Quercetin in Idiopathic Pulmonary Fibrosis and Its Comorbidities: Gene Regulatory Mechanisms and Therapeutic Implications
Abstract
1. Introduction
2. Treatments for IPF
3. Alternative Treatment for IPF and Its Comorbidities
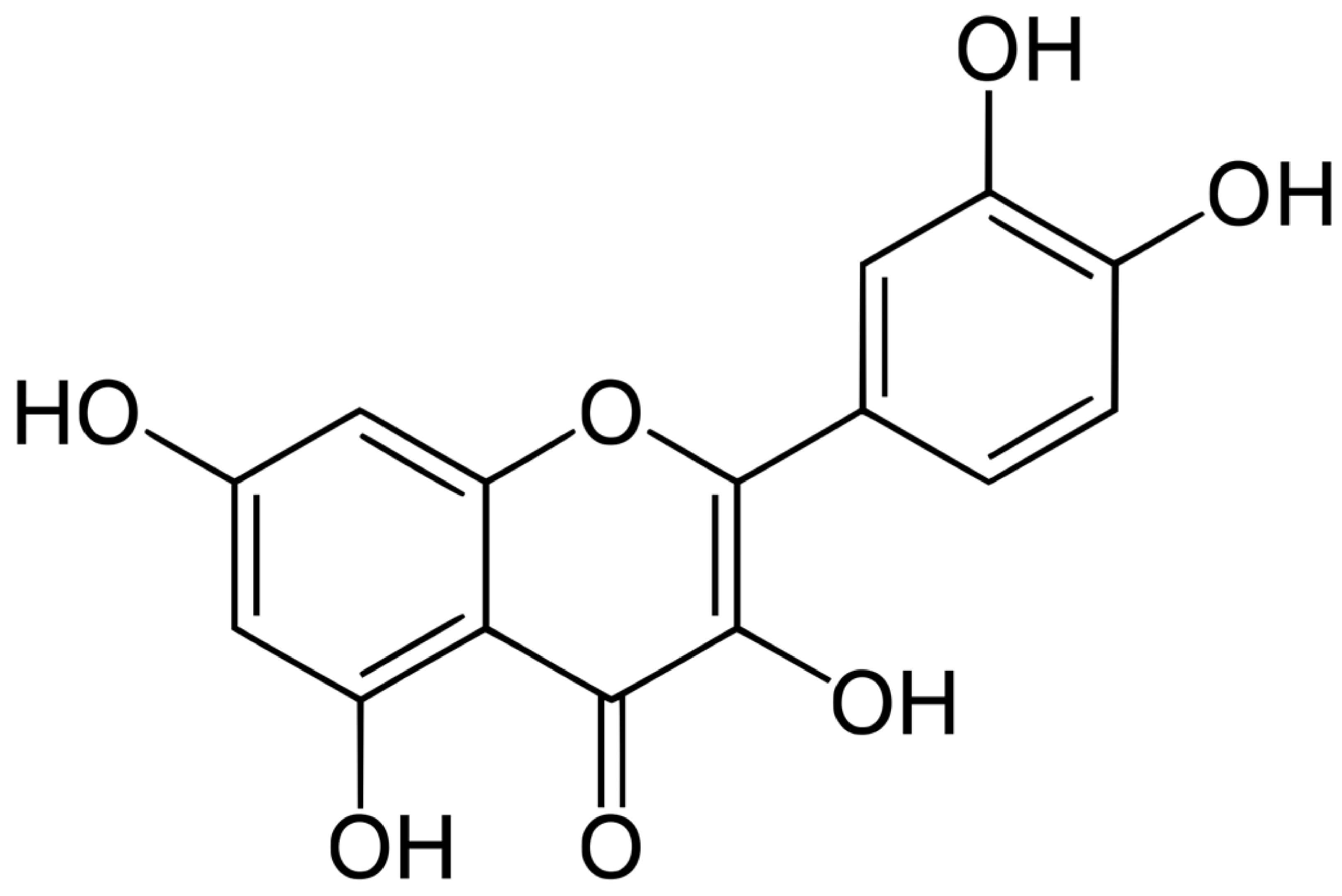
4. Quercetin: Chemical Properties, Pharmacokinetics, and Mechanistic Insights
5. Quercetin and IPF
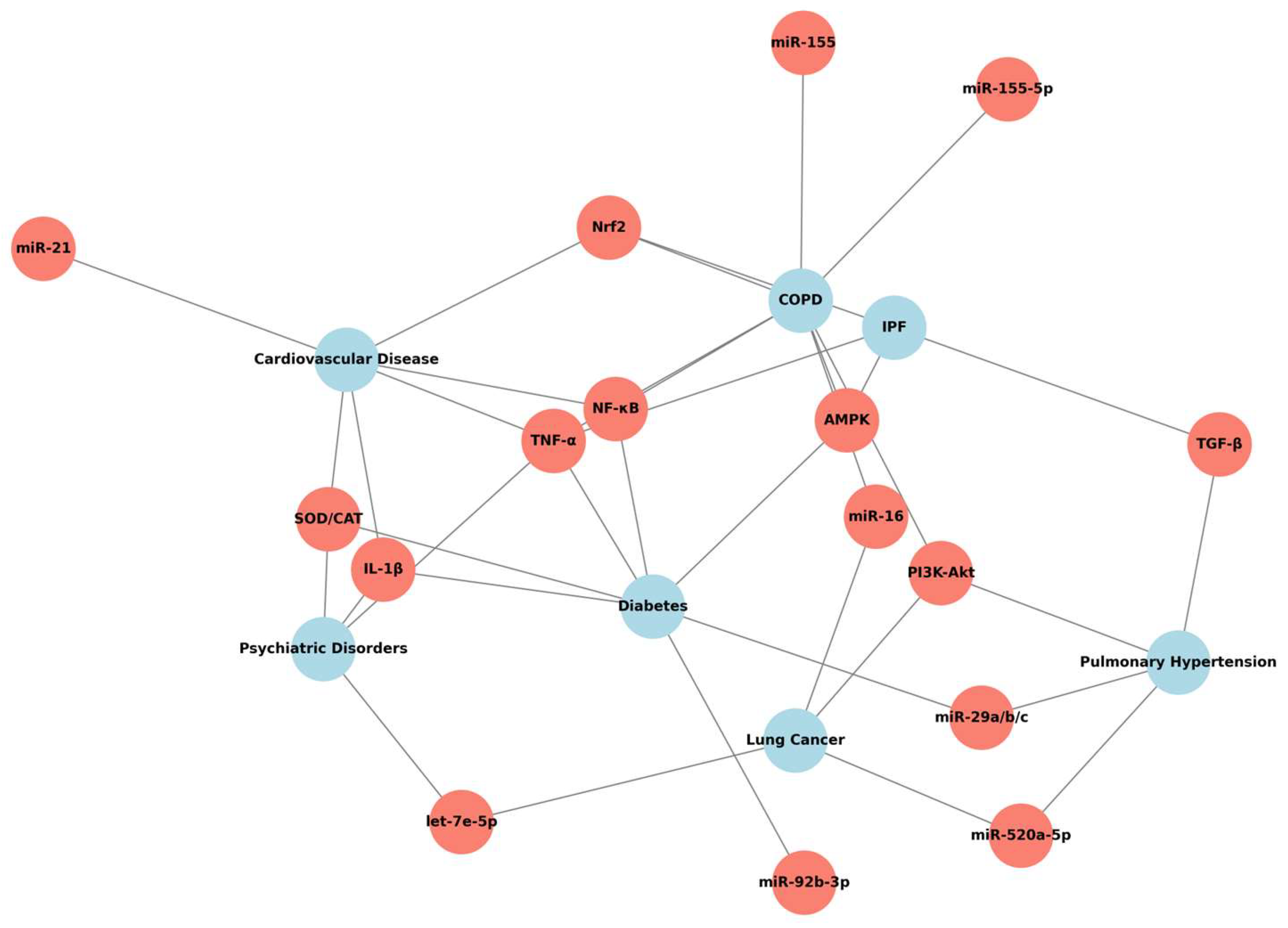
5.1. Pulmonary Comorbidities
5.1.1. Quercetin and COPD
5.1.2. Quercetin and Lung Cancer
5.1.3. Quercetin and Pulmonary Hypertension
5.2. Extrapulmonary Comorbidities
5.2.1. Quercetin and Cardiovascular Disease
5.2.2. Quercetin and Diabetes
5.2.3. Quercetin and Psychiatric Diseases
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IPF | Idiopathic Pulmonary Fibrosis |
| COPD | Chronic Obstructive Pulmonary Disease |
| PH | Pulmonary Hypertension |
| LC | Lung Cancer |
| CVDs | Cardiovascular Diseases |
| CAD | Coronary Artery Disease |
| FDA | Food and Drug Administration |
| TGF-β | Transforming Growth Factor Beta |
| TNF-α | Tumor Necrosis Factor-alpha |
| PDGF | Platelet-Derived Growth Factor |
| FGF | Fibroblast Growth Factor |
| VEGF | Vascular Endothelial Growth Factor |
| miRNAs | microRNAs |
| ECM | Extracellular Matrix |
| EGCG | Epigallocatechin Gallate |
| SASP | Senescence-Associated Secretory Phenotypes |
| BLM | Bleomycin |
| AMPK | AMP-activated protein kinase |
| GCs | Glucocorticoids |
| NSCLC | Non-Small-Cell Lung Cancer |
| MIAT | Myocardial Infarction-Associated Transcript |
| PASMCs | Pulmonary Artery Smooth Muscle Cells |
| H/R | Hypoxia/Reoxygenation |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| DM | Diabetes Mellitus |
| GLUT | Glucose Transporter |
| G6 Pase | Glucose-6-phosphatase |
| CaMKKs | Calcium/Calmodulin-Mediated Protein Kinases |
| IGF-1 | Insulin-Like Growth Factor 1 |
| HMCs | Human Mesangial Cells |
| MDA | Malondialdehyde |
| HPA | Hypothalamic‒Pituitary‒Adrenal |
| CRF | Corticotropin-Releasing Factor |
| MA | Methamphetamine |
| lncRNAs | Long Noncoding RNAs |
| ceRNA | Competitor RNA |
References
- Kreuter, M.; Ehlers-Tenenbaum, S.; Schaaf, M.; Oltmanns, U.; Palmowski, K.; Hoffmann, H.; Schnabel, P.A.; Heußel, C.P.; Puderbach, M.; Herth, F.J.; et al. Treatment and outcome of lung cancer in idiopathic interstitial pneumonias. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2015, 31, 266–274. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Rotunno, M.; Lubin, J.H.; Wacholder, S.; Consonni, D.; Pesatori, A.C.; Bertazzi, P.A.; Chanock, S.J.; Burdette, L.; Goldstein, A.M.; et al. Dietary quercetin, quercetin-gene interaction, metabolic gene expression in lung tissue and lung cancer risk. Carcinogenesis 2009, 31, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Amatto, V.C.; Behr, J.; Stowasser, S. Comorbidities in idiopathic pulmonary fibrosis patients: A systematic literature review. Eur. Respir. J. 2015, 46, 1113–1130. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, T.M.; Robalo Cordeiro, C. Comorbidity in idiopathic pulmonary fibrosis-what can biomarkers tell us? Ther. Adv. Respir. Dis. 2020, 14, 1753466620910092. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, D.M.; Šterclová, M.; Mogulkoc, N.; Lewandowska, K.; Müller, V.; Hájková, M.; Studnicka, M.; Tekavec-Trkanjec, J.; Littnerová, S.; Vašáková, M.; et al. Comorbidity burden and survival in patients with idiopathic pulmonary fibrosis: The EMPIRE registry study. Respir. Res. 2022, 23, 135. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.; Ehlers-Tenenbaum, S.; Palmowski, K.; Bruhwyler, J.; Oltmanns, U.; Muley, T.; Heussel, C.P.; Warth, A.; Kolb, M.; Herth, F.J. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PLoS ONE 2016, 11, e0151425. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.M.; Leong, M.C.H.; Montesi, S.B.; Ryerson, C.J.; Khor, Y.H. Comorbidities in the idiopathic pulmonary fibrosis and progressive pulmonary fibrosis trial population: A systematic review and meta-analysis. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2025, 34, 240238. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Buendia-Roldan, I.; Pardo, A. Decoding the complexity: Mechanistic insights into comorbidities in idiopathic pulmonary fibrosis. Eur. Respir. J. 2025, 65, 2402418. [Google Scholar] [CrossRef] [PubMed]
