Additional Effects of Xbox Kinect Training on Upper Limb Function in Chronic Stroke Patients: A Randomized Control Trial
Abstract
1. Introduction
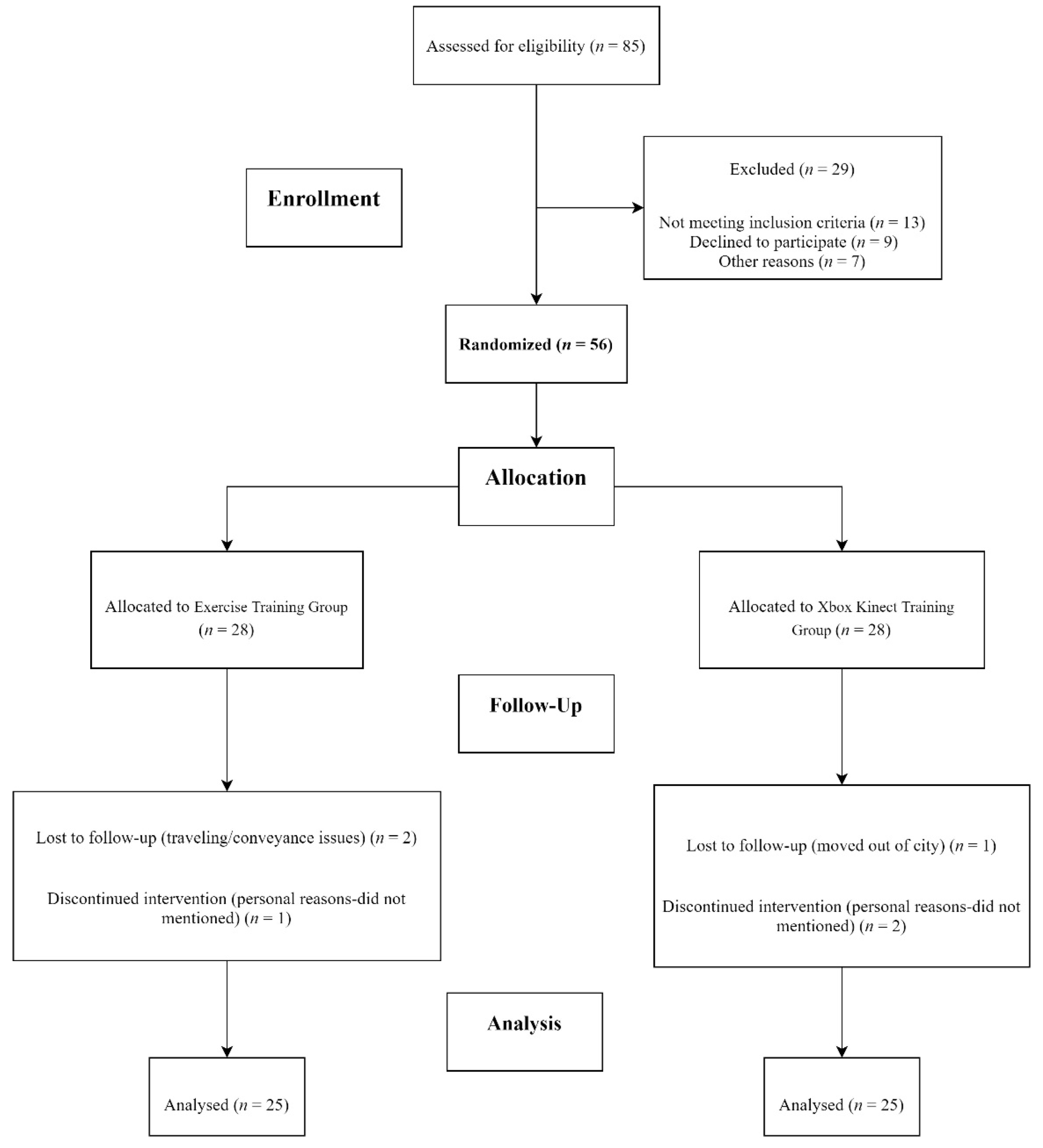
2. Methodology
2.1. Intervention
2.1.1. Experimental Group (Xbox Kinect Training Group)
2.1.2. Control Group (Exercise Training Group)
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolf, T.J.; Polatajko, H.; Baum, C.; Rios, J.; Cirone, D.; Doherty, M.; McEwen, S. Combined cognitive-strategy and task-specific training affects cognition and upper-extremity function in subacute stroke: An exploratory randomized con-trolled trial. Am. J. Occup. Ther. 2016, 70. [Google Scholar] [CrossRef] [PubMed]
- Licher, S.; Darweesh, S.K.L.; Wolters, F.J.; Fani, L.; Heshmatollah, A.; Mutlu, U.; Koudstaal, P.J.; Heeringa, J.; Leening, M.J.G.; Ikram, M.K. Lifetime risk of common neurological diseases in the elderly population. J. Neurol. Neurosurg. Psychiatry 2019, 90, 148–156. [Google Scholar] [CrossRef]
- Ward, N.S.; Brander, F.; Kelly, K. Intensive upper limb neurorehabilitation in chronic stroke: Outcomes from the Queen Square programme. J. Neurol. Neurosurg. Psychiatry 2019, 90, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Teka, W.W.; Hamade, K.C.; Barnett, W.H.; Kim, T.; Markin, S.N.; Rybak, I.A.; Molkov, Y.I. From the motor cortex to the movement and back again. PLoS ONE 2017, 12, e0179288. [Google Scholar] [CrossRef]
- Xu, G.-Q.; Lan, Y.; Zhang, Q.; Liu, D.-X.; He, X.-F.; Lin, T. 1-Hz Repetitive Transcranial Magnetic Stimulation over the Posterior Parietal Cortex Modulates Spatial Attention. Front. Hum. Neurosci. 2016, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.; Bosnell, R.; Dawes, H.; Howells, K.; Cockburn, J.; Kischka, U.; Matthews, P.; Johansen-Berg, H. Cognitive Context Determines Dorsal Premotor Cortical Activity During Hand Movement in Patients After Stroke. Stroke 2011, 42, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Sin, H.; Lee, G. Additional Virtual Reality Training Using Xbox Kinect in Stroke Survivors with Hemiplegia. Am. J. Phys. Med. Rehabil. 2013, 92, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Kiper, P.; Szczudlik, A.; Agostini, M.; Opara, J.; Nowobilski, R.; Ventura, L.; Tonin, P.; Turolla, A. Virtual Reality for Upper Limb Rehabilitation in Subacute and Chronic Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 834–842.e4. [Google Scholar] [CrossRef]
- Lang, C.E.; Bland, M.D.; Bailey, R.R.; Schaefer, S.Y.; Birkenmeier, R.L. Assessment of upper extremity impairment, function, and activity after stroke: Foundations for clinical decision making. J. Hand Ther. 2013, 26, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, D.; Zollo, L.; Milighetti, S.; Miccinilli, S.; Bravi, M.; Ranieri, F.; Magrone, G.; Guglielmelli, E.; Di Lazzaro, V.; Sterzi, S. Literature Review on the Effects of tDCS Coupled with Robotic Therapy in Post Stroke Upper Limb Rehabilitation. Front. Hum. Neurosci. 2017, 11, 268. [Google Scholar] [CrossRef]
