Assessment of Health Information Technology Interventions in Evidence-Based Medicine: A Systematic Review by Adopting a Methodological Evaluation Framework
Abstract
1. Introduction
- RQ1. Are there any classification methods for the detailed description of the results associated with HITs, and to what extent? It remains unclear whether the advances of HITs in recent years take into account or suggest such evaluation models or standards.
- RQ2. Is there any framework or methodology to follow for the assessment of HITs in order to evaluate, compare or extend results from other studies and systematic reviews? It is uncertain whether comprehensive and standardized assessment frameworks/methodologies have been proposed, or if further investigation and study is required in the field.
- RQ3. Which are the most established HITs (i.e., HIT forms in accordance with their functional capabilities and the categories of applied Information science) that support evidence-based medicine and are integrated in medical and nursing practices?
- RQ4. What are the features, the outcomes and the types of HIT interventions of the included studies in this research, and their classification in accordance with the medical/health domain?
2. Materials and Methods
2.1. Related Work
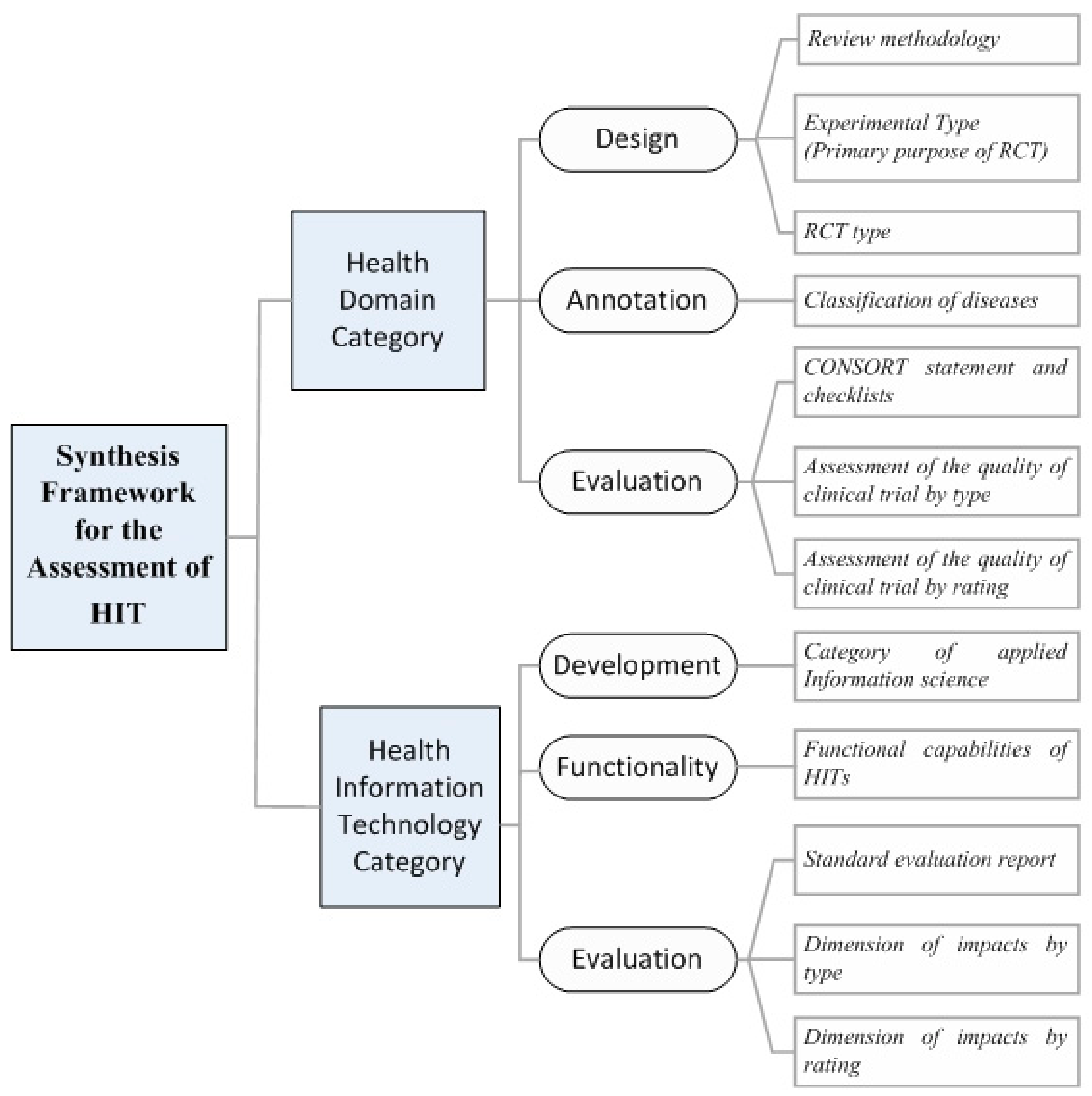
2.2. The Classification Scheme for the Synthesis of Results
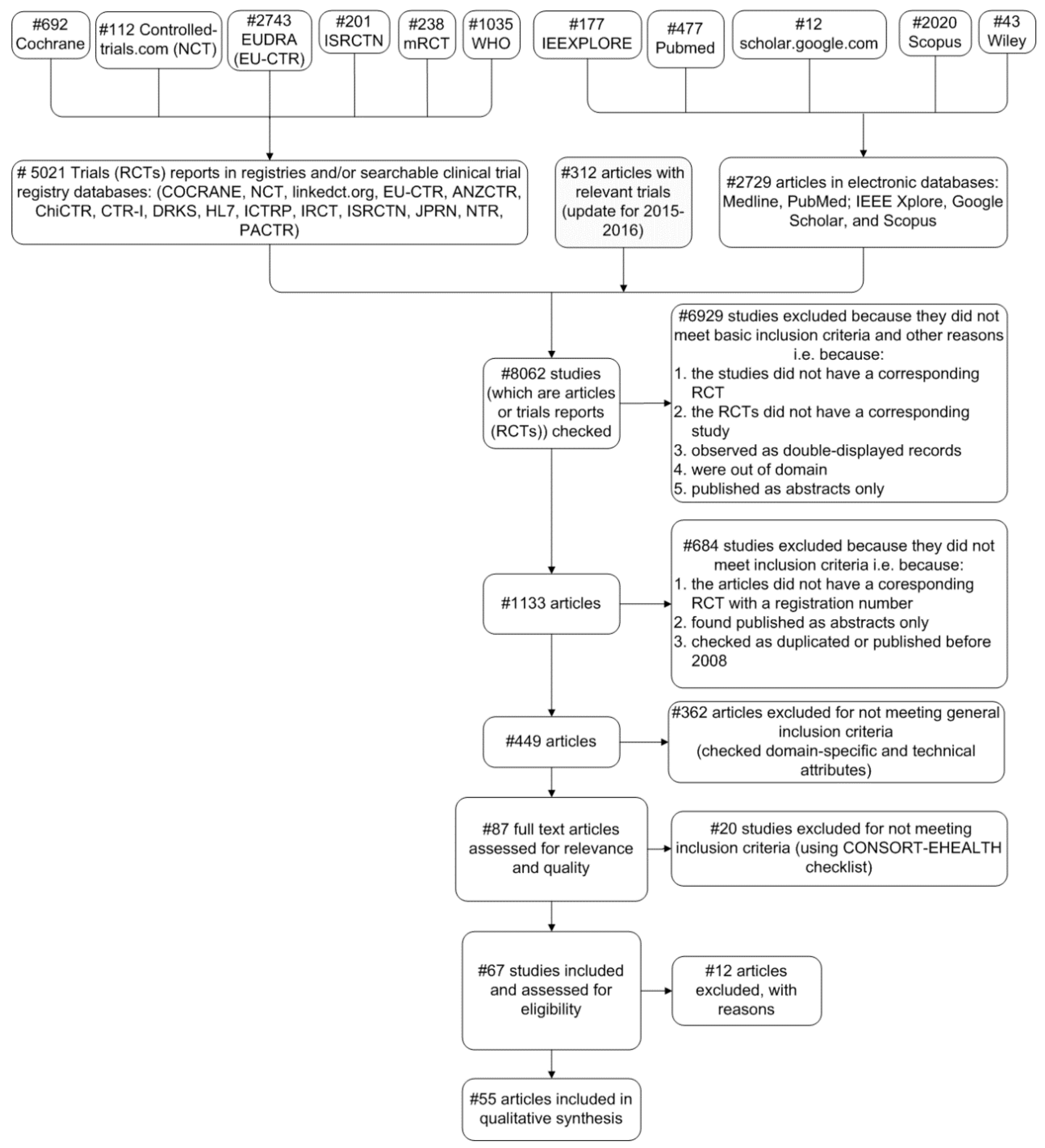
2.3. Systematic Review Search Strategy