- Barratt, S.L.; Creamer, A.; Hayton, C.; Chaudhuri, N. Idiopathic Pulmonary Fibrosis (IPF): An Overview. J. Clin. Med. 2018, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Bonella, F.; Spagnolo, P.; Ryerson, C. Current and Future Treatment Landscape for Idiopathic Pulmonary Fibrosis. Drugs 2023, 83, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J. Cellular and Molecular Mechanisms in Idiopathic Pulmonary Fibrosis. Adv. Respir. Med. 2023, 91, 26–48. [Google Scholar] [CrossRef] [PubMed]
- Millan-Billi, P.; Serra, C.; Alonso Leon, A.; Castillo, D. Comorbidities, Complications and Non-Pharmacologic Treatment in Idiopathic Pulmonary Fibrosis. Med. Sci. 2018, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Imenshahidi, M.; Hosseinzadeh, H.; Karimi, G. Effects of plant extracts and bioactive compounds on attenuation of bleomycin-induced pulmonary fibrosis. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 107, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Santos-Álvarez, J.C.; Velázquez-Enríquez, J.M.; García-Carrillo, R.; Rodríguez-Beas, C.; Ramírez-Hernández, A.A.; Reyes-Jiménez, E.; González-García, K.; López-Martínez, A.; Pérez-Campos Mayoral, L.; Aguilar-Ruiz, S.R.; et al. miRNAs Contained in Extracellular Vesicles Cargo Contribute to the Progression of Idiopathic Pulmonary Fibrosis: An In Vitro Aproach. Cell 2022, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010, 207, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liang, Y.; Lin, Q.; Liu, J.; Luo, F.; Li, X.; Zhou, H.; Zhuang, S.; Zhang, H. miR-29 mediates TGFβ1-induced extracellular matrix synthesis through activation of PI3K-AKT pathway in human lung fibroblasts. J. Cell. Biochem. 2013, 114, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, H.; Zhao, F.; Li, H.; Gao, R.; Yan, B.; Ren, J.; Yang, J. Decrypting the crosstalk of noncoding RNAs in the progression of IPF. Mol. Biol. Rep. 2020, 47, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xie, Y.; Wei, Q.; Zhang, X.; Xu, Z. Revisiting the role of MicroRNAs in the pathogenesis of idiopathic pulmonary fibrosis. Front. Cell Dev. Biol. 2024, 12, 1470875. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Shan, H.; Liang, H. MicroRNAs in idiopathic pulmonary fibrosis: Involvement in pathogenesis and potential use in diagnosis and therapeutics. Acta Pharm. Sin. B 2016, 6, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Iannitti, R.; Palumbo, R. Quercetin: A pleiotropic kinase inhibitor against cancer. Cancer Treat. Res. 2014, 159, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Parvaresh, A.; Razavi, R.; Rafie, N.; Ghiasvand, R.; Pourmasoumi, M.; Miraghajani, M. Quercetin and ovarian cancer: An evaluation based on a systematic review. J. Res. Med. Sci. 2016, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.F.; Ribeiro, M.; Abrantes, A.M.; Pires, A.S.; Teixo, R.J.; Tralhão, J.G.; Botelho, M.F. Quercetin in Cancer Treatment, Alone or in Combination with Conventional Therapeutics? Curr. Med. Chem. 2015, 22, 3025–3039. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Soleti, R.; Porro, C. The Potential Neuroprotective Role of Free and Encapsulated Quercetin Mediated by miRNA against Neurological Diseases. Nutrients 2021, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Khan, H.; Ullah, H.; Hassan, S.T.S.; Šmejkal, K.; Efferth, T.; Mahomoodally, M.F.; Xu, S.; Habtemariam, S.; Filosa, R.; et al. MicroRNA targeting by quercetin in cancer treatment and chemoprotection. Pharmacol. Res. 2019, 147, 104346. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Xiao, N.; Li, X.W.; Fan, Y.; Alolga, R.N.; Sun, X.Y.; Wang, S.L.; Li, P.; Qi, L.W. Pharmacokinetic comparison between quercetin and quercetin 3-O-β-glucuronide in rats by UHPLC-MS/MS. Sci. Rep. 2016, 6, 35460. [Google Scholar] [CrossRef] [PubMed]