- Lohse, K.R.; Lang, C.E.; Boyd, L.A. Is More Better? Using Metadata to Explore Dose–Response Relationships in Stroke Rehabilitation. Stroke 2014, 45, 2053–2058. [Google Scholar] [CrossRef]
- Winstein, C.J.; Wolf, S.L.; Dromerick, A.W.; Lane, C.J.; Nelsen, M.A.; Lewthwaite, R.; Cen, S.Y.; Azen, S.P. Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: The ICARE randomized clinical trial. JAMA 2016, 315, 571–581. [Google Scholar] [CrossRef]
- Lang, C.E.; Strube, M.J.; Bland, M.D.; Waddell, K.J.; Cherry-Allen, K.M.; Nudo, R.J.; Dromerick, A.W.; Birkenmeier, R.L. Dose response of task-specific upper limb training in people at least 6 months poststroke: A phase II, single-blind, randomized, controlled trial. Ann. Neurol. 2016, 80, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Klamroth-Marganska, V.; Blanco, J.; Campen, K.; Curt, A.; Dietz, V.; Ettlin, T.; Felder, M.; Fellinghauer, B.; Guidali, M.; Kollmar, A.; et al. Three-dimensional, task-specific robot therapy of the arm after stroke: A multicentre, parallel-group randomised trial. Lancet Neurol. 2014, 13, 159–166. [Google Scholar] [CrossRef]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; Van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 11, CD010820. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; Macdonald, J.R.; Gnip, C. Counting Repetitions: An Observational Study of Outpatient Therapy for People with Hemiparesis Post-Stroke. J. Neurol. Phys. Ther. 2007, 31, 3–10. [Google Scholar] [CrossRef]
- Lee, G. Effects of Training Using Video Games on the Muscle Strength, Muscle Tone, and Activities of Daily Living of Chronic Stroke Patients. J. Phys. Ther. Sci. 2013, 25, 595–597. [Google Scholar] [CrossRef]
- Afsar, S.I.; Mirzayev, I.; Yemisci, O.U.; Saracgil, S.N.C. Virtual Reality in Upper Extremity Rehabilitation of Stroke Patients: A Randomized Controlled Trial. J. Stroke Cerebrovasc. Dis. 2018, 27, 3473–3478. [Google Scholar] [CrossRef] [PubMed]
- Xavier-Rocha, T.B.; Carneiro, L.; Martins, G.C.; Vilela-Júnior, G.D.B.; Passos, R.P.; Pupe, C.C.B.; do Nascimento, O.J.M.; Haikal, D.S.A.; Monteiro-Junior, R.S. The Xbox/Kinect use in poststroke reha-bilitation settings: A systematic review. ARQ Neuropsiquiatr. 2020, 78, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Aşkın, A.; Atar, E.; Koçyiğit, H.; Tosun, A. Effects of Kinect-based virtual reality game training on upper extremity motor recovery in chronic stroke. Somatosens. Mot. Res. 2018, 35, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Chen, S.-F.; Huang, J.-D. A Kinect-based system for physical rehabilitation: A pilot study for young adults with motor disabilities. Res. Dev. Disabil. 2011, 32, 2566–2570. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Bird, M.-L.; Muthalib, M.; Teo, W.