Smokers’ Views on Personal Carbon Monoxide Monitors, Associated Apps, and Their Use: An Interview and Think-Aloud Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participant Recruitment
2.3. Interview Procedure and Materials
2.4. Data Analysis
3. Results
3.1. Participants
3.2. Findings from the Interviews
3.2.1. Theme 1: CO Testing—General Views and Motivation to Use
3.2.2. Theme 2: Practicalities of CSS Use
3.2.3. Theme 3: Factors Potentially Affecting Preferences, Views and Engagement with CSSs
3.2.4. Theme 4: Personal CO Monitor: Features and Qualities
3.2.5. Theme 5: CSSs Apps: Features and Qualities
4. Discussion
4.1. Summary of Findings
4.2. Implications for Development and Evaluation of CSSs in the Future
4.3. Strengths and Limitations
4.4. Future Research Directions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Interview Focus and Specific Topics | Method and Prompts | |
|---|---|---|
| Needs assessment | Part 1: Participant profile and background information (about 5 min) Brief discussion about past and current smoking and quitting history Prior use of any digital programs (e.g., apps, devices) Any experience with CO monitors and testing in the past | Open-ended questions + verbal prompts No visual materials or prompts |
| Part 2: Assessment of needs and expectations for CO testing (about 10 min) Interest in CO testing? Why? Interested to use CO Smartphone System? Why? Expectations/general views on CSS Expectations towards CO monitor and associated apps Desired functionality/features of CO monitor and associated apps Expectations for CSS use (When?/Where?/How?/Why?) Readiness to share results (With whom? How?) Reminders (Email, Push notifications, Text) Expectations for information/advice (topic, location, timing of delivery) | Open-ended questions + verbal prompts No visual materials or prompts | |
| Demonstration | Part 3: Views and reactions to personal CO monitor General views on iCOTM Smokerlyzer® Designs/looks Size Features and functionality Battery life (about 200 tests or 3 years) Suggestions for improvement | Open-ended questions + verbal prompts Visual prompts used: iCOTM Smokerlyzer® No app shown |
| User testing of apps or functioning app prototypes | Part 4A (only in 2016): Views on, and reactions to, available apps or app prototypes developed to work with a personal CO monitor (about 15 min). Overall impressions (Likes and Dislikes) Whether would like to use it (when? where? how often?) User journey through the app Journey to complete CO testing Displaying CO results history Other content, information and advice Other issues (language, terminology, amount of texts) Views on ease of use How to improve? Discussion of additional suggestions and possible features (e.g., sharing results, using reminders, setting targets, scheduling testing, etc.) | Usability tests (exploring the apps naturally, navigating the different content and features) + think-aloud procedure Visual prompts used: Smokerlyzer® app by Bedfont® CO Monitor App prototypes (V1–V2) and designs developed for UCL |
| Part 4B: (only in 2017): Usability testing of CO Monitor app (V3) developed for UCL and discussion of piloting use of iCOTM Smokerlyzer® and CO Monitor App V3 at home Specific prompts as in 4A above | Usability testing and think-aloud. Visual prompts used: CO Monitor app (V3) developed for UCL |
| 2016 | 2017 | ||
|---|---|---|---|
| Bedfont® Scientific Ltd. Smokerlyzer® App | UCL CO Monitor App (V1) | UCL CO Monitor App (V3) | |
| Think-aloud of functioning apps or app prototypes | 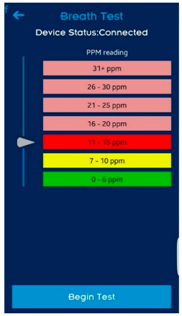 |  |  |
| UCL CO Monitor Interactive Design (V2) | UCL Different CO Monitor Designs | ||
| Think-aloud about click-through or static designs of new app prototypes | 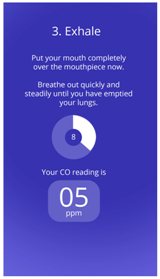 | 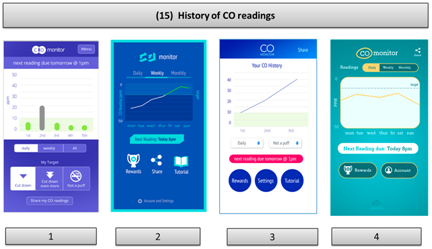 | N/A |
References