- Ishisaka, A.; Sugimoto, R.; Marumo, H.; Doi, T.; Hamada, K.; Fujimoto, M.; Fujiwara, N.; Yamasaki, M.; Murakami, A. Role of Extracellular Vesicles in Absorption and Functional Mechanisms of Quercetin. Mol. Nutr. Food Res. 2023, 67, e2300225. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Jabeen, S.; Attar, R.; Yaylim, I.; Xu, B. Quercetin-mediated regulation of signal transduction cascades and microRNAs: Natural weapon against cancer. J. Cell. Biochem. 2018, 119, 9664–9674. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Hayes, A.W.; Pressman, P.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Quercetin induces its chemoprotective effects via hormesis. Food Chem. Toxicol. 2024, 184, 114419. [Google Scholar] [CrossRef] [PubMed]
- Milackova, I.; Rackova, L.; Majekova, M.; Mrvova, N.; Stefek, M. Protection or cytotoxicity mediated by a novel quinonoid-polyphenol compound? Gen. Physiol. Biophys. 2015, 34, 51–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sang, A.; Wang, Y.; Wang, S.; Wang, Q.; Wang, X.; Li, X.; Song, X. Quercetin attenuates sepsis-induced acute lung injury via suppressing oxidative stress-mediated ER stress through activation of SIRT1/AMPK pathways. Cell. Signal. 2022, 96, 110363. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, A.; Kellogg, D., 3rd; Justice, J.; Goros, M.; Gelfond, J.; Pascual, R.; Hashmi, S.; Masternak, M.; Prata, L.; LeBrasseur, N.; et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: Results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine 2023, 90, 104481. [Google Scholar] [CrossRef] [PubMed]
- Veith, C.; Drent, M.; Bast, A.; van Schooten, F.J.; Boots, A.W. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. Toxicol. Appl. Pharmacol. 2017, 336, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiao, Y.; Wu, Z.; Liu, H.; Li, Y.; Cai, Y.; Wei, W.; Cao, F. The role of quercetin in ameliorating bleomycin-induced pulmonary fibrosis: Insights into autophagy and the SIRT1/AMPK signaling pathway. Mol. Biol. Rep. 2024, 51, 795. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, Y.; Zhang, W.; Chen, X. Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochem. Cell Biol. 2018, 96, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Veith, C.; Albrecht, C.; Bartholome, R.; Drittij, M.J.; Claessen, S.M.H.; Bast, A.; Rosenbruch, M.; Jonkers, L.; van Schooten, F.J.; et al. The dietary antioxidant quercetin reduces hallmarks of bleomycin-induced lung fibrogenesis in mice. BMC Pulm. Med. 2020, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Toker, Ç.; Kuyucu, Y.; Şaker, D.; Kara, S.; Güzelel, B.; Mete, U. Investigation of miR-26b and miR-27b expressions and the effect of quercetin on fibrosis in experimental pulmonary fibrosis. J. Mol. Histol. 2024, 55, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L. Traditional Herbal Plants and their Phytoconstituents Based Remedies for Respiratory Diseases: A Review. Open Respir. Med. J. 2025, 19, e18743064341009. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Hogg, J.C. Update on the Pathogenesis of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2019, 381, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.P.; Parmenter, B.H.; Dalgaard, F.; Murray, K.; Rasmussen, D.B.; Kyrø, C.; Cassidy, A.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; et al. Flavonoid intakes inversely associate with COPD in smokers. Eur. Respir. J. 2022, 60, 2102604. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Barreto, T.A.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020, 7, e000392. [Google Scholar] [CrossRef] [PubMed]
- Farazuddin, M.; Mishra, R.; Jing, Y.; Srivastava, V.; Comstock, A.T.; Sajjan, U.S. Quercetin prevents rhinovirus-induced progression of lung disease in mice with COPD phenotype. PLoS ONE 2018, 13, e0199612. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.; de Matos, N.A.; Oliveira, M.; de Souza, A.B.F.; Castro, T.F.; Machado-Júnior, P.A.; de Souza, D.M.S.; Talvani, A.; Cangussú, S.D.; de Menezes, R.C.A.; et al. Quercetin Improves Pulmonary Function and Prevents Emphysema Caused by Exposure to Cigarette Smoke in Male Mice. Antioxidants 2022, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Mitani, A.; Azam, A.; Vuppusetty, C.; Ito, K.; Mercado, N.; Barnes, P.J. Quercetin restores corticosteroid sensitivity in cells from patients with chronic obstructive pulmonary disease. Exp. Lung Res. 2017, 43, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Zhang, P.A.; Chen, M.Z.; Du, W.S.; Zhang, Y.; Jiao, Y.; Li, X. Network Pharmacology and Experimental Validation of Jinwei Decoction for Enhancement of Glucocorticoid Anti-Inflammatory Effect in COPD through miR-155-5p. Comb. Chem. High Throughput Screen. 