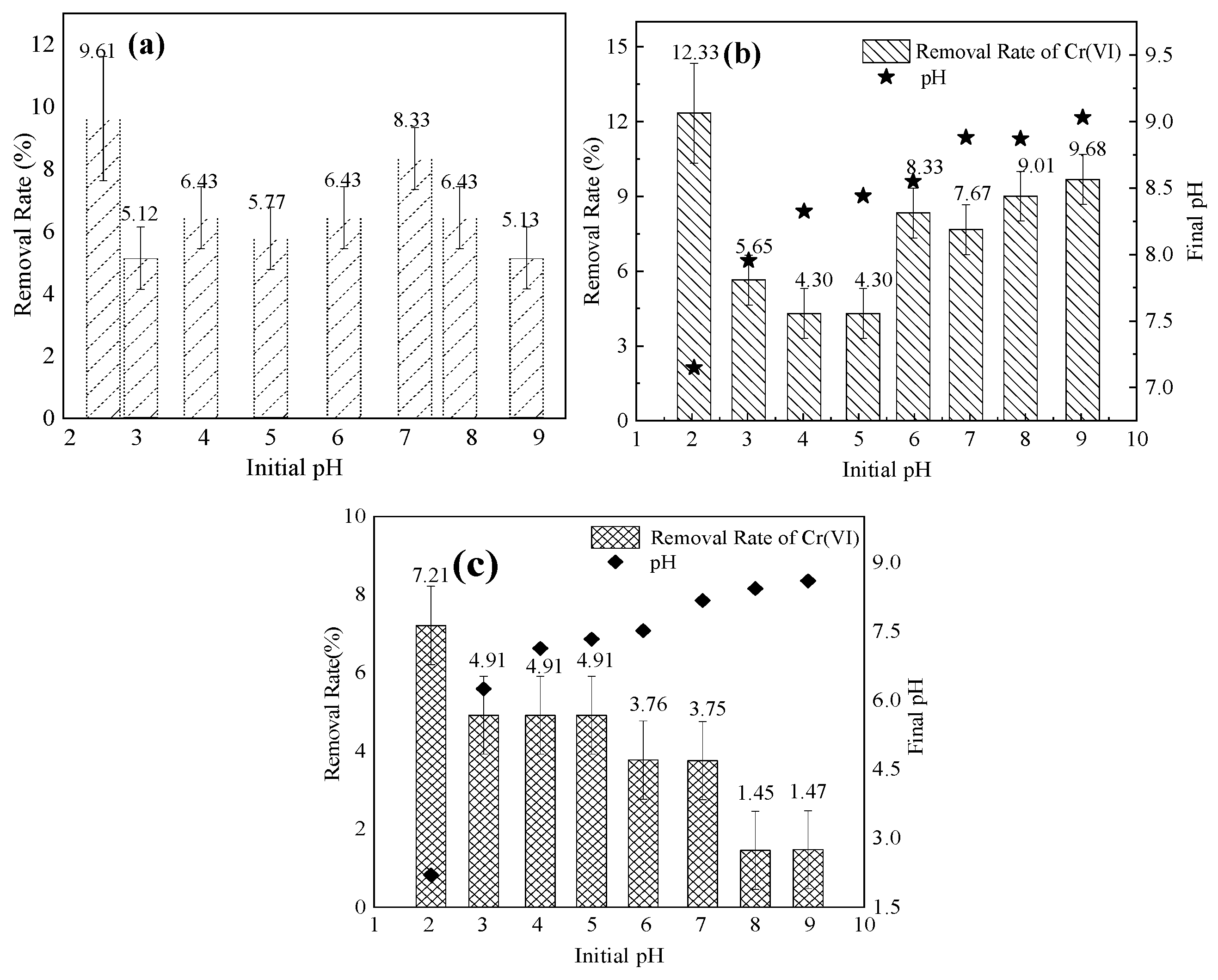
3.4.3. Effect of pH on Adsorption
3.5. Cr-Contaminated Soil Remediation by the Mineral Materials
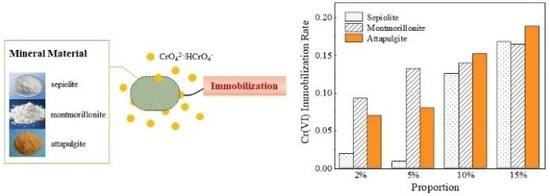
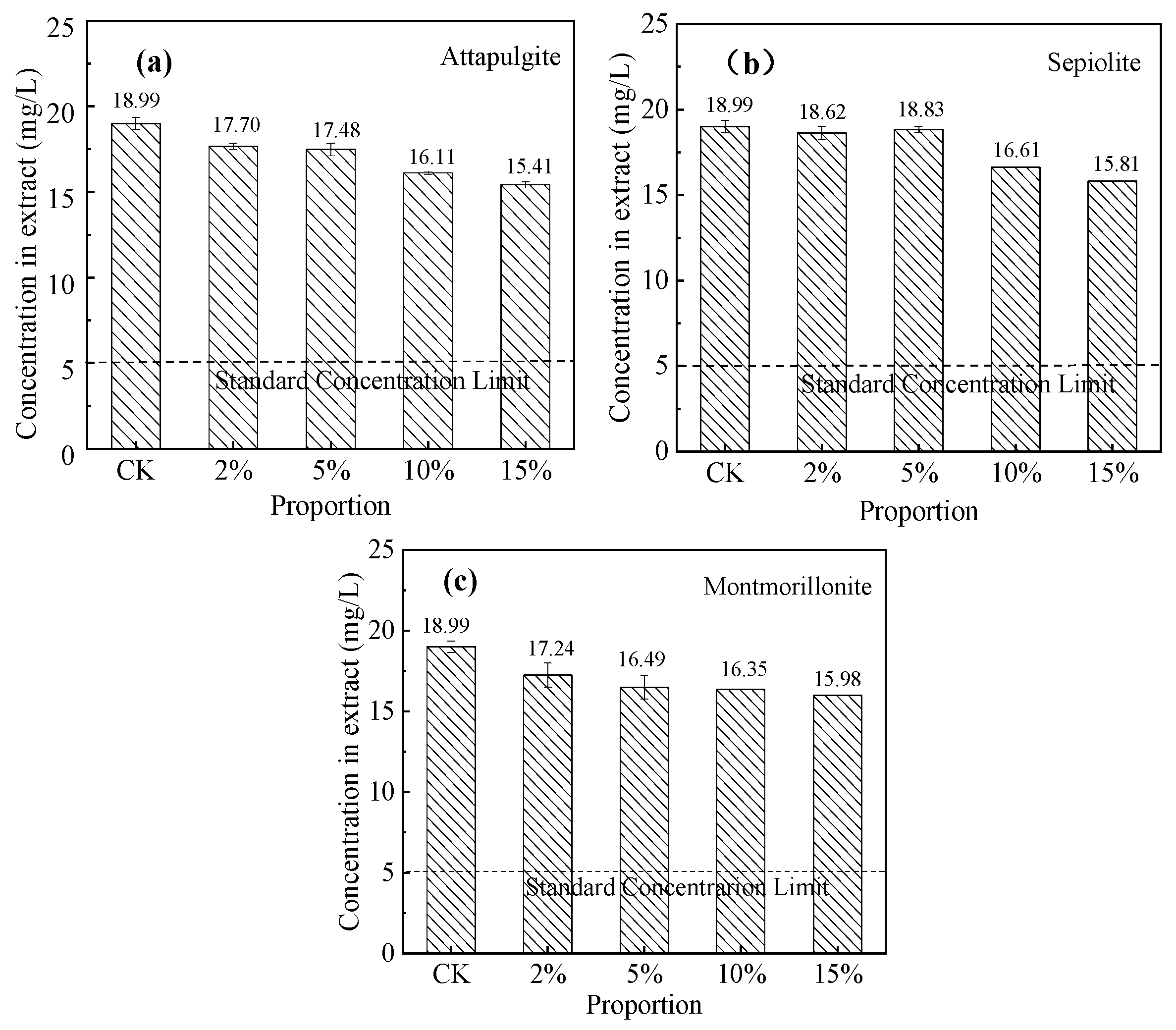
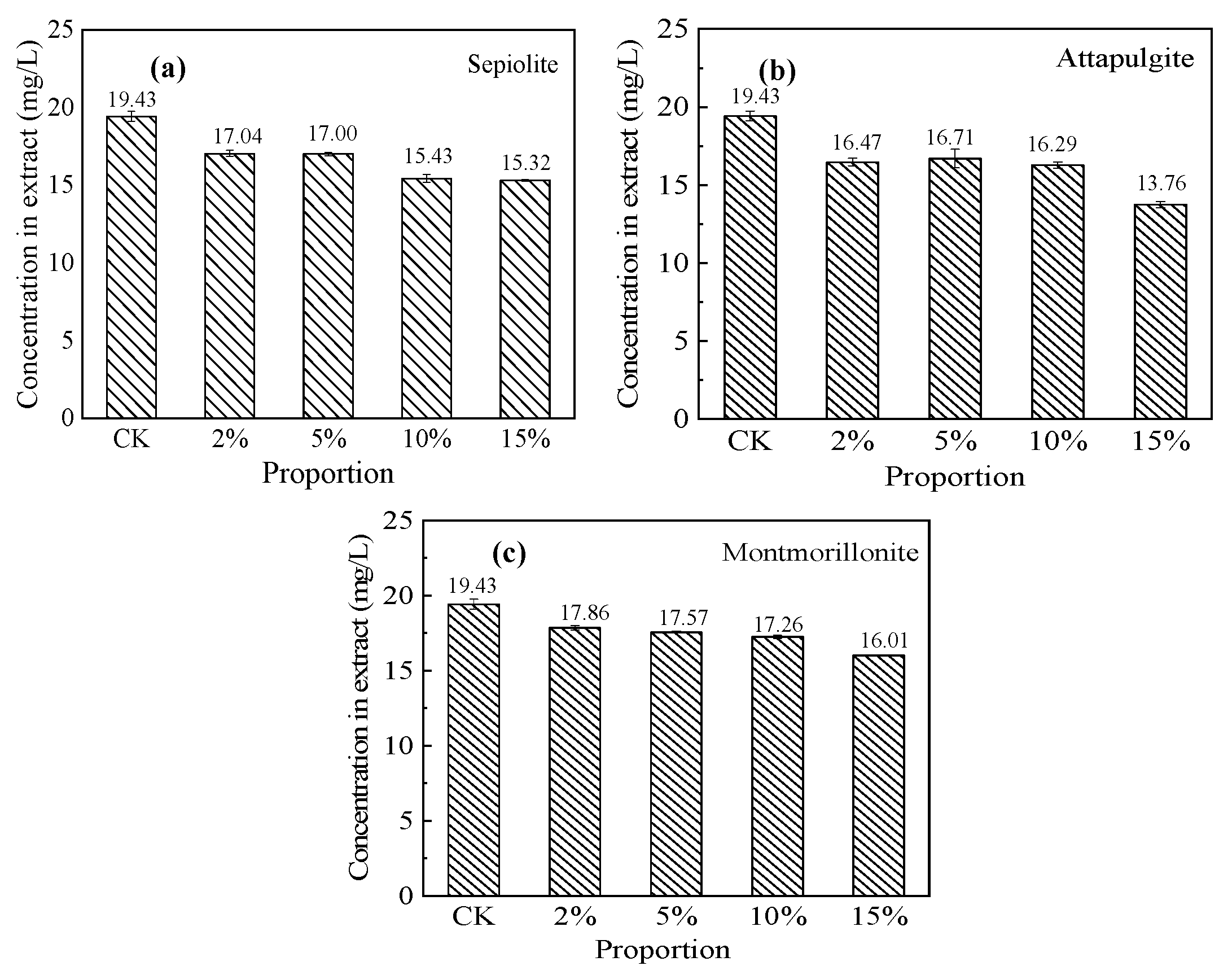
3.5.1. Cr(VI) Immobilization Effect
3.5.2. Total Cr Immobilization Effect
3.6. Effect of Mineral Materials on Soil pH
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| XRD | X-ray diffraction |
| FTIR | Fourier-transform infrared |
| BET | Brunauer–Emmett–Teller |
References