- Kotz, D.; Fidler, J.; West, R. Factors associated with the use of aids to cessation in English smokers. Addiction 2009, 104, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Raupach, T.; West, R.; Brown, J. The most “successful” method for failing to quit smoking is unassisted cessation. Nicotine Tob. Res. 2013, 15, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Ubhi, H.K.; Michie, S.; Kotz, D.; Wong, W.C.; West, R. A mobile app to aid smoking cessation: Preliminary evaluation of SmokeFree28. J. Med. Internet Res. 2015, 17, e17. [Google Scholar] [CrossRef] [PubMed]
- Zeng, E.Y.; Vilardaga, R.; Heffner, J.L.; Mull, K.E.; Bricker, J.B. Predictors of Utilization of a Novel Smoking Cessation Smartphone App. Telemed. J. E-Health 2015, 21, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Bricker, J.B.; Copeland, W.; Mull, K.E.; Zeng, E.Y.; Watson, N.L.; Akioka, K.J.; Heffner, J.L. Single-arm trial of the second version of an acceptance & commitment therapy smartphone application for smoking cessation. Drug Alcohol Depend. 2017, 170, 37–42. [Google Scholar] [PubMed]
- Bricker, J.B.; Mull, K.E.; Kientz, J.A.; Vilardaga, R.; Mercer, L.D.; Akioka, K.J.; Heffner, J.L. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug Alcohol Depend. 2014, 143, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; West, W. A Guide to Development and Evaluation of Digital Behaviour Change Interventions in Healthcare; Silverback Publishing: London, UK, 2016. [Google Scholar]
- Yardley, L.; Morrison, L.; Bradbury, K.; Muller, I. The person-based approach to intervention development: Application to digital health-related behavior change interventions. J. Med. Internet Res. 2015, 17, e30. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.O.; Gans, S.P.; Ripley-Moffitt, C.; Kotsen, C.; Bars, M. Use of Expired Air Carbon Monoxide Testing in Clinical Tobacco Treatment Settings. Chest 2017. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.T.; Morice, A.H. Breath Carbon Monoxide as an Indication of Smoking Habit. Chest 2000, 117, 758–763. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Hajek, P.; Stead, L.; Stapleton, J. Outcome criteria in smoking cessation trials: Proposal for a common standard. Addiction 2005, 100, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Brose, L.S.; Tombor, I.; Shahab, L.; West, R. The effect of reducing the threshold for carbon monoxide validation of smoking abstinence—Evidence from the English Stop Smoking Services. Addict. Behav. 2013, 38, 2529–2531. [Google Scholar] [CrossRef] [PubMed]
- Beard, E.; West, R. Pilot Study of the Use of Personal Carbon Monoxide Monitoring to Achieve Radical Smoking Reduction. J. Smok. Cessat. 2012, 7, 12–17. [Google Scholar] [CrossRef]
- Jarvis, C.M.; Russell, M.A.; Feyerabend, C. Absorption of nicotine and carbon monoxide from passive smoking under natural conditions of exposure. Thorax 1983, 38, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Shahab, L.; West, R.; McNeill, A. A randomized, controlled trial of adding expired carbon monoxide feedback to brief stop smoking advice: Evaluation of cognitive and behavioral effects. Health Psychol. 2011, 30, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, L.; Smith, M.; Guo, Y.; Whitlock, G.; Bian, Z.; Kurmi, O.; Collins, R.; Chen, J.; Lv, S.; et al. Exhaled carbon monoxide and its associations with smoking, indoor household air pollution and chronic respiratory diseases among 512,000 Chinese adults. Int. J. Epidemiol. 2013, 42, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Lee, K.K.; McAllister, D.A.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N.L. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Chen, W.K.; Lin, C.L.; Kao, C.H. Carbon monoxide poisoning and subsequent cardiovascular disease risk: A nationwide population-based cohort study. Medicine 2015, 94, e624. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.; Magnus Sköld, C.; Grunewald, J.; Eklund, A.; Wheelock, A.M. Assessing Recent Smoking Status by Measuring Exhaled Carbon Monoxide Levels. PLoS ONE 2011, 6, e28864. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.; Jacob, P.; Ahijevych, K.L.; Jarvis, M.; Hall, S.; LeHouezec, J.; Hansson, A.; Lichtenstein, E.; Henningfield, J.; Tsoh, J.Y.; et al. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002, 4, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Yamaya, M. Relation between exhaled carbon monoxide levels and clinical severity of asthma. Clin. Exp. Allergy 2001, 31, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Nishimura, K.; Koyama, H.; Tsukino, M.; Oga, T.; Hajiro, T.; Mishima, M. Optimal cutoff level of breath carbon monoxide for assessing smoking status in patients with asthma and COPD. Chest 2003, 124, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Raiff, B.R.; Faix, C.; Turturici, M.; Dallery, J. Breath carbon monoxide output is affected by speed of emptying the lungs: Implications for laboratory and smoking cessation research. Nicotine Tob. Res. 2010, 12, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Bittoun, R. Carbon Monoxide Meter: The Essential Clinical Tool—The ‘Stethoscope’—Of Smoking Cessation. J. Smok. Cessat. 2012, 3, 69–70. [Google Scholar] [CrossRef]
- Louwagie, G.M.; Okuyemi, K.S.; Ayo-Yusuf, O.A. Efficacy of brief motivational interviewing on smoking cessation at tuberculosis clinics in Tshwane, South Africa: A randomized controlled trial. Addiction 2014, 109, 1942–1952. [Google Scholar] [CrossRef] [PubMed]
- Lorencatto, F.; West, R.; Michie, S. Specifying evidence-based behavior change techniques to aid smoking cessation in pregnancy. Nicotine Tob. Res. 2012, 14, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Walia, A.; Hyder, N.; Shahab, L.; Michie, S. Behavior change techniques used by the English Stop Smoking Services and their associations with short-term quit outcomes. Nicotine Tob. Res. 2010, 12, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Meredith, S.E.; Robinson, A.; Erb, P.; Spieler, C.A.; Klugman, N.; Dutta, P.; Dallery, J. A mobile-phone-based breath carbon monoxide meter to detect cigarette smoking. Nicotine Tob. Res. 2014, 16, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Hyder, N.; Walia, A.; West, R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict. Behav. 2011, 36, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, Y.K.; Sheeran, P.; Hawley, M.S. Effective behaviour change techniques in smoking cessation interventions for people with chronic obstructive pulmonary disease: A meta-analysis. Br. J. Health Psychol. 2014, 19, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Mairs, L.; Mullan, B. Self-Monitoring vs. Implementation Intentions: A Comparison of Behaviour Change Techniques to Improve Sleep Hygiene and Sleep Outcomes in Students. Int. J. Behav. Med. 2015, 22, 635–644. [Google Scholar] [CrossRef] [PubMed]
- French, D.P.; Olander, E.K.; Chisholm, A.; Mc Sharry, J. Which behaviour change techniques are most effective at increasing older adults’ self-efficacy and physical activity behaviour? A systematic review. Ann. Behav. Med. 2014, 48, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Samdal, G.B.; Eide, G.E.; Barth, T.; Williams, G.; Meland, E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Raiff, B.R.; Jarvis, B.P.; Turturici, M.; Dallery, J. Acceptability of an Internet-based contingency management intervention for smoking cessation: Views of smokers, nonsmokers, and healthcare professionals. Exp. Clin. Psychopharmacol. 2013, 21, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Ashton, K.; Phillips, R. Foucault, Surveillance, and Carbon Monoxide Testing within Stop-Smoking Services. Qual. Health Res. 2015, 25, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Deloitte, L. State of the Smart. Consumer and Business Usage Patterns. Global Mobile Consumer Survey 2017: The UK Cut; Deloitte: London, UK, 2017. [Google Scholar]
- Krebs, P.; Duncan, D.T. Health App Use among US Mobile Phone Owners: A National Survey. JMIR mHealth uHealth 2015, 3, e101. [Google Scholar] [CrossRef] [PubMed]
- Beard, E.; Brown, J.; Michie, S.; Kaner, E.; Meier, P.; West, R. Use of aids for smoking cessation and alcohol reduction: A population survey of adults in England. BMC Public Health 2016, 16, 1237. [Google Scholar] [CrossRef] [PubMed]
- Bedfont® Scienctific Ltd. iCO Smokerlyzer. Available online: http://www.webcitation.org/6x1w0tkmo (accessed on 6 February 2018).