2025, 28, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; Fadda, L.M.; Attia, H.A.; Ali, H.M.; Al-Rasheed, N.M. Quercetin inhibits sodium nitrite-induced inflammation and apoptosis in different rats organs by suppressing Bax, HIF1-α, TGF-β, Smad-2, and AKT pathways. J. Biochem. Mol. Toxicol. 2017, 31, e21883. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Shao, S.; Zhao, Y.; Marincola, F.; Pesatori, A.; Bertazzi, P.A.; Caporaso, N.E.; Wang, E.; Landi, M.T. Influence of quercetin-rich food intake on microRNA expression in lung cancer tissues. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Lu, H.; Wang, H.; Feng, H.; Xu, J.; Zhang, B. Quercetin radiosensitizes non-small cell lung cancer cells through the regulation of miR-16-5p/WEE1 axis. IUBMB Life 2020, 72, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Su, T.; Ye, J.; Yang, C.; Zhang, S.; Xie, C. The miR-15 family enhances the radiosensitivity of breast cancer cells by targeting G2 checkpoints. Radiat. Res. 2015, 183, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Yue, X.; Ren, G.; Li, H.; Ping, L.; Wang, Y.; Xia, T. miR-15a/16 enhances radiation sensitivity of non-small cell lung cancer cells by targeting the TLR1/NF-κB signaling pathway. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, H.; Sato, T.; Endo, S.; Matsunaga, T.; Yamaguchi, M.; Yamazaki, Y.; Sugatani, J.; Ikari, A. Quercetin Decreases Claudin-2 Expression Mediated by Up-Regulation of microRNA miR-16 in Lung Adenocarcinoma A549 Cells. Nutrients 2015, 7, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Zhao, W.; Xiong, J.; Cao, R. Downregulation of miR-16 promotes growth and motility by targeting HDGF in non-small cell lung cancer cells. FEBS Lett. 2013, 587, 3153–3157. [Google Scholar] [CrossRef]
- Arslan, A.; Smith, J.; Qureshi, M.R.; Uysal, A.; Patel, K.K.; Herazo-Maya, J.D.; Bandyopadhyay, D. Evolution of pulmonary hypertension in interstitial lung disease: A journey through past, present, and future. Front. Med. 2023, 10, 1306032. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cano, D.; Menendez, C.; Moreno, E.; Moral-Sanz, J.; Barreira, B.; Galindo, P.; Pandolfi, R.; Jimenez, R.; Moreno, L.; Cogolludo, A.; et al. The flavonoid quercetin reverses pulmonary hypertension in rats. PLoS ONE 2014, 9, e114492. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, S.; Najafipour, H.; Jafarinejad Farsangi, S.; Joukar, S.; Beik, A.; Iranpour, M.; Kordestani, Z. Perillyle alcohol and Quercetin ameliorate monocrotaline-induced pulmonary artery hypertension in rats through PARP1-mediated miR-204 down-regulation and its downstream pathway. BMC Complement. Med. Ther. 2020, 20, 218. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, S.; Najafipour, H.; Sheikholeslami, M.; Jafarinejad-Farsangi, S.; Beik, A.; Askaripour, M.; Karam, Z.M. Perillyl alcohol and quercetin modulate the expression of non-coding RNAs MIAT, H19, miR-29a, and miR-33a in pulmonary artery hypertension in rats. Non-Coding RNA Res. 2022, 7, 27–33. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cao, X.; Guo, P.; Li, X.; Shang, H.; Liu, J.; Xie, M.; Xu, Y.; Liu, X. Quercetin induces autophagy via FOXO1-dependent pathways and autophagy suppression enhances quercetin-induced apoptosis in PASMCs in hypoxia. Free. Radic. Biol. Med. 2017, 103, 165–176. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cao, X.; Liu, X.; Li, X.; Xu, Y.; Liu, J.; Shi, J. Quercetin reverses experimental pulmonary arterial hypertension by modulating the TrkA pathway. Exp. Cell Res. 2015, 339, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.-J.; Aikeremu, N.; Cao, N.; Chen, C.; Ma, K.-T.; Li, L.; Zhang, A.-M.; Si, J.-Q. Quercetin regulates pulmonary vascular remodeling in pulmonary hypertension by downregulating TGF-β1-Smad2/3 pathway. BMC Cardiovasc. Disord. 2024, 24, 535. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Basavaraj, A.; Reichner, C.; Shlobin, O.A.; Ahmad, S.; Kiernan, J.; Burton, N.; Barnett, S.D. Prevalence and impact of coronary artery disease in idiopathic pulmonary fibrosis. Respir. Med. 2010, 104, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Kizer, J.R.; Zisman, D.A.; Blumenthal, N.P.; Kotloff, R.M.; Kimmel, S.E.; Strieter, R.M.; Arcasoy, S.M.; Ferrari, V.A.; Hansen-Flaschen, J. Association between pulmonary fibrosis and coronary artery disease. Arch. Intern. Med. 2004, 164, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid intake and coronary mortality in Finland: A cohort study. BMJ 1996, 312, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Chekalina, N.; Burmak, Y.; Petrov, Y.; Borisova, Z.; Manusha, Y.; Kazakov, Y.; Kaidashev, I. Quercetin reduces the transcriptional activity of NF-kB in stable coronary artery disease. Indian Heart J. 2018, 70, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Hoeth, M.; Hofer-Warbinek, R.; Schmid, J.A. The Transcription Factor NF-κB and the Regulation of Vascular Cell Function. Arterioscler. Thromb. Vasc. Biol. 2000, 20, e83–e88. [Google Scholar] [CrossRef]
- Song, J.; Li, S.; Zhang, B.; Wu, J.; Zhong, A. Quercetin protects human coronary artery endothelial cells against hypoxia/reoxygenation-induced mitochondrial apoptosis via the Nrf2/HO-1 axis. Biomed. Res. 2024, 45, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Liu, L. Hyperoside prevents sepsis-associated cardiac dysfunction through regulating cardiomyocyte viability and inflammation via inhibiting miR-21. Biomed. Pharmacother. 2021, 138, 111524. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yan, M.; Tan, L.; Zhao, X.; Liu, G.; Zhang, Z.; Zhang, J.; Gao, H.; Qin, W. Non-coding RNAs: Targets for Chinese herbal medicine in treating myocardial fibrosis. Front. Pharmacol. 2024, 15, 1337623. [Google Scholar] [CrossRef] [PubMed]
- Garelnabi, M.; Mahini, H. Modulation of microRNA 21, 125 b and 451 expression by quercetin intake and exercise in mice fed atherogenic diet. Biomed. Prev. Nutr. 2014, 4, 359–363. [Google Scholar] [CrossRef]
- Tu, Y.; Wan, L.; Fan, Y.; Wang, K.; Bu, L.; Huang, T.; Cheng, Z.; Shen, B. Ischemic Postconditioning-Mediated miRNA-21 Protects against Cardiac ischemia/reperfusion Injury via PTEN/Akt Pathway. PLoS ONE 2013, 8, e75872. [Google Scholar] [CrossRef] [PubMed]
- Bandres, E.; Bitarte, N.; Arias, F.; Agorreta, J.; Fortes, P.; Agirre, X.; Zarate, R.; Diaz-Gonzalez, J.A.; Ramirez, N.; Sola, J.J.; et al. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin. Cancer Res. 2009, 15, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-h.; Li, H.; Li, J.-p.; Zhong, H.; Zhang, H.-c.; Chen, J.; Xiao, T. miR-125b suppresses the proliferation and migration of osteosarcoma cells through down-regulation of STAT3. Biochem. Biophys. Res. Commun. 2011, 416, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, Y.; Tong, X.; Zhang, Y.; Fan, H. Diabetes Mellitus Contributes to Idiopathic Pulmonary Fibrosis: A Review From Clinical Appearance to Possible Pathogenesis. Front. Public Health 2020, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, C.; Hilberg, O.; Bendstrup, E. How does comorbidity influence survival in idiopathic pulmonary fibrosis? Respir. Med. 2014, 108, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhang, L.; Pan, T.; Wang, W.; Wang, D.; Turner, C.; Zhou, X.; He, H. Idiopathic pulmonary fibrosis and diabetes mellitus: A meta-analysis and systematic review. Respir Res 2021, 22, 175. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiao, Y.; Hu, J.; Hu, Z.; Yan, J.; Zhou, Z.; Mei, Z. Associations Between Diabetes and Idiopathic Pulmonary Fibrosis: A Study-level Pooled Analysis of 26 Million People. J. Clin. Endocrinol. Metab. 2021, 106, 3367–3380. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Meerza, D.; Naseem, I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Kang, M.J.; Choi, H.N.; Kim, J.H.; Kim, J.I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012, 6, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Mifune, Y.; Inui, A.; Nishimoto, H.; Yamaura, K.; Mukohara, S.; Shinohara, I.; Kuroda, R. Quercetin treatment protects the Achilles tendons of rats from oxidative stress induced by hyperglycemia. BMC Musculoskelet. Disord. 