-P. Innovative STRoke Interactive Virtual thErapy (STRIVE) online platform for community-dwelling stroke survivors: A randomised controlled trial protocol. BMJ Open 2018, 8, e018388. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Mao, Y.; Lin, Q.; Qiu, Y.; Chen, S.; Li, L.; Cates, R.S.; Zhou, S.; Huang, D. Mechanism of Kinect-based virtual reality training for motor functional recovery of upper limbs after subacute stroke. Neural Regen. Res. 2013, 8, 2904–2913. [Google Scholar]
- You, S.H.; Jang, S.H.; Kim, Y.-H.; Hallett, M.; Ahn, S.H.; Kwon, Y.-H.; Kim, J.H.; Lee, M.Y.L. Virtual reality–induced cortical reorganization and associated locomotor recovery in chronic stroke: An experimenter-blind randomized study. Stroke 2005, 36, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- August, K.; Lewis, J.A.; Chandar, G.; Merians, A.; Biswal, B.; Adamovich, S. analysis of neural mechanisms underlying rehabilitation in virtual reality: Activating secondary motor areas. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE, New York, NY, USA, 30 August–3 September 2006; pp. 3692–3695. [Google Scholar]
- Saposnik, G.; Levin, M.; Outcome Research Canada (SORCan) Working Group. Virtual reality in stroke rehabilitation: A meta-analysis and implications for clinicians. Stroke 2011, 42, 1380–1386. [Google Scholar] [CrossRef]
- Laver, E.K.; Lange, B.; George, S.; Deutsch, E.J.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-S.; Lee, D.-G.; Lee, K.; Lee, G. Effects of virtual reality training using Xbox Kinect on motor function in stroke sur-vivors: A preliminary study. J. Stroke Cerebrovasc. Dis. 2017, 26, 2313–2319. [Google Scholar] [CrossRef]
- Merians, A.S.; Poizner, H.; Boian, R.; Burdea, G.; Adamovich, S. Sensorimotor Training in a Virtual Reality Environment: Does It Improve Functional Recovery Poststroke? Neurorehabilit. Neural Repair 2006, 20, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-S.; Park, M.-J.; Yoon, I.-J.; Park, S.-H. Effects of virtual reality training on upper extremity function and activities of daily living in patients with sub-acute stroke: A double-blind randomized clinical trial. NeuroRehabilitation 2019, 17, 271–278. [Google Scholar]
- Schuster-Amft, C.; Eng, K.; Suica, Z.; Thaler, I.; Signer, S.; Lehmann, I.; Schmid, L.; McCaskey, M.A.; Hawkins, M.; Verra, M.L.; et al. Effect of a four-week virtual reality-based training versus conventional therapy on upper limb motor function after stroke: A multicenter parallel group randomized trial. PLoS ONE 2018, 13, e0204455. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health; Version 3.01; Epi Info™, Division of Health Informatics & Surveillance (DHIS), Center for Surveillance, Epidemiology & Laboratory Services (CSELS): Atlanta, GE, USA, 2019. [Google Scholar]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabilit. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Duncan, P.W.; Propst, M.; Nelson, S.G. Reliability of the Fugl-Meyer Assessment of Sensorimotor Recovery Following Cerebrovascular Accident. Phys. Ther. 1983, 63, 1606–1610. [Google Scholar] [CrossRef]