2.3.1. Eligibility Criteria
- Interventional completed trials (RCTs) published between 2008 and 2016;
- Relative trials are assigned an official registration number, as this is a fundamental requirement from 2004 (i.e., the International Committee of Medical Journal Editors announced that RCTs will be considered for publication only if they are registered before the enrolment of the first patient [33]);
- Results of the trials are published in at least one article that belongs to well-known and established scholar databases. Therefore, every article is related to one or more trials and vice-versa;
- Publication language is English.
2.3.2. Data Extraction and Analysis Process
- Category of disease/health domain (e.g., neoplasm, mental, behavioral disorders, etc.) (i.e., Sub-category 1.2.1. of SF/HIT).
- RCT type (i.e., open label, single blind, double blind, not blinded and unclear) (i.e., Sub-category 1.1.1. of SF/HIT).
- RCT Experimental type (prevention, screening, treatment, supportive care and health services research) (i.e., Sub-category 1.1.2. of SF/HIT).
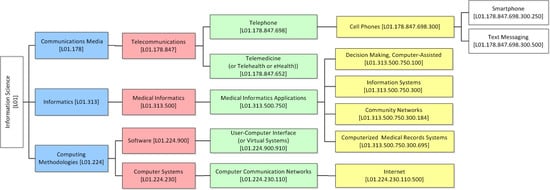
- ICT category—under the MeSH classification system (e.g., telemedicine, medical informatics applications, etc.) (i.e., Sub-category 2.1.1. of SF/HIT).
- Outcomes/impacts by type (e.g., effectiveness, acceptability, usefulness, etc.) and rating (e.g., positive, negative) (i.e., Sub-categories 2.3.2. and 2.3.3. of SF/HIT).