2022, 23, 563. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.C.; Martinez, K.; Xie, G.; Kennedy, A.; Bumrungpert, A.; Overman, A.; Jia, W.; McIntosh, M.K. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-α-mediated inflammation and insulin resistance in primary human adipocytes. Am. J. Clin. Nutr. 2010, 92, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.Q.; Wang, Y.; Hong, S.H.; Xiao, L.X. Anti-diabetic effect of quercetin in type 2 diabetes mellitus by regulating the microRNA-92b-3p/EGR1 axis. J. Physiol. Pharmacol. 2023, 74. [Google Scholar] [CrossRef]
- Dini, S.; Zakeri, M.; Ebrahimpour, S.; Dehghanian, F.; Esmaeili, A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles modulate glucose metabolism-related genes and miR-29 family in the hippocampus of diabetic rats. Sci. Rep. 2021, 11, 8618. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Wang, Y.; Pan, Q.; Chen, X.; Chen, S.; Li, X.; Yao, W. Quercetin attenuates the proliferation, inflammation, and oxidative stress of high glucose-induced human mesangial cells by regulating the miR-485-5p/YAP1 pathway. Int. J. Immunopathol. Pharmacol. 2022, 36, 20587384211066440. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Fiore, J.F., Jr.; Bell, E.C.; Goh, N.; Westall, G.; Symons, K.; Dowman, L.; Glaspole, I. Dyspnoea and comorbidity contribute to anxiety and depression in interstitial lung disease. Respirology 2014, 19, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jiaqi, J.; Zongmin, P.; Zeli, L.; Siyu, F.; Xiaoqin, L.; Yan, L.; Haiying, Y.; and Guo, L. Anxiety and depression status in patients with idiopathic pulmonary fibrosis and outcomes of nintedanib treatment: An observational study. Ann. Med. 2024, 56, 2323097. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Choi, S.M.; Lee, Y.J.; Cho, Y.-J.; Yoon, H.I.; Lee, J.-H.; Lee, C.-T.; Park, J.S. Clinical impact of depression and anxiety in patients with idiopathic pulmonary fibrosis. PLoS ONE 2017, 12, e0184300. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, H.J.; Kim, S.; Kim, Y.-J.; Kim, H.C. Epidemiology and comorbidities in idiopathic pulmonary fibrosis: A nationwide cohort study. BMC Pulm. Med. 2023, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Ubgade, A.; Quazi, M.; Umathe, S.; Mundhada, D. Reversal by quercetin of corticotrophin releasing factor induced anxiety- and depression-like effect in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Sun, J.; Chen, C.; Zhang, Y.; Zou, L.; Zhang, Z.; Chen, M.; Wu, H.; Tian, W.; Liu, Y.; et al. Quercetin Mitigates Methamphetamine-Induced Anxiety-Like Behavior Through Ameliorating Mitochondrial Dysfunction and Neuroinflammation. Front. Mol. Neurosci. 2022, 15, 829886. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Donaldson, J.; Tomaszewska, E.; Baranowska-Wójcik, E. Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols—Polyphenols as an Element of Diet Therapy in Depressive Disorders. Int. J. Mol. Sci. 2023, 24, 2258. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ouyang, T.; Xu, A.; Bian, Q.; Zhu, B.; Zhao, M. Quercetin Improves Hippocampal Neurogenesis in Depression by Regulating the Level of Let-7e-5p in Microglia Exosomes. Drug Des. Dev. Ther. 2025, 19, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Yu, W.; Cheng, Y.; Lin, Q.; Jiang, Y.; Wang, D.; Che, L.; Du, M.; Wang, S.; Zhen, H. Quercetin can improve anesthesia induced neuroinflammation and cognitive dysfunction by regulating miR-138-5p/ LCN2. BMC Anesthesiol. 2025, 25, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yi, P.; Yi, M.; Tong, X.; Cheng, X.; Yang, J.; Hu, Y.; Peng, W. Protective Effect of Quercetin against H(2)O(2)-Induced Oxidative Damage in PC-12 Cells: Comprehensive Analysis of a lncRNA-Associated ceRNA Network. Oxidative Med. Cell. Longev. 2020, 2020, 6038919. [Google Scholar] [CrossRef] [PubMed]