- Domínguez-Téllez, P.; Moral-Muñoz, J.A.; Salazar, A.; Casado-Fernández, E.; Lucena-Antón, D. Game-Based Virtual Reality Interventions to Improve Upper Limb Motor Function and Quality of Life After Stroke: Systematic Review and Meta-analysis. Games Heal. J. 2020, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karamians, R.; Proffitt, R.; Kline, D.; Gauthier, L.V. Effectiveness of virtual reality-and gaming-based interventions for upper extremity rehabilitation poststroke: A meta-analysis. Arch. Phys. Med. Rehabil. 2020, 101, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Amedi, A.; Fregni, F.; Merabet, L.B. The plastic human brain cortex. Annu. Rev. Neurosci. 2005, 28, 377–401. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Štětkářová, I.; Macri, V.; Kühn, S.; Pětioký, J.; Gualeni, S.; Simmons, С.D.; Arthanat, S.; Zilber, P. Virtual reality-based treatment for regaining upper extremity function induces cortex grey matter changes in persons with acquired brain injury. J. Neuroeng. Rehabil. 2020, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Rand, D. Proprioception deficits in chronic stroke—Upper extremity function and daily living. PLoS ONE 2018, 13, e0195043. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.-L.; Kim, C.-W. Cognition and Upper-extremity Function Influence on Performance of Activities of Daily Living in Patients with Chronic Stroke. J. Korean Soc. Phys. Med. 2019, 14, 115–123. [Google Scholar] [CrossRef]
| Variable | Xbox Kinect Training Group | Exercise Training Group |
|---|---|---|
| Age (Mean ± SD) | 57.48 ± 10.60 | 57.68 ± 10.43 |
| Gender (Male, Female) | 23 (92%), 2 (8%) | 20 (76%), 5 (24%) |
| Modified Ashworth Scale (Mean ± SD) | 1.44 ± 1.00 | 1.56 ± 1.08 |
| Montreal Cognitive Assessment (Mean ± SD) | 27.04 ± 2.31 | 27.00 ± 2.70 |
| Fugl-Meyer Assessment UE (Mean ± SD) | 29.16 ± 14.33 | 26.96 ± 12.44 |
| Box and Block Test Dominant Hand (Mean ± SD) | 22.80 ± 15.00 | 22.48 ± 18.42 |
| Box and Block Test Non-Dominant Hand (Mean ± SD | 32.44 ± 13.85 | 33.96 ± 13.84 |
| Across Group Analysis for Fugl-Meyer Assessment Upper Extremity (FMA-UE) | |||||
|---|---|---|---|---|---|
| Variable | Xbox Kinect Training Group | Exercise Training Group | Effect Size | ||
| Group | Mean ± SD | Mean ± SD | p-Value | Partial Eta Squared | |
| FMA-Total Score | Pre | 29.16 ± 14.33 | 26.96 ± 12.45 | 0.565 | |
| Post | 52.20 ± 10.69 | 35.36 ± 12.73 | <0.001 | ||
| Training Effect Difference | 23.04 ± 11 | 8.40 ± 5.48 | <0.001 | 0.425 | |
| Reflex Activity | Pre | 3.40 ± 1.32 | 3.36 ± 1.60 | 0.924 | |
| Post | 3.92 ± 0.40 | 3.56 ± 0.92 | 0.78 | ||
| Training Effect Difference | 0.52 ± 1.41 | 0.20 ± 1.73 | 0.478 | 0.011 | |
| Volitional movement within synergies | Pre | 8.28 ± 5.47 | 5.48 ± 3.83 | 0.042 | |