3. Results
3.1. Descriptive Elements of the Studies and the Types of HIT in Use
3.2. Synthesis of the Characteristics and Findings
4. Discussion
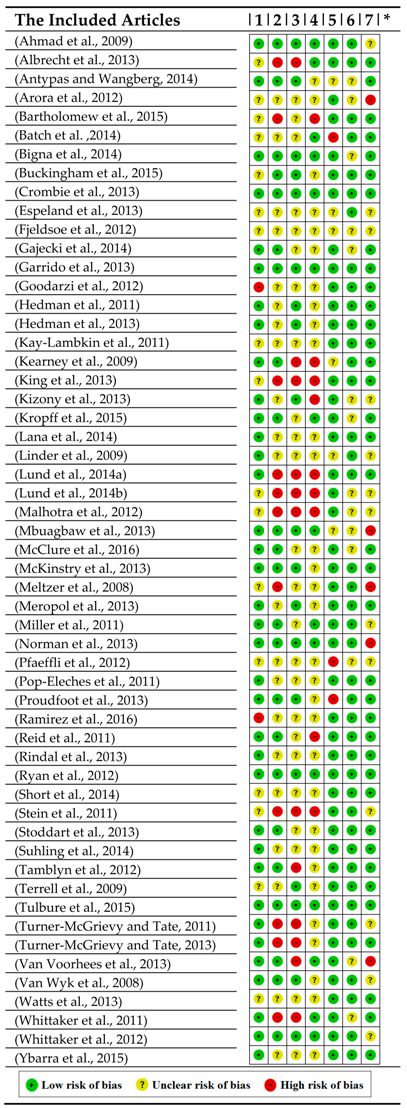
4.1. Study Quality of the Literature Review
4.2. Study Quality of the Systematic Review
4.3. Limitations on the Composition of this Research Work
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Interpretation |
| ANZCTR | Australian and New Zealand Clinical Trials Registry |
| AHRQ's | Agency for Healthcare Research and Quality’s |
| CBA | Computer-based alerts and reminders systems |
| ChiCTR | Chinese Clinical trial registry |
| ClinicalTrials.gov | United States Trials Registry |
| CMS | Centers for Medicare & Medicaid Services |
| Cochrane library | Cochrane Central Register of Controlled Trials |
| CONSORT | Consolidated Standards of Reporting Trials |
| CPOE | Computerized Physician Order Entry |
| CTRI | Clinical Trials Registry – India |
| DRKS | Deutsches Register Klinischer Studien (German Clinical Trials Register) |
| DSS | Decision Support Systems |
| EBHI | Evidence-Based Health Informatics |
| EHR | Electronic Health Record |
| EU Clinical Trials Register | European Clinical Trials Database |
| EudraCT | The European Clinical Trials Database |
| Google Scholar | Google Scholar |
| HIT | Health Information Technology |
| HITECH | Health Information Technology for Economic and Clinical Health |
| HL7 | Health Level 7 |
| HTA | Health Technology Assessment |
| ICD | International Statistical Classification of Diseases and Related Health Problems |
| ICMJE | International Committee of Medical Journal Editors |
| ICT | Information & Computer Technology |
| ICTRP | International Clinical Trials Registry Platform |
| IEEE Xplore | IEEE Xplore |
| IRCT | Iranian Registry of Clinical Trials |
| ISRCTN | International Standardised Randomised Controlled Trial Number |
| JPRN | Japan Primary Registries Network |
| LinkedCT | Linked Clinical Trials |
| Medline/PubMed | Medline/PubMed |
| MeSH | Medical Subject Headings |
| NTR | The Netherlands National Trial Register |
| PACTR | Pan African Clinical Trials Registry |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized Controlled Trials |
| RQ | Research Question |
| Scopus | Scopus |
| SF/HIT | Synthesis Framework for the Assessment of Health Information Technology |
| WHO ICTRP | WHO International Clinical Trials Registry Platform |
| WHO library databases | World Health Organization library databases |
| Wiley Online Library | Wiley Online Library |
References
- Ammenwerth, E.; De Keizer, N. A viewpoint on evidence-based health informatics, based on a pilot survey on evaluation studies in health care informatics. J. Am. Med. Inform. Assoc. 2007, 14, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, J.M.; Kelton, D.F.; O’Connor, A.M. Study designs and systematic reviews of interventions: Building evidence across study designs. Zoonoses Public Health 2014, 61, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, S.C.; Kotsilieris, T.; Anagnostopoulos, I. Evidence-based health and clinical informatics: A systematic review on randomized controlled trials. Health Technol. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Dalkey, N.; Helmer, O. An experimental application of the Delphi method to the use of experts. Manag. Sci. 1963, 9, 458–467. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Petticrew, M.; Roberts, H. Evidence, hierarchies, and typologies: Horses for courses. J. Epidemiol. Community Health 2003, 57, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.L.; Rosenberg, W.M.; Gray, J.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ Br. Med. J. 1996, 312, 71–72. [Google Scholar] [CrossRef]
- Jonas, W.B. The evidence house: How to build an inclusive base for complementary medicine. West. J. Med. 2001, 175, 79. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Eysenbach, G.; Group, C.-E. CONSORT-EHEALTH: Improving and standardizing evaluation reports of Web-based and mobile health interventions. J. Med. Internet Res. 2011, 13, e126. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Appel, L.; Morra, D.; Lo, V.; Kitto, S.; Quan, S. Short message service or disService: Issues with text messaging in a complex medical environment. Int. J. Med. Inf. 2014, 83, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Sittig, D.F.; Gonzalez, D.; Singh, H. Contingency planning for electronic health record-based care continuity: A survey of recommended practices. Int. J. Med. Inform. 2014, 83, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Law, L.M.; Wason, J.M. Design of telehealth trials-Introducing adaptive approaches. Int. J. Med. Inform. 2014, 83, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Brooks, D.; Marques, A. Home telemonitoring in COPD: A systematic review of methodologies and patients’ adherence. Int. J. Med. Inform. 2014, 83, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Ammenwerth, E.; Duftschmid, G.; Gall, W.; Hackl, W.O.; Hoerbst, A.; Janzek-Hawlat, S.; Jeske, M.; Jung, M.; Woertz, K.; Dorda, W. A nationwide computerized patient medication history: Evaluation of the Austrian pilot project “e-Medikation”. Int. J. Med. Inform. 2014, 83, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Häyrinen, K.; Saranto, K.; Nykänen, P. Definition, structure, content, use and impacts of electronic health records: A review of the research literature. Int. J. Med. Inform. 2008, 77, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Shekelle, P.G.; Goldzweig, C.L. Costs and Benefits of Health Information Technology: An Updated Systematic Review; Health Foundation: London, UK, 2009. [Google Scholar]
- WHO. Medical Devices Health Technology Assessment. Available online: http://www.who.int/medical_devices/assessment/en/ (accessed on 10 August 2018).