- Tomou, E.M.; Papakyriakopoulou, P.; Saitani, E.M.; Valsami, G.; Pippa, N.; Skaltsa, H. Recent Advances in Nanoformulations for Quercetin Delivery. Pharmaceutics 2023, 15, 1656. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Silva, M.; Faria-Silva, C.; Carvalheiro, M.C.; Simões, S.; Marinho, H.S.; Marcelino, P.; Campos, M.C.; Metselaar, J.M.; Fernandes, E.; Baptista, P.V.; et al. Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment. Pharmaceutics 2022, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Wangsawangrung, N.; Choipang, C.; Chaiarwut, S.; Ekabutr, P.; Suwantong, O.; Chuysinuan, P.; Techasakul, S.; Supaphol, P. Quercetin/Hydroxypropyl-β-Cyclodextrin Inclusion Complex-Loaded Hydrogels for Accelerated Wound Healing. Gels 2022, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, M.; Fu, J.; Ao, H.; Wang, W.; Wang, X. Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions. Drug Deliv. 2021, 28, 1226–1236. [Google Scholar] [CrossRef] [PubMed]


| Model/Study | Molecules or Pathways Regulated | Observed Effect | Therapeutic Category | Type of Evidence | Reference |
|---|---|---|---|---|---|
| Murine model of COPD exposed to rhinovirus + quercetin | ↓ inflammation, ↓ goblet cell metaplasia, ↓ cholinergic response | ↓ airway inflammation and dysfunction | Anti-inflammatory | In vivo | [45] |
| Murine model of COPD induced by cigarette smoke + quercetin | ↓ cellular infiltration, ↓ IL-10, IL-13 e IL-22, ↑ SOD, CAT | ↓ lung inflammation and tissue damage, ↓ oxidative stress, ↓ emphysema | Anti-inflammatory, Antioxidant Immunomodulator | In vivo | [46] |
| Human monocytic U937 cells + cigarette smoke extract + quercetin Human PBMCs from COPD patients + quercetin | ↑ AMPK/Nrf2, ↓ corticosteroid resistance | ↑ steroid sensitivity, ↓ oxidative stress | Antioxidant, Corticosteroid Sensitizer | In vitro | [47] |
| BEAS-2B cells + cigarette smoke extract + Jinwei decoction (quercetin, luteolin, stigmasterol) | ↓ miR-155-5p, ↓ PI3K-Akt, ↑ HDAC2 | ↓ inflammation, ↑ glucocorticoid response | Anti-inflammatory, Epigenetic Modulator | In vitro | [48] |
| LPS-activated macrophages + quercetin | ↓ miR-155, ↑ Nrf2/HO-1 | ↓ NF-κB, iNOS, TNF-α, IL1β, and IL-6 | Anti-inflammatory, Antioxidant | In vitro | [50] |
| Model/Study | Molecules or Pathways Regulated | Observed Effect | Therapeutic Category | Type of Evidence | Reference |
|---|---|---|---|---|---|
| GLC-82 and HTB-182 cell lines + quercetin treatment | ↑ miR-16-5p, ↓ WEE1 | ↑ radiosensitivity | Radiosensitizer | In vitro | [52] |
| A549 + quercetin treatment | ↑ miR-16, ↓ claudin-2 | ↓ proliferation, ↓ migration ↓ invasión | Antitumor, Antimetastatic | In vitro | [55] |
| Lung cancer tissue after quercetin-rich diet | ↑ miR-let-7, miR-146, miR-26, miR-17, miR-125a, miR-503, miR-16 | ↓ proliferation, ↑ apoptosis | Chemopreventive, Epigenetic modulator | In vivo | [51] |
| Model/Study | Molecules or Pathways Regulated | Observed Effect | Therapeutic Category | Type of Evidence | Reference |
|---|---|---|---|---|---|
| MCT-induced PH rats + quercetin | ↓ lncRNA H19, ↓ MIAT, ↓ miR-29a/33a | ↓ vascular smooth muscle cell proliferation and fibrosis | Antiproliferative, Antifibrotic, Epigenetic Modulator | In vivo | [60] |
| MCT-induced PH rats + quercetin | ↑ miR-204, ↓ PARP1, ↓ HIF1α, ↓ NFATc2, ↓ α-SMA | ↓ pulmonary artery pressure, ↓ vascular remodeling, and inflammation | Anti-inflammatory, Antiproliferative, Epigenetic Modulator | In vivo | [59] |
| Hypoxia-induced PASMCs + quercetin | ↑ LC3-II, Beclin-1, Atg5, SESN3, FOXO1; ↓ p-mTOR, ↓ 4E-BP1, ↓ p70S6K | ↑ autophagy, ↑ apoptosis; inhibition of mTOR pathway; FOXO1-dependent mechanism | Pro-autophagic, Pro-apoptotic | In vitro | [61] |
| Hypoxia-induced PH in rats + quercetin | ↑ FOXO1, ↑ LC3-II, ↑ apoptosis; ↓ RVSP, ↓ vascular remodeling | ↓ pulmonary artery pressure and wall thickness; ↑ lung cell apoptosis | Antiproliferative, Pro-Apoptotic | In vivo | [61] |
| Hypoxia-induced PH in rats + quercetin | ↓ RVSP, ↓ RV/LV+S, ↓ vascular remodeling, ↓ PCNA/Ki67, ↑ apoptosis, ↓ p-TrkA, ↓ p-AKT | ↓ proliferation and remodeling, ↑ apoptosis in PASMCs | Antiproliferative, Pro-Apoptotic | In vivo | [62] |
| Hypoxia-induced PASMCs + quercetin | ↓ MMP2/9, ↓ CXCR4, ↓ integrins α5/β1, ↓ p-TrkA, ↓ p-AKT, ↑ Bax/Bcl-2 | ↓ proliferation and migration, ↑ apoptosis | Antiproliferative, Pro-Apoptotic | In vitro | [62] |