- Perski, O.; Blandford, A.; Ubhi, H.K.; West, R.; Michie, S. Smokers’ and drinkers’ choice of smartphone applications and expectations of engagement: A think aloud and interview study. BMC Med. Inform. Decis. Mak. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.A.; Hallyburton, M.B.; Pacek, L.R.; Mitchell, J.T.; Vilardaga, R.; Fuemmeler, B.F.; Joseph McClernon, F. What Do Smokers Want in a Smartphone-Based Cessation Application? Nicotine Tob. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Herbec, A.; Beard, E.; Brown, J.; Gardner, B.; Tombor, I.; West, R. The needs and preferences of pregnant smokers regarding tailored Internet-based smoking cessation interventions: A qualitative interview study. BMC Public Health 2014, 14, 1070. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tombor, I.; Shahab, L.; West, R. Usability testing of a smoking cessation smartphone application (‘SmokeFree Baby’): A think-aloud study with pregnant smokers. Digit. Health 2017, 3. [Google Scholar] [CrossRef]
- Perski, O.; Blandford, A.; West, R.; Michie, S. Conceptualising engagement with digital behaviour change interventions: A systematic review using principles from critical interpretive synthesis. Transl. Behav. Med. 2017, 7, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Clark, D.J.; Morgan, S.P.; Crowe, J.A.; Murphy, E. A user-centred approach to requirements elicitation in medical device development: A case study from an industry perspective. Appl. Ergon. 2012, 43, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, B.; Glenton, C. What about N? A methodological study of sample-size reporting in focus group studies. BMC Med. Res. Methodol. 2011, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.J.; Johnston, M.; Robertson, C.; Glidewell, L.; Entwistle, V.; Eccles, M.P.; Grimshaw, J.M. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol. Health 2010, 25, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.; Lewis, J. Qualitative Research Practice: A Guide for Social Science Students and Researchers; Sage Publications: London, UK, 2003. [Google Scholar]
- Parkinson, S.; Eatough, V.; Holmes, J.; Stapley, E.; Midgley, N. Framework analysis: A worked example of a study exploring young people’s experiences of depression. Qual. Res. Psychol. 2015, 13, 109–129. [Google Scholar] [CrossRef]
- Madill, A.; Jordon, A.; Shirley, C. Objectivity and reliability in qualitative analysis: Realist, contextualist and radical constructionist epistemologies. Br. J. Psychol. 2000, 90, 1–20. [Google Scholar] [CrossRef]
- Mays, N.; Pope, C. Qualitative research in health care: Assessing quality in qualitative research. Br. Med. J. 2000, 320, 50–52. [Google Scholar] [CrossRef]
- Birt, L.; Scott, S.; Cavers, D.; Campbell, C.; Walter, F. Member Checking: A Tool to Enhance Trustworthiness or Merely a Nod to Validation? Qual. Health Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Nahum-Shani, I.; Smith, S.N.; Spring, B.J.; Collins, L.M.; Witkiewitz, K.; Tewari, A.; Murphy, S.A. Just-in-Time Adaptive Interventions (JITAIs) in Mobile Health: Key Components and Design Principles for Ongoing Health Behavior Support. Ann. Behav. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Robert, G.; Bate, P.; Macfarlane, F.; Kyriakidou, O. Adopters and Adoption. In Diffusion of Innovations in Health Service Organisations; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; pp. 100–113. [Google Scholar]
- Braun, V.; Clarke, V.S. Successful Qualitative Research: A Ractical Guide for Beginners; Sage: London, UK, 2013. [Google Scholar]
- Cooper, A.; Reimann, R.; Cronin, D.; Noessel, C. About Face: The Essentials of Interaction Design, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Popova, L. The extended parallel process model: Illuminating the gaps in research. Health Educ. Behav. 2012, 39, 455–473. [Google Scholar] [CrossRef] [PubMed]

| ID | Sex | Age | Post-16 Education | Employment | Cigarettes per Day | Last Quit Attempt | Ever Quit for >1 Week | Ever Tested Carbon Monoxide Levels before | Ever Used Cessation Apps before | Ever Used Evidence-Based Cessation Support |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 34 | Yes | Employed (non-manual) | 10–15 | Never | - | - | - | - |