- Chen, T.; Chang, Q.R.; Liu, J.; Clevers, J.G.P.W.; Kooistra, L. Identification of soil heavy metal sources and improvement in spatial mapping based on soil spectral information: A case study in northwest China. Sci. Total Environ. 2016, 565, 155–164. [Google Scholar] [CrossRef]
- Un, U.T.; Onpeker, S.E.; Ozel, E. The treatment of chromium containing wastewater using electrocoagulation and the production of ceramic pigments from the resulting sludge. J. Environ. Manag. 2017, 200, 196–203. [Google Scholar]
- Ziati, M.; Khemmari, F.; Aitbara, A.; Hazourli, S. Reduction of turbidity and chromium content of tannery wastewater by electrocoagulation process. Water Environ. Res. 2018, 90, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.X.; Li, L.N.; Tang, G.G.; Jing, L.X. Source, regional distribution and treatment status of chromium-containing waste in China, and regulatory recommendations. Environ. Monitor. China 2013, 29, 201–204. (In Chinese) [Google Scholar]
- Ministry of Environmental Protection & Ministry of Land and Resources of the People’s Republic of China. The Report on the National General Survey of Soil Contamination; Ministry of Environmental Protection & Ministry of Land and Resources of the People’s Republic of China: Beijing, China, 2014.