| HPASMCs + PDGF-BB + quercetin | ↓ PCNA, ↓ OPN, ↓ TGF-β1, ↓ p-Smad2/3, ↑ α-SMA | ↓ cell proliferation and migration; ↑ apoptosis; ↓ phenotypic switching | Antiproliferative, Antifibrotic | In vitro | [63] |
| MCT-induced PH rats + quercetin | ↓ PCNA, ↓ OPN, ↓ TGF-β1, ↓ p-Smad2/3, ↑ α-SMA | ↓ pulmonary artery pressure and wall thickness (mPAP, WT%, WA%), ↓ RV hypertrophy index, ↓ vascular remodeling | Antiproliferative, Antifibrotic | In vivo | [63] |
| Model/Study | Molecules or Pathways Regulated | Observed Effect | Therapeutic Category | Type of Evidence | Reference |
|---|---|---|---|---|---|
| Stable CAD patients + quercetin | ↓ IL-1β, ↓ TNF-α, ↓ IκBα ↓ NF-κB | ↓ inflammatory signaling | Anti-inflammatory | Clinical | [67] |
| CAECs + H/R + quercetin | ↓ ROS, ↑ SOD, ↑ CAT, ↑ Nrf2/HO-1, ↓ caspase-3 | ↓ oxidative damage, ↓ apoptosis, ↑ antioxidant | Antioxidant, Antiapoptotic | In vitro | [69] |
| Mice (LDL−/− + atherogenic diet) + quercetin + exercise | ↑ miR-21, ↑ miR-125b | Modulation of miRNAs associated with vascular health | Epigenetic Modulator, Cardioprotective | In vivo | [72] |
| Sepsis-induced cardiac dysfunction + hyperoside | ↓ miR-21, ↓ IL-6, ↓ TNF-α, ↓ cTnI, ↓ CK-MB | ↓ inflammation, improved cardiac function | Anti-inflammatory, Epigenetic Modulator | In vivo | [70] |
| H9C2 cell line + LPS + hyperoside | ↓ miR-21, ↓ IL-6, ↓ TNF-α ↑ cell viability | ↓ inflammation | Anti-inflammatory, Epigenetic Modulator | In vitro | [70] |
| Model/Study | Molecules or Pathways Regulated | Observed Effect | Therapeutic Category | Type of Evidence | Reference |
|---|---|---|---|---|---|
| C57BL/KsJ-db/db mice with quercetin-enriched diet | ↓ plasma glucose, ↓ HOMA-IR, ↑ adiponectin, ↓ TG, ↓ CT, ↑ HDL, ↓ TBARS, ↑ SOD/CAT/GSH-Px | ↓ hyperglycemia, ↓ dyslipidemia, ↓ oxidative stress, ↑ insulin sensitivity | Hypoglycemic, Antioxidant, Insulin Sensitizer | In vivo | [81] |
| Model of DM induced by aloxane + quercetin | ↓ fasting blood glucose, ↑ GLUT4, ↑ GSH, ↑ SOD, ↑ CAT, ↓ TBARS, ↓ DNA damage | ↓ hyperglycemia, ↓ oxidative stress, ↓ cytotoxicity | Hypoglycemic, Antioxidant | In vivo | [80] |
| L6 skeletal muscle cells, H4IIE and HepG2 cell lines | ↑ AMPK, ↑ GLUT4 translocation, ↓ G6Pase. | ↑ muscle glucose uptake, ↓ hepatic gluconeogenesis | Hypoglycemic, Insulin Sensitizer | In vitro | [82,83] |
| Achilles tendon cells from diabetic rats + quercetin | ↓ Nox1, ↓ Nox4, ↓ Il6 | ↓ ROS, ↓ inflammation, ↓ cell apoptosis. | Antioxidant, Anti-inflammatory | In vitro | [84] |
| Primary human adipocytes treated with TNF-α + quercetin | ↓ IL-6, ↓ IL-1β, ↓ IL-1β, ↓ IL-8, ↓ MCP-1, ↓ ERK/JNK/NF-κB, ↓ PTP-1B, ↓ p-IRS-1(Ser), ↑ PPARγ | ↓ inflammation, ↓ insulin resistance, ↑ glucose uptake | Anti-inflammatory, Insulin Sensitizer | In vitro | [85] |
| DM model induced by high-fat/-glucose diet and STZ + quercetin | ↑ miR-92b-3p, ↓ EGR1 | ↓ inflammation, ↓ insulin resistance, ↓ pancreatic damage | Epigenetic Modulator, Anti-inflammatory | In vivo | [86] |
| Model of DM induced by STZ + quercetin | ↓ miR-29a/b/c, ↑ GLUT1-4, ↑ IGF-1. | ↑ cerebral glucose metabolism, ↓ neurological alterations | Epigenetic Modulator, Neuroprotector | In vivo | [87] |
| HMCs + HG + quercetin | ↑ miR-485-5p, ↓YAP1, ↓ YAP1, ↓ TNF-α, ↓ IL-1β, ↓ IL-6, ↓ MDA, ↑ SOD, ↑ GSH-Px | ↓ proliferation, ↓ inflammation, ↓ oxidative stress, ↑ antioxidant function | Epigenetic Modulator, Antioxidant, Anti-inflammatory | In vitro | [88] |
| Model/Study | Molecules or Pathways Regulated | Observed Effect | Therapeutic Category | Type of Evidence | Reference |
|---|---|---|---|---|---|
| CRF-induced stress in mice + quercetin | Modulation of HPA axis | ↓ anxiety and depression-like behavior (comparable to fluoxetine/diazepam) | Anxiolytic, Antidepressant | In vivo | [93] |
| MA-induced anxiety in mice + quercetin | ↓ ROS, ↑ mitochondrial potential, ↑ ATP, ↓ IL-1β, ↓ TNF-α, ↓ astrocyte activation | ↓ anxiety-like behavior, ↓ neuroinflammation, ↑ mitochondrial function | Antioxidant, Neuroprotective, Anti-inflammatory | In vivo | [94] |
| CUMS-induced depression in mice + quercetin | ↑ neurogenesis (↑ Wnt1/β-catenin), ↓ let-7e-5p from microglial exosomes | ↓ depressive behavior, ↑ hippocampal neurogenesis | Antidepressant, Neurogenic, Epigenetic Modulator | In vivo | [96