| Post | 14.08 ± 4.15 | 6.88 ± 3.89 | <0.001 | ||
| Training Effect Difference | 5.80 ± 5.97 | 1.40 ± 3.41 | 0.002 | 0.175 | |
| Volitional movement mixing synergies | Pre | 2.84 ± 1.88 | 2.56 ± 1.73 | 0.587 | |
| Post | 5.32 ± 2.92 | 3.92 ± 1.91 | 0.051 | ||
| Training Effect Difference | 2.48 ± 2.36 | 1.36 ± 1.15 | 0.038 | 0.086 | |
| Volitional movement no synergy | Pre | 3.12 ± 2.67 | 2.56 ± 1.93 | 0.4 | |
| Post | 4.32 ± 1.90 | 3.44 ± 1.58 | 0.082 | ||
| Training Effect Difference | 1.20 ± 2.91 | 0.88 ± 1.73 | 0.64 | 0.005 | |
| FMA-Wrist | Pre | 4.72 ± 3.76 | 3.36 ± 3.31 | 0.182 | |
| Post | 7.48 ± 2.64 | 5.44 ± 3.37 | 0.021 | ||
| Training Effect Difference | 2.76 ± 2.81 | 2.08 ± 3.17 | 0.427 | 0.013 | |
| FMA-Hand | Pre | 2.20 ± 1.73 | 2.20 ± 2.06 | 1.001 | |
| Post | 3.92 ± 2.46 | 2.52 ± 2.40 | 0.047 | ||
| Training Effect Difference | 1.72 ± 3.11 | 0.32 ± 2.59 | 0.05 | 0.058 | |
| FMA-Grasp | Pre | 4.96 ± 3.79 | 4.36 ± 2.77 | 0.526 | |
| Post | 8.96 ± 3.36 | 6.08 ± 3.68 | 0.006 | ||
| Training Effect Difference | 4.00 ± 2.14 | 1.72 ± 3.07 | 0.004 | 0.162 | |
| FMA-Coordination/Speed | Pre | 2.88 ± 1.53 | 2.80 ± 1.75 | 0.865 | |
| Post | 4.88 ± 1.30 | 3.48 ± 1.87 | 0.004 | ||
| Training Effect Difference | 2.00 ± 2.12 | 0.68 ± 1.54 | 0.015 | 0.116 | |
| N = 25 Each Group | |||||
| Within Group Analysis for Fugl-Meyer Assessment Upper Extremity (FMA-UE) | ||||
|---|---|---|---|---|
| Variable | Group | Baseline | Post Intervention | p-Value |
| Mean ± SD | Mean ± SD | |||
| FMA-Total Score | XKTG | 29.16 ± 14.33 | 52.20 ± 10.69 | <0.001 |
| ETG | 26.96 ± 12.45 | 35.36 ± 12.73 | <0.001 | |
| Reflex Activity | XKTG | 3.40 ± 1.32 | 3.92 ± 0.40 | 0.079 |
| ETG | 3.36 ± 1.60 | 3.56 ± 0.92 | 0.569 | |
| Volitional movement within synergies | XKTG | 8.28 ± 5.47 | 14.08 ± 4.15 | <0.001 |
| ETG | 5.48 ± 3.83 | 6.88 ± 3.89 | 0.052 | |
| Volitional movement mixing synergies | XKTG | 2.84 ± 1.88 | 5.32 ± 2.92 | <0.001 |
| ETG | 2.56 ± 1.73 | 3.92 ± 1.91 | <0.001 | |
| Volitional movement no synergy | XKTG | 3.12 ± 2.67 | 4.32 ± 1.90 | 0.051 |
| ETG | 2.56 ± 1.93 | 3.44 ± 1.58 | 0.018 | |
| FMA-Wrist | XKTG | 4.72 ± 3.76 | 7.48 ± 2.64 | <0.001 |
| ETG | 3.36 ± 3.31 | 5.44 ± 3.37 | 0.003 | |
| FMA-Hand | XKTG | 2.20 ± 1.73 | 3.92 ± 2.46 | 0.011 |
| ETG | 2.20 ± 2.06 | 2.52 ± 2.40 | 0.543 | |
| FMA-Grasp | XKTG | 4.96 ± 3.79 | 8.96 ± 3.36 | <0.001 |
| ETG | 4.36 ± 2.77 | 6.08 ± 3.68 | 0.01 | |
| FMA-Coordination/Speed | XKTG | 2.88 ± 1.53 | 4.88 ± 1.30 | <0.001 |
| ETG | 2.80 ± 1.75 | 3.48 ± 1.87 | 0.038 | |
| N = 25 Each Group | ||||
| Across Group Analysis for Box and Block Test (BBT) | ||||||
| Variable | Group | Xbox Kinect Training Group | Exercise Training Group | |||
| Mean ± SD | Mean Rank | Mean ± SD | Mean Rank | p-Value | ||
| Dominant Hand | Baseline | 22.80 ± 15.00 | 26.32 | 22.48 ± 18.42 | 24.68 | 0.691 |