- Busse, R.; Orvain, J.; Velasco, M.; Perleth, M.; Drummond, M.; Jørgensen, T.; Jovell, A.; Malone, J.; Alric, R.; Wild, C.; et al. Best practice in undertaking and reporting health technology assessments: Working Group 4 report. Int. J. Technol. Assess. Health Care 2002, 18, 361–422. [Google Scholar] [CrossRef]
- Kristensen, F.B.; Lampe, K.; Chase, D.L.; Lee-Robin, S.H.; Wild, C.; Moharra, M.; Garrido, M.V.; Nielsen, C.P.; Røttingen, J.-A.; Neikter, S.A.; et al. Practical tools and methods for health technology assessment in Europe: Structures, methodologies, and tools developed by the European network for Health Technology Assessment, EUnetHTA. Int. J. Technol. Assess. Health Care 2009, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.E.; Zafar, A.; McGowan, J.J. Development of a taxonomy for health information technology. Stud. Health Technol. Inform. 2007, 129, 616–620. [Google Scholar] [PubMed]
- Jamal, A.; McKenzie, K.; Clark, M.J. The impact of health information technology on the quality of medical and health care: A systematic review. Health Inf. Manag. J. 2009, 38, 26–37. [Google Scholar] [CrossRef]
- Chaudhry, B.; Wang, J.; Wu, S.; Maglione, M.; Mojica, W.; Roth, E.; Morton, S.C.; Shekelle, P.G. Systematic review: Impact of health information technology on quality, efficiency, and costs of medical care. Ann. Intern. Med. 2006, 144, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D. Launching hitech. N. Engl. J. Med. 2010, 2010, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.S.; Rudin, R.S.; Perry, T.; Shekelle, P.G. Health information technology: An updated systematic review with a focus on meaningful use. Ann. Intern. Med. 2014, 160, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Buntin, M.B.; Burke, M.F.; Hoaglin, M.C.; Blumenthal, D. The benefits of health information technology: A review of the recent literature shows predominantly positive results. Health Aff. 2011, 30, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Tang, P. Key Capabilities of an Electronic Health Record System; Institute of Medicine of the National Academies: Washington, DC, USA, 2003. [Google Scholar]
- National Institute of Health. NIH CLINICAL RESEARCH TRIALS AND YOU—Glossary of Common Terms. Available online: www.nih.gov/health/clinicaltrials/glossary.htm (accessed on 29 November 2014).
- National Institutes of Health, Department of Health and Human Services. Clinical Trials Registration and Results Information Submission. Final rule. Fed. Regist. 2016, 81, 64981–65157. [Google Scholar]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems (International Classification of Diseases) (ICD) 10th Revision—Version:2010; World Health Organization: Geneva, Switzerland, 2010; Volume 1. [Google Scholar]
- Begg, C.; Cho, M.; Eastwood, S.; Horton, R.; Moher, D.; Olkin, I.; Pitkin, R.; Rennie, D.; Schulz, K.F.; Simel, D.; et al. Improving the quality of reporting of randomized controlled trials: The CONSORT statement. JAMA 1996, 276, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, C.E. Medical subject headings (MeSH). Bull. Med. Libr. Assoc. 2000, 88, 265–266. [Google Scholar] [PubMed]
- De Angelis, C.D.; Drazen, J.M.; Frizelle, F.A.; Haug, C.; Hoey, J.; Horton, R.; Kotzin, S.; Laine, C.; Marusic, A.; Overbeke, A.J.P.; et al. Is this clinical trial fully registered?—A statement from the International Committee of Medical Journal Editors. N. Engl. J. Med. 2005, 352, 2436–2438. [Google Scholar] [CrossRef] [PubMed]
- Clancy, M.J. How to conduct an appraisal of a senior house officer. J. Accid. Emerg. Med. 1999, 16, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.C.; Navab, B.; Frazee, B.; Tebb, K.; Hendey, G.; Maselli, J.; Gonzales, R. A randomized trial of computer kiosk—Expedited management of cystitis in the emergency department. Acad. Emerg. Med. 2011, 18, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Pop-Eleches, C.; Thirumurthy, H.; Habyarimana, J.P.; Zivin, J.G.; Goldstein, M.P.; De Walque, D.; Mackeen, L.; Haberer, J.; Kimaiyo, S.; Sidle, J.; et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: A randomized controlled trial of text message reminders. AIDS 2011, 25, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Bigna, J.J.R.; Noubiap, J.J.N.; Kouanfack, C.; Plottel, C.S.; Koulla-Shiro, S. Effect of mobile phone reminders on follow-up medical care of children exposed to or infected with HIV in Cameroon (MORE CARE): A multicentre, single-blind, factorial, randomised controlled trial. Lancet Infect. Dis. 2014, 14, 600–608. [Google Scholar] [CrossRef]