- Chrysochoou, M.; Johnston, C.P.; Dahal, G. A comparative evaluation of hexavalent chromium treatment in contaminated soil by calcium polysulfide and green-tea nanoscale zero-valent iron. J. Hazard. Mater. 2012, 201, 33–42. [Google Scholar] [CrossRef]
- Speer, R.M.; Wise, C.F.; Young, J.L.; Aboueissa, A.; Bras, M.M.; Barandiaran, M.; Bermudez, E.; Marquez-D’Acunti, L.; Wise, J.P. The cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in leatherback sea turtle lung cells. Aquat. Toxicol. 2018, 198, 149–157. [Google Scholar] [CrossRef]
- Helena, O. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012. [Google Scholar] [CrossRef]
- Fendorf, S.E. Surface reactions of chromium in soils and waters. Geoderma 1995, 67, 55–71. [Google Scholar] [CrossRef]
- Gad, S.C. Acute and chronic systemic chromium toxicity. Sci. Total Environ. 1989, 86, 149–157. [Google Scholar] [CrossRef]
- Lee, K.P.; Ulrich, C.E.; Geil, R.G.; Trochimowicz, H.J. Inhalation toxicity of chromium dioxide dust to rats after two years exposure. Sci. Total Environ. 1989, 86, 83–108. [Google Scholar] [CrossRef]
- Kotas, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Amin, A.S.; Kassem, M.A. Chromium speciation in environmental samples using a solid phase spectrophotometric method. Spectrochim. Acta. A 2012, 96, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Bag, H.; Turker, A.R.; Lale, M.; Tunceli, A. Separation and speciation of Cr(III) and Cr(VI) with Saccharomyces cerevisiae immobilized on sepiolite and determination of both species in water by FAAS. Talanta 2000, 51, 895–902. [Google Scholar] [CrossRef]
- Choppala, G.; Kunhikrishnan, A.; Seshadri, B.; Park, J.H.; Bush, R.; Bolan, N. Comparative sorption of chromium species as influenced by pH, surface charge and organic matter content in contaminated soils. J. Geochem. Explor. 2018, 184, 255–260. [Google Scholar] [CrossRef]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250, 272–291. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Lu, J.S.; Wu, Q.; Jing, C.Y. Reduction and immobilization of chromate in chromite ore processing residue with nanoscale zero-valent iron. J. Hazard. Mater. 2012, 215, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Geelhoed, J.S.; Meeussen, J.C.L.; Roe, M.J.; Hillier, S.; Thomas, R.; Farmer, J.; Paterson, E. Chromium remediation or release effect of iron(II) sulfate addition on chromium(VI) leaching from columns of chromite ore processing residue. Environ. Sci. Technol. 2003, 37, 3206–3213. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Misra, V.; Singh, R.P. Removal of Cr(VI) by nanoscale zero-valent iron (nZVI) from soil contaminated with tannery wastes. Bull. Environ. Contam. Toxicol. 2012, 88, 210–214. [Google Scholar] [CrossRef]
- Su, C.Q.; Li, L.Q.; Yang, Z.H.; Chai, L.Y.; Liao, Q.; Shi, Y.; Li, J.W. Cr(VI) reduction in chromium-contaminated soil by indigenous microorganisms under aerobic condition. Trans. Nonferrous Met. Soc. China 2019, 29, 1304–1311. [Google Scholar] [CrossRef]