| Post Intervention | 40.64 ± 13.03 | 30.5 | 31.20 ± 20.82 | 20.5 | 0.719 | |
| Training Effect Difference | 17.84 ± 9.24 | 33.52 | 8.72 ± 8.22 | 17.48 | <0.001 | |
| Non-Dominant Hand | Baseline | 32.44 ± 13.85 | 24.76 | 33.96 ± 13.84 | 26.24 | 0.015 |
| Post Intervention | 50.28.32 ± 17.69 | 29.16 | 42.20 ± 15.56 | 21.84 | 0.076 | |
| Training Effect Difference | 17.84 ± 9.64 | 33.4 | 8.24 ± 4.53 | 17.6 | <0.001 | |
| Within Group Analysis for Box and Block Test (BBT) | ||||||
| Variable | Group | Baseline | Post Int | Mean Rank (+) | Mean Rank (−) | p-value |
| Mean ± SD | Mean ± SD | |||||
| Dominant Hand | Xbox Kinect Training Group | 22.80 ± 15.00 | 40.64 ± 13.03 | 13 | 0 | <0.001 |
| Exercise Training Group | 22.48 ± 18.42 | 31.20 ± 20.82 | 13 | 0 | <0.001 | |
| Non-Dominant Hand | Xbox Kinect Training Group | 32.44 ± 13.85 | 50.28.32 ± 17.69 | 13 | 0 | <0.001 |
| Exercise Training Group | 33.96 ± 13.84 | 42.20 ± 15.56 | 13 | 0 | <0.001 | |
| N = 25 Each Group | ||||||
| Across Group Analysis for Montreal Cognitive Assessment (MOCA) | |||||
| Variable | Xbox Kinect Training Group | Exercise Training Group | |||
| Mean ± SD | Mean Rank | Mean ± SD | Mean Rank | p-Value | |
| Baseline | 27.04 ± 2.32 | 25.2 | 27.00 ± 2.69 | 25.8 | 0.883 |
| Post Intervention | 27.32 ± 1.75 | 24.06 | 27.32 ± 2.87 | 26.94 | 0.477 |
| Training Effect Difference | 0.28 ± 1.88 | 23.5 | 0.32 ± 0.90 | 27.5 | 0.293 |
| Within Group Analysis for Montreal Cognitive Assessment (MOCA) | |||||
| Treatment Groups | Baseline | Post Int | Mean Rank (+) | Mean Rank (−) | p-value |
| Mean ± SD | Mean ± SD | ||||
| Xbox Kinect Training Group | 27.04 ± 2.32 | 27.32 ± 1.75 | 7 | 5.8 | 0.417 |
| Exercise Training Group | 27.00 ± 2.69 | 27.32 ± 2.87 | 6.09 | 12 | 0.113 |
| N = 25 Each Group | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ain, Q.U.; Khan, S.; Ilyas, S.; Yaseen, A.; Tariq, I.; Liu, T.; Wang, J. Additional Effects of Xbox Kinect Training on Upper Limb Function in Chronic Stroke Patients: A Randomized Control Trial. Healthcare 2021, 9, 242. https://doi.org/10.3390/healthcare9030242
Ain QU, Khan S, Ilyas S, Yaseen A, Tariq I, Liu T, Wang J. Additional Effects of Xbox Kinect Training on Upper Limb Function in Chronic Stroke Patients: A Randomized Control Trial. Healthcare. 2021; 9(3):242. https://doi.org/10.3390/healthcare9030242
Chicago/Turabian StyleAin, Qurat Ul, Sara Khan, Saad Ilyas, Amna Yaseen, Iqbal Tariq, Tian Liu, and Jue Wang. 2021. "Additional Effects of Xbox Kinect Training on Upper Limb Function in Chronic Stroke Patients: A Randomized Control Trial" Healthcare 9, no. 3: 242. https://doi.org/10.3390/healthcare9030242
APA StyleAin, Q. U., Khan, S., Ilyas, S., Yaseen, A., Tariq, I., Liu, T., & Wang, J. (2021). Additional Effects of Xbox Kinect Training on Upper Limb Function in Chronic Stroke Patients: A Randomized Control Trial. Healthcare, 9(3), 242. https://doi.org/10.3390/healthcare9030242