- Mbuagbaw, L.; Thabane, L.; Ongolo-Zogo, P. Opening communication channels with people living with HIV using mobile phone text messaging: Insights from the CAMPS trial. BMC Res. Notes 2013, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Ybarra, M.L.; Korchmaros, J.D.; Prescott, T.L.; Birungi, R. A randomized controlled trial to increase HIV preventive information, motivation, and behavioral skills in Ugandan adolescents. Ann. Behav. Med. 2015, 49, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Lana, A.; Faya-Ornia, G.; López, M.L. Impact of a web-based intervention supplemented with text messages to improve cancer prevention behaviors among adolescents: Results from a randomized controlled trial. Prev. Med. 2014, 59, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Kearney, N.; McCann, L.; Norrie, J.; Taylor, L.; Gray, P.; McGee-Lennon, M.; Sage, M.; Miller, M.; Maguire, R. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Support. Care Cancer 2009, 17, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Meropol, N.J.; Egleston, B.L.; Buzaglo, J.S.; Balshem, A.; Benson, A.B.; Cegala, D.J.; Cohen, R.B.; Collins, M.; Diefenbach, M.A.; Miller, S.M.; et al. A Web-based communication aid for patients with cancer. Cancer 2013, 119, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.P., Jr.; Spangler, J.G.; Case, L.D.; Goff, D.C., Jr.; Singh, S.; Pignone, M.P. Effectiveness of a web-based colorectal cancer screening patient decision aid: A randomized controlled trial in a mixed-literacy population. Am. J. Prev. Med. 2011, 40, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, J.T.; Van Wijk, M.A.; Sturkenboom, M.C.; Mosseveld, M.; Moorman, P.W.; van der Lei, J. Electronic Alerts Versus On-Demand Decision Support to Improve Dyslipidemia Treatment A Cluster Randomized Controlled Trial. Circulation 2008, 117, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, B.A.; Raghinaru, D.; Cameron, F.; Bequette, B.W.; Chase, H.P.; Maahs, D.M.; Slover, R.; Wadwa, R.P.; Wilson, D.M.; Ly, T.; et al. Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care 2015, 38, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Peters, A.L.; Agy, C.; Menchine, M. A mobile health intervention for inner city patients with poorly controlled diabetes: Proof-of-concept of the TExT-MED program. Diabetes Technol. Ther. 2012, 14, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.; Ebrahimzadeh, I.; Rabi, A.; Saedipoor, B.; Jafarabadi, M.A. Impact of distance education via mobile phone text messaging on knowledge, attitude, practice and self efficacy of patients with type 2 diabetes mellitus in Iran. J. Diabetes Metab. Disord. 2012, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Wu, S.; Jin, H.; Ell, K.; Gross-Schulman, S.; Sklaroff, L.M.; Guterman, J. Automated remote monitoring of depression: Acceptance among low-income patients in diabetes disease management. JMIR Ment. Health 2016, 3, e6. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, M.L.; Soules, K.; Church, K.; Shaha, S.; Burlingame, J.; Graham, G.; Sauvage, L.; Zalud, I. Managing diabetes in pregnancy using cell phone/internet technology. Clin. Diabetes 2015, 33, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kropff, J.; Del Favero, S.; Place, J.; Toffanin, C.; Visentin, R.; Monaro, M.; Messori, M.; Di Palma, F.; Lanzola, G.; Farret, A.; et al. 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: A randomised crossover trial. Lancet Diabetes Endocrinol. 2015, 3, 939–947. [Google Scholar] [CrossRef]
- Kay-Lambkin, F.; Baker, A.; Lewin, T.; Carr, V. Acceptability of a clinician-assisted computerized psychological intervention for comorbid mental health and substance use problems: Treatment adherence data from a randomized controlled trial. J. Med. Internet Res. 2011, 13, e11. [Google Scholar] [CrossRef] [PubMed]
- Garrido, G.; Barrios, M.; Penadés, R.; Enriquez, M.; Garolera, M.; Aragay, N.; Pajares, M.; Vallès, V.; Delgado, L.; Alberni, J.; et al. Computer-assisted cognitive remediation therapy: Cognition, self-esteem and quality of life in schizophrenia. Schizophr. Res. 2013, 150, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Katula, J.A.; Rushing, J.; Kramer, A.F.; Jennings, J.M.; Sink, K.M.; Nadkarni, N.K.; Reid, K.F.; Castro, C.M.; Church, T.; et al. Performance of a computer-based assessment of cognitive function measures in two cohorts of seniors. Int. J. Geriatr. Psychiatry 2013, 28, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Hedman, E.; Andersson, E.; Lindefors, N.; Andersson, G.; Rück, C.; Ljótsson, B. Cost-effectiveness and long-term effectiveness of Internet-based cognitive behaviour therapy for severe health anxiety. Psychol. Med. 2013, 43, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Hedman, E.; Andersson, G.; Andersson, E.; Ljótsson, B.; Rück, C.; Asmundson, G.J.; Lindefors, N. Internet-based cognitive-behavioural therapy for severe health anxiety: Randomised controlled trial. Br. J. Psychiatry 2011, 198, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, J.; Clarke, J.; Birch, M.-R.; Whitton, A.E.; Parker, G.; Manicavasagar, V.; Harrison, V.; Christensen, H.; Hadzi-Pavlovic, D. Impact of a mobile phone and web program on symptom and functional outcomes for people with mild-to-moderate depression, anxiety and stress: A randomised controlled trial. BMC Psychiatry 2013, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.C.; Kauer, S.D.; Hearps, S.J.; Crooke, A.H.; Khor, A.S.; Sanci, L.A.; Patton, G.C. A mobile phone application for the assessment and management of youth mental health problems in primary care: A randomised controlled trial. BMC Fam. Pract. 2011, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Tulbure, B.T.; Szentagotai, A.; David, O.; Ștefan, S.; Månsson, K.N.; David, D.; Andersson, G. Internet-delivered cognitive-behavioral therapy for social anxiety disorder in Romania: A randomized controlled trial. PLoS ONE 2015, 10, e0123997. [Google Scholar] [CrossRef] [PubMed]