- Saravanan, A.; Jayasree, R.; Hemavathy, R.V.; Jeevanantham, S.; Hamsini, S.; Senthil Kumar, P.; Yaashikaa, P.R.; Manivasagan, V.; Yuvaraj, D. Phytoremediation of Cr(VI) ion contaminated soil using Black gram (Vigna mungo): Assessment of removal capacity. J. Environ. Chem. Eng. 2019, 7, 103052. [Google Scholar]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.F.; Wang, S.; Sun, W.; Sun, Z.M.; Zheng, S.L. Synthesis of a novel illite@carbon nanocomposite adsorbent for removal of Cr(VI) from wastewater. J. Environ. Sci. 2017, 57, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kobya, M. Removal of Cr(VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: Kinetic and equilibrium studies. Bioresour. Technol. 2004, 91, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, K.; Das, D.; Biswas, M.N. Preparation and characterization of activated carbons from Sterculia alata nutshell by chemical activation with zinc chloride to remove phenol from wastewater. Adsorption 2006, 12, 119–132. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Dawodu, F.A. Acid-modified montmorillonite for sorption of heavy metals from automobile effluent. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Volzone, C. Retention of pollutant gases: Comparison between clay minerals and their modified products. Appl. Clay Sci. 2007, 36, 191–196. [Google Scholar] [CrossRef]
- Sdiri, A.; Khairy, M.; Bouaziz, S.; El-Safty, S. A natural clayey adsorbent for selective removal of lead from aqueous solutions. Appl. Clay Sci. 2016, 126, 89–97. [Google Scholar] [CrossRef]
- Zotiadis, V.; Argyraki, A. Development of innovative environmental applications of attapulgite clay. Bull. Geol. Soc. Greece 2013, 47, 992–1001. [Google Scholar] [CrossRef] [Green Version]
- Kocaoba, S. Adsorption of Cd(II), Cr(III) and Mn(II) on natural sepiolite. Desalination 2009, 244, 24–30. [Google Scholar] [CrossRef]
- Padilla-Ortega, E.; Leyva-Ramos, R.; Mendoza-Barron, J.; Guerrero-Coronado, R.M.; Jacobo-Azuara, A.; Aragon-Pina, A. Adsorption of Heavy Metal Ions from Aqueous Solution onto Sepiolite. Adsorpt. Sci. Technol. 2011, 29, 569–584. [Google Scholar] [CrossRef]
- Yu, K.; Xu, J.; Jiang, X.H.; Liu, C.; McCall, W.; Lu, J.L. Stabilization of heavy metals in soil using two organo-bentonites. Chemosphere 2017, 184, 884–891. [Google Scholar] [CrossRef]
- Yang, J.; Yu, K.; Liu, C. Chromium immobilization in soil using quaternary ammonium cations modified montmorillonite: Characterization and mechanism. J. Hazard. Mater. 2017, 321, 73–80. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, Q.; Wang, X.Z.; Liang, J.H.; Chen, C.; Luo, H.; Mou, H.M.; Deng, Q.L.; Zhang, T.H.; Jiang, J.L. Removal of Cr(III) using humic acid-modified attapulgite. J. Environ. Eng. 2019, 145, 04019028. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Anayurt, R.A.; Sari, A.; Tuzen, M. Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem. Eng. J. 2009, 151, 255–261. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen. Zeitschrift Physikalische Chemie (Leipzig) 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. Solid Waste-Extraction Procedure for Leaching Toxicity—Acetic Acid Buffer Solution Method; HJ/T 300-2007; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2007.
- Ministry of Environmental Protection of the People’s Republic of China. Soil Quality-Determination of Cation Exchange Capacity (CEC)-Hexamminecobalt Trichloride Solution—Spectrophotometric Method; HJ 889-2017; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2017.
- Ministry of Environmental Protection of the People’s Republic of China. Soil-Determination of Organic Carbon—Potassium Dichromate Oxidation Spectrophotometric Method; HJ 615-2011; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2011.
- Zhu, K.C.; Jia, H.Z.; Wang, F.; Zhu, Y.Q.; Wang, C.Y.; Ma, C.Y. Efficient Removal of Pb(II) from Aqueous Solution by Modified Montmorillonite/Carbon Composite: Equilibrium, Kinetics, and Thermodynamics. J. Chem. Eng. Data 2017, 62, 333–340. [Google Scholar] [CrossRef]
- Wang, M.M.; Qian, R.; Bao, M.; Gu, C.X.; Zhu, P.Z. Raman, FT-IR and XRD study of bovine bone mineral and carbonated apatites with different carbonate levels. Mater. Lett. 2018, 210, 203–206. [Google Scholar] [CrossRef]
- Weiss, Y.; Kiflawi, I.; Navon, O. IR spectroscopy: Quantitative determination of the mineralogy and bulk composition of fluid microinclusions in diamonds. Chem. Geol. 2010, 275, 26–34. [Google Scholar] [CrossRef]
- Gheju, M.; Balcu, I.; Mosoarca, G. Removal of Cr(VI) from aqueous solutions by adsorption on MnO2. J. Hazard. Mater. 2016, 310, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Glatstein, D.A.; Francisca, F.M. Influence of pH and ionic strength on Cd, Cu and Pb removal from water by adsorption in Na-bentonite. Appl. Clay Sci. 2015, 118, 61–67. [Google Scholar] [CrossRef]
- Gheju, M. Hexavalent Chromium Reduction with Zero-Valent Iron (ZVI) in Aquatic Systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Lee, S.; Kim, W.; Laldawngliana, C.; Tiwari, D. Removal Behavior of Surface Modified Sand for Cd(II) and Cr(VI) from Aqueous Solutions. J. Chem. Eng. Data 2010, 55, 3089–3094. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Khan, T.A.; Ali, I. Equilibrium, kinetic and thermodynamic studies of Cr(VI) adsorption from aqueous solution onto manganese oxide coated sand grain (MOCSG). J. Mol. Liq. 2017, 236, 320–330. [Google Scholar] [CrossRef]
| Soil Properties | Redox Potential (mV) | Organic Matter (g/kg) | Cation Exchange Capacity (cmol/kg) | pH | Cr(VI) Content (mg/kg) | Total Cr Content (mg/kg) |
|---|---|---|---|---|---|---|
| Test values | 227 ± 21 | 15.6 ± 3 | 26.0 ± 4 | 8.16 ± 0.02 | 424.59 ±17 | 718.05 ± 28 |
| Mineral Material | BET Specific Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| Sepiolite | 5.73 ± 0.05 | 0.018 ± 0.03 |
| Attapulgite | 119.82 ± 0.1 | 0.31 ± 0.05 |
| Montmorillonite | 84.24 ± 0.08 | 0.11 ± 0.02 |
| Mineral Material | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL (L/mg) | Qm (mg/g) | R2 | KF (mg1−1/nL1/n/g) | n | R2 | |
| Sepiolite | 0.0133 | 4.35 | 0.9961 ** | 21.54 | 0.92 | 0.9988 ** |
| Attapulgite | 0.0133 | 2.94 | 0.8594 * | 22.42 | 1.27 | 0.9355 ** |
| Montmorillonite | 0.0162 | 0.39 | 0.9955 ** | 67.36 | 1.30 | 0.9904 ** |
| Material | pH | Group | pH |
|---|---|---|---|
| Untreated Soil | 8.16 | Blank | 8.16 |
| Sepiolite | 8.59 | Soil + 5% Sepiolite | 8.10 ± 0.02 |
| Soil + 10% Sepiolite | 8.09 ± 0.02 | ||
| Soil + 15% Sepiolite | 8.09 ± 0.02 | ||
| Attapulgite | 6.74 | Soil + 5% Attapulgite | 8.09 ± 0.02 |
| Soil + 10% Attapulgite | 7.97 ± 0.02 | ||
| Soil + 15% Attapulgite | 7.96 ± 0.02 | ||
| Montmorillonite | 8.02 | Soil + 5% Montmorillonite | 8.01 ± 0.02 |
| Soil + 10% Montmorillonite | 8.03 ± 0.03 | ||
| Soil + 15% Montmorillonite | 8.06 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Xu, Y.; Li, X.; Wang, L.; He, X.; Ma, Y.; Zou, D. The Immobilization Effect of Natural Mineral Materials on Cr(VI) Remediation in Water and Soil. Int. J. Environ. Res. Public Health 2020, 17, 2832. https://doi.org/10.3390/ijerph17082832
Zhang D, Xu Y, Li X, Wang L, He X, Ma Y, Zou D. The Immobilization Effect of Natural Mineral Materials on Cr(VI) Remediation in Water and Soil. International Journal of Environmental Research and Public Health. 2020; 17(8):2832. https://doi.org/10.3390/ijerph17082832
Chicago/Turabian StyleZhang, Dading, Yanqiu Xu, Xiaofei Li, Lina Wang, Xuwen He, Yan Ma, and Dexun Zou. 2020. "The Immobilization Effect of Natural Mineral Materials on Cr(VI) Remediation in Water and Soil" International Journal of Environmental Research and Public Health 17, no. 8: 2832. https://doi.org/10.3390/ijerph17082832