| 2 | Female | 25 | Yes | Student | 5 | Past year | Yes | Yes, once | Yes | Yes |
| 3 | Female | 31 | - | Employed (manual) | 10–12 | Past year | Yes | Yes, once | - | Yes |
| 4 | Male | 24 | Yes | Student | 3–20 | Past year | Yes | Yes, once | - | Yes |
| 5 | Female | 26 | Yes | Employed (non-manual) | 10 | >1 year ago | Yes | - | - | - |
| 6 | Female | 30 | Yes | Employed (non-manual) | 10 | Past year | Yes | - | - | Yes |
| 7 | Female | 28 | Yes | Employed (non-manual) | 15 | >1 year ago | Yes | - | - | - |
| 8 | Female | 40 | Yes | Employed (non-manual) | 1–2 | >1 year ago | Yes | - | - | Yes |
| 9 | Male | 20 | Yes | Employed (manual) and Student | 6–20 | Past year | Yes | - | Yes | - |
| 10 | Male | 28 | Yes | Employed (non-manual) | 7–10 | Past year | Yes | Yes, >once | - | Yes |
| 11 | Male | 51 | Yes | Employed (non-manual) | 15–20 | Past year | - | Yes, once | - | Yes |
| 12 | Female | 26 | Yes | Employed (non-manual) | 16 | Past year | Yes | Yes, >once | - | Yes |
| 13 | Male | 39 | Yes | Student | 5–8 | Past year | Yes | Yes, >once | - | Yes |
| 14 | Male | 46 | Yes | Employed (non-manual) | 15 | >1 year ago | Yes | - | - | Yes |
| 15 | Male | 29 | Yes | Employed (manual) | 10 | Past year | Yes | - | Yes | Yes |
| 16 | Male | 35 | - | Employed (non-manual) | 15 | Past year | Yes | - | - | - |
| Theme 1 | CO Testing—General Views and Motivation to Use |
|---|---|
| 1.1. | General Views on CSSs |
| 1.2. | Motivation to use—a novel cessation aid
|
| 1.3. | Motivation to use—other reasons
|
| 1.3. | Concerns over CSS
|
| Theme 2 | Practicalities of CSS Use |
| 2.1. | Commercial use vs. use as part of study
|
| 2.2. | Smoking status and CO testing
|
| 2.3. | Location of use
|
| 2.4. | Sharing the device
|
| 2.5. | Timing and duration of use
|
| 2.6. | Barriers to CSS use
|
| Theme 3 | Factors Potentially Affecting Preferences, Views and Engagement with CSSs |
| 3.1. | Smoking profile
|
| 3.2. | Barriers to quitting
|
| 3.3. | Views on, and plans for quitting
|
| 3.4. | Prior experience with digital programs and user digital behaviors
|
| 3.5. | Prior experience with CO testing |
| Theme 4 | Personal CO Monitor: Features and Qualities | |
|---|---|---|
| 4.1.–4.2. |
| |
| Theme 5 | App: Features and Qualities | |
| 5.1. | CO testing and display of CO results | |
| 5.1.1. | CO testing journey |
|
| 5.1.2. | Feedback on CO results |
|
| 5.1.3. | Recording contextual data |
|
| 5.2. | Interactive infographics |
|
| 5.3. | Factual content |
|
| 5.4. | Additional features |
|
| 5.5. | External expert support |
|
| 5.6. | General app qualities and Information architecture |
|
| 5.7. | Onboarding and registration |
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbeć, A.; Perski, O.; Shahab, L.; West, R. Smokers’ Views on Personal Carbon Monoxide Monitors, Associated Apps, and Their Use: An Interview and Think-Aloud Study. Int. J. Environ. Res. Public Health 2018, 15, 288. https://doi.org/10.3390/ijerph15020288
Herbeć A, Perski O, Shahab L, West R. Smokers’ Views on Personal Carbon Monoxide Monitors, Associated Apps, and Their Use: An Interview and Think-Aloud Study. International Journal of Environmental Research and Public Health. 2018; 15(2):288. https://doi.org/10.3390/ijerph15020288
Chicago/Turabian StyleHerbeć, Aleksandra, Olga Perski, Lion Shahab, and Robert West. 2018. "Smokers’ Views on Personal Carbon Monoxide Monitors, Associated Apps, and Their Use: An Interview and Think-Aloud Study" International Journal of Environmental Research and Public Health 15, no. 2: 288. https://doi.org/10.3390/ijerph15020288
APA StyleHerbeć, A., Perski, O., Shahab, L., & West, R. (2018). Smokers’ Views on Personal Carbon Monoxide Monitors, Associated Apps, and Their Use: An Interview and Think-Aloud Study. International Journal of Environmental Research and Public Health, 15(2), 288. https://doi.org/10.3390/ijerph15020288