- Van Voorhees, B.W.; Hsiung, R.C.; Marko-Holguin, M.; Houston, T.K.; Fogel, J.; Lee, R.; Ford, D.E. Internal versus external motivation in referral of primary care patients with depression to an internet support group: Randomized controlled trial. J. Med. Internet Res. 2013, 15, e42. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.; Mackenzie, A.; Thomas, C.; Griskaitis, A.; Mewton, L.; Williams, A.; Andrews, G. CBT for depression: A pilot RCT comparing mobile phone vs. computer. BMC Psychiatry 2013, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.; Merry, S.; Stasiak, K.; McDowell, H.; Doherty, I.; Shepherd, M.; Dorey, E.; Parag, V.; Ameratunga, S.; Rodgers, A. MEMO—A mobile phone depression prevention intervention for adolescents: Development process and postprogram findings on acceptability from a randomized controlled trial. J. Med. Internet Res. 2012, 14, e13. [Google Scholar] [CrossRef] [PubMed]
- Linder, J.A.; Rigotti, N.A.; Schneider, L.I.; Kelley, J.H.; Brawarsky, P.; Haas, J.S. An Electronic Health Record-Based Intervention to Improve Tobacco Treatment in Primary Care: A Cluster-Randomized Controlled Trial. Arch. Intern. Med. 2009, 169, 781–787. [Google Scholar] [CrossRef] [PubMed]
- McClure, J.B.; Anderson, M.L.; Bradley, K.; An, L.C.; Catz, S.L. Evaluating an adaptive and interactive mHealth Smoking Cessation and Medication Adherence Program: A randomized pilot feasibility study. JMIR mHealth uHealth 2016, 4, e94. [Google Scholar] [CrossRef] [PubMed]
- Rindal, D.B.; Rush, W.A.; Schleyer, T.K.; Kirshner, M.; Boyle, R.G.; Thoele, M.J.; Asche, S.E.; Thyvalikakath, T.; Spallek, H.; Durand, E.C.; et al. Computer-assisted guidance for dental office tobacco-cessation counseling: A randomized controlled trial. Am. J. Prev. Med. 2013, 44, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.; Dorey, E.; Bramley, D.; Bullen, C.; Denny, S.; Elley, C.R.; Maddison, R.; McRobbie, H.; Parag, V.; Rodgers, A.; et al. A theory-based video messaging mobile phone intervention for smoking cessation: Randomized controlled trial. J. Med. Internet Res. 2011, 13, e10. [Google Scholar] [CrossRef] [PubMed]
- Crombie, I.; Falconer, D.; Irvine, L.; Williams, B.; Ricketts, I.; Humphris, G.; Norrie, J.; Rice, P.; Slane, P.W. Reducing alcohol-related harm in disadvantaged men: Development and feasibility assessment of a brief intervention delivered by mobile telephone. Public Health Res. 2013, 1, 1–158. [Google Scholar] [CrossRef] [PubMed]
- Gajecki, M.; Berman, A.H.; Sinadinovic, K.; Rosendahl, I.; Andersson, C. Mobile phone brief intervention applications for risky alcohol use among university students: A randomized controlled study. Addict. Sci. Clin. Pract. 2014, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Tamblyn, R.; Eguale, T.; Buckeridge, D.L.; Huang, A.; Hanley, J.; Reidel, K.; Shi, S.; Winslade, N. The effectiveness of a new generation of computerized drug alerts in reducing the risk of injury from drug side effects: A cluster randomized trial. J. Am. Med. Inform. Assoc. 2012, 19, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Antypas, K.; Wangberg, S.C. Combining users’ needs with health behavior models in designing an internet-and mobile-based intervention for physical activity in cardiac rehabilitation. JMIR Res. Protoc. 2014, 3, e4. [Google Scholar] [CrossRef] [PubMed]
- Pfaeffli, L.; Maddison, R.; Whittaker, R.; Stewart, R.; Kerr, A.; Jiang, Y.; Kira, G.; Carter, K.; Dalleck, L. A mHealth cardiac rehabilitation exercise intervention: Findings from content development studies. BMC Cardiovasc. Disord. 2012, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Fjeldsoe, B.S.; Miller, Y.D.; O’Brien, J.L.; Marshall, A.L. Iterative development of MobileMums: A physical activity intervention for women with young children. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- McKinstry, B.; Hanley, J.; Wild, S.; Pagliari, C.; Paterson, M.; Lewis, S.; Sheikh, A.; Krishan, A.; Stoddart, A.; Padfield, P. Telemonitoring based service redesign for the management of uncontrolled hypertension: Multicentre randomised controlled trial. BMJ Br. Med. J. 2013, 346, f3030. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, A.; Hanley, J.; Wild, S.; Pagliari, C.; Paterson, M.; Lewis, S.; Sheikh, A.; Krishan, A.; Padfield, P.; McKinstry, B. Telemonitoring-based service redesign for the management of uncontrolled hypertension (HITS): Cost and cost-effectiveness analysis of a randomised controlled trial. BMJ Open 2013, 3, e002681. [Google Scholar] [CrossRef] [PubMed]
- Kizony, R.; Weiss, P.; Feldman, Y.; Shani, M.; Elion, O.; Kizony, R.; Weiss, P.L.; Kizony, R.; Harel, S.; Baum-Cohen, I. Evaluation of a Tele-Health System for upper extremity stroke rehabilitation. In Proceedings of the 2013 International Conference on Virtual Rehabilitation (ICVR), Philadelphia, PA, USA, 26–29 August 2013; pp. 80–86. [Google Scholar]
- Malhotra, S.; Musgrave, S.D.; Pinnock, H.; Price, D.; Ryan, D.P. The challenge of recruiting in primary care for a trial of telemonitoring in asthma: An observational study. Pragmat. Obs. Res. 2012, 3, 51–55. [Google Scholar] [PubMed]
- Meltzer, E.O.; Kelley, N.; Hovell, M.F. Randomized, cross-over evaluation of mobile phone vs paper diary in subjects with mild to moderate persistent asthma. Open Respir. Med. J. 2008, 2, 72–79. [Google Scholar] [CrossRef] [PubMed]
- King, A.C.; Bickmore, T.W.; Campero, M.I.; Pruitt, L.A.; Yin, J.L. Employing virtual advisors in preventive care for underserved communities: Results from the COMPASS study. J. Health Commun. 2013, 18, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Turner-McGrievy, G.; Tate, D. Tweets, apps, and pods: Results of the 6-month Mobile Pounds Off Digitally (Mobile POD) randomized weight-loss intervention among adults. J. Med. Internet Res. 2011, 13, e120. [Google Scholar] [CrossRef] [PubMed]
- Turner-McGrievy, G.M.; Tate, D.F. Weight loss social support in 140 characters or less: Use of an online social network in a remotely delivered weight loss intervention. Transl. Behav. Med. 2013, 3, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Batch, B.C.; Tyson, C.; Bagwell, J.; Corsino, L.; Intille, S.; Lin, P.-H.; Lazenka, T.; Bennett, G.; Bosworth, H.B.; Voils, C.; et al. Weight loss intervention for young adults using mobile technology: Design and rationale of a randomized controlled trial—Cell Phone Intervention for You (CITY). Contemp. Clin. Trials 2014, 37, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.J.; Kolodziejczyk, J.K.; Adams, M.A.; Patrick, K.; Marshall, S.J. Fruit and vegetable intake and eating behaviors mediate the effect of a randomized text-message based weight loss program. Prev. Med. 2013, 56, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U.-V.; Folta-Schoofs, K.; Behrends, M.; von Jan, U. Effects of mobile augmented reality learning compared to textbook learning on medical students: Randomized controlled pilot study. J. Med. Internet Res. 2013, 15, e182. [Google Scholar] [CrossRef] [PubMed]
- Suhling, H.; Rademacher, J.; Zinowsky, I.; Fuge, J.; Greer, M.; Warnecke, G.; Smits, J.M.; Bertram, A.; Haverich, A.; Welte, T.; et al. Conventional vs. tablet computer-based patient education following lung transplantation—A randomized controlled trial. PLoS ONE 2014, 9, e90828. [Google Scholar] [CrossRef] [PubMed]
- Short, C.E.; Vandelanotte, C.; Dixon, M.W.; Rosenkranz, R.; Caperchione, C.; Hooker, C.; Karunanithi, M.; Kolt, G.S.; Maeder, A.; Ding, H.; et al. Examining participant engagement in an information technology-based physical activity and nutrition intervention for men: The manup randomized controlled trial. JMIR Res. Protocols 2014, 3, e2. [Google Scholar] [CrossRef] [PubMed]
- Terrell, K.M.; Perkins, A.J.; Dexter, P.R.; Hui, S.L.; Callahan, C.M.; Miller, D.K. Computerized decision support to reduce potentially inappropriate prescribing to older emergency department patients: A randomized, controlled trial. J. Am. Geriatr. Soc. 2009, 57, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.; Nielsen, B.B.; Hemed, M.; Boas, I.M.; Said, A.; Said, K.; Makungu, M.H.; Rasch, V. Mobile phones improve antenatal care attendance in Zanzibar: A cluster randomized controlled trial. BMC Pregnancy Childbirth 2014, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.; Rasch, V.; Hemed, M.; Boas, I.M.; Said, A.; Said, K.; Makundu, M.H.; Nielsen, B.B. Mobile phone intervention reduces perinatal mortality in Zanzibar: Secondary outcomes of a cluster randomized controlled trial. JMIR mHealth uHealth 2014, 2, e15. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Hogg-Johnson, S.; Stewart, D.E.; Skinner, H.A.; Glazier, R.H.; Levinson, W. Computer-assisted screening for intimate partner violence and control: A randomized trial. Ann. Intern. Med. 2009, 151, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Boulkedid, R.; Abdoul, H.; Loustau, M.; Sibony, O.; Alberti, C. Using and reporting the Delphi method for selecting healthcare quality indicators: A systematic review. PLoS ONE 2011, 6, e20476. [Google Scholar] [CrossRef] [PubMed]
- Mallett, R.; Hagen-Zanker, J.; Slater, R.; Duvendack, M. The benefits and challenges of using systematic reviews in international development research. J. Dev. Eff. 2012, 4, 445–455. [Google Scholar] [CrossRef]
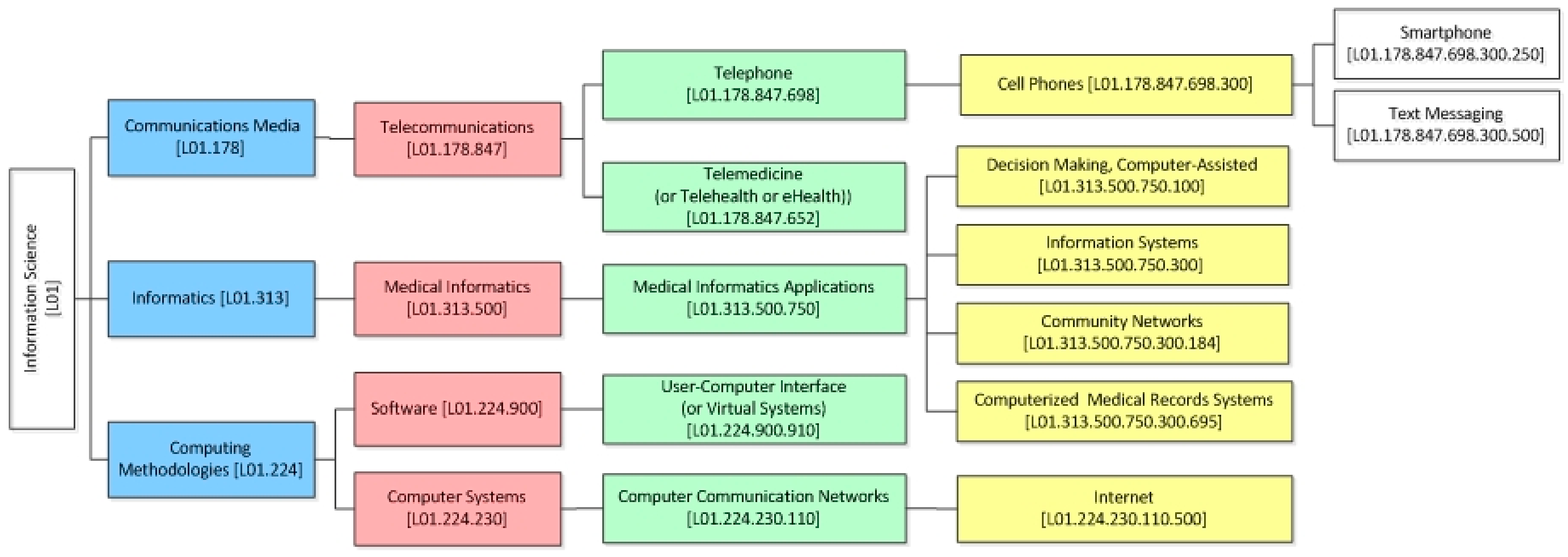
| Classification of the Studies Based on the ICD-10 Classification System | Duration (Year(s)) | RCT Type | Population | Experimental Type | Functional Capabilities of HITs | Category of Applied Information Science | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Open Label | Single Blind | Double Blind | Not Blinded | Unclear | Prevention | Screening | Treatment | Supportive Care | Health services Research | CBA | CPOE | DSS | EHR | Other | L01.224.230.110 | L01.178.847.652 | L01.178.847.698 | L01.224.900.910 | L01.313.500.750 | |||
| A00–B99: Certain infectious and parasitic diseases Infections (5 studies/5 trials) | 2.4 | 2 | 0 | 3 | 0 | 0 | 1916 | 1 | 0 | 2 | 1 | 1 | 3 | 0 | 0 | 0 | 2 | 1 | 0 | 3 | 0 | 1 |
| C00–D49: Neoplasms (4 studies/4 trials) | 5.25 | 0 | 1 | 0 | 0 | 3 | 4220 | 1 | 1 | 1 | 0 | 1 | 3 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 0 | 0 |
| E00–E89: Endocrine, nutritional and metabolic diseases (7 studies/7 trials) | 1.71 | 6 | 0 | 0 | 1 | 0 | 90,042 | 1 | 1 | 4 | 0 | 1 | 4 | 0 | 3 | 0 | 1 | 1 | 1 | 4 | 0 | 3 |
| F01-F99: Mental, Behavioral and Neurodevelopmental disorders (18 studies /18 trials) | 2.72 | 4 | 6 | 6 | 0 | 2 | 20,827 | 4 | 0 | 11 | 2 | 1 | 8 | 1 | 1 | 3 | 9 | 4 | 2 | 8 | 0 | 7 |
| I00–I99: Diseases of the circulatory system (6 studies/5 trials) | 2.60 | 0 | 1 | 2 | 0 | 2 | 1249 | 0 | 1 | 1 | 3 | 0 | 4 | 0 | 1 | 0 | 1 | 3 | 2 | 3 | 1 | 0 |
| J00–J99: Diseases of the respiratory system (3 studies/2 trials) | 3.50 | 1 | 1 | 0 | 0 | 0 | 324 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 0 |
| Z00–Z99: Factors influencing health status and contact with health services (8 studies/7 trials) | 2.86 | 3 | 1 | 2 | 0 | 0 | 1040 | 0 | 0 | 3 | 1 | 2 | 3 | 0 | 0 | 0 | 2 | 2 | 0 | 5 | 1 | 1 |
| Other subjects (Older adults, Reproductive Health and Childbirth, Screening for Partner Violence) (4 studies/3 trials) | 2.67 | 2 | 0 | 1 | 0 | 0 | 3784 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 2 |
| Number of trials | 18 | 11 | 14 | 1 | 7 | - | 8 | 4 | 24 | 8 | 7 | 27 | 2 | 6 | 4 | 19 | 14 | 6 | 26 | 3 | 14 | |
| Percentage of total | 35.3% | 21.6% | 27.5% | 2.0% | 13.7% | 15.7% | 7.8% | 47.1% | 15.7% | 13.7% | 52.9% | 3.9% | 11.8% | 7.8% | 37.3% | 27.5% | 11.8% | 51.0% | 5.9% | 27.5% | ||
| Percentage of studies with positive outcome summary | 66.7% | 63.6% | 57.1 % | 100 % | 42.9% | 75% | 100% | 58.3% | 25% | 71.4% | 51.9% | 50% | 100% | 50% | 68.4% | 64.3% | 66.7% | 50% | 33.3% | 64.3% | ||
| Summary of trials: | 51 | |||||||||||||||||||||
| Classification of the Studies Based on the ICD-10 Classification System | Impact by Type | Positive Outcome Summary | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Safety | Efficacy/Effectiveness | Organizational/Professional | Psychological/Social/Ethical | Economic | ||||||||
| Safety/Privacy/Security | Efficiency | Efficacy/Effectiveness | Preventive Care | Adherence/Attendance | Service Delivery/Performance | Appropriateness | Perceived Ease of Use/Usefulness | Acceptability | Satisfaction | Cost Effectiveness | ||
| A00–B99: Certain infectious and parasitic diseases Infections (5 studies/5 trials) | 1 | 2 | 3 | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 2 | 1 |
| C00–D49: Neoplasms (4 studies/4 trials) | 0 | 0 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 3 |
| E00–E89: Endocrine, nutritional and metabolic diseases (7 studies/7 trials) | 2 | 0 | 7 | 1 | 0 | 2 | 0 | 2 | 2 | 1 | 0 | 7 |
| F01–F99: Mental, Behavioral and Neurodevelopmental disorders (18 studies/18 trials) | 0 | 0 | 16 | 3 | 4 | 2 | 0 | 1 | 4 | 1 | 1 | 12 |
| I00–I99: Diseases of the circulatory system (6 studies/5 trials) | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 2 |
| J00–J99: Diseases of the respiratory system (3 studies/2 trials) | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Z00–Z99: Factors influencing health status and contact with health services (8 studies/7 trials) | 1 | 1 | 6 | 1 | 1 | 0 | 2 | 2 | 0 | 1 | 1 | 4 |
| Other subjects (Older adults, Reproductive Health and Childbirth, Screening for Partner Violence) (4 studies/3 trials) | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Number of trials that examined this impact | 6 | 3 | 45 | 7 | 10 | 7 | 3 | 7 | 8 | 5 | 6 | |
| Number wherein the examined impact contribute to positive outcome summary | 4 | 1 | 26 | 6 | 5 | 4 | 2 | 4 | 6 | 3 | 1 | 31 |
| Rate wherein the examined impact contribute to positive outcome summary | 66.7% | 33.3% | 57.8% | 85.7% | 50% | 57.1% | 66.7% | 57.1% | 75% | 60% | 16.7% | 60.8% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulou, S.C.; Kotsilieris, T.; Anagnostopoulos, I. Assessment of Health Information Technology Interventions in Evidence-Based Medicine: A Systematic Review by Adopting a Methodological Evaluation Framework. Healthcare 2018, 6, 109. https://doi.org/10.3390/healthcare6030109
Christopoulou SC, Kotsilieris T, Anagnostopoulos I. Assessment of Health Information Technology Interventions in Evidence-Based Medicine: A Systematic Review by Adopting a Methodological Evaluation Framework. Healthcare. 2018; 6(3):109. https://doi.org/10.3390/healthcare6030109
Chicago/Turabian StyleChristopoulou, Stella C., Theodore Kotsilieris, and Ioannis Anagnostopoulos. 2018. "Assessment of Health Information Technology Interventions in Evidence-Based Medicine: A Systematic Review by Adopting a Methodological Evaluation Framework" Healthcare 6, no. 3: 109. https://doi.org/10.3390/healthcare6030109
APA StyleChristopoulou, S. C., Kotsilieris, T., & Anagnostopoulos, I. (2018). Assessment of Health Information Technology Interventions in Evidence-Based Medicine: A Systematic Review by Adopting a Methodological Evaluation Framework. Healthcare, 6(3), 109. https://doi.org/10.3390/healthcare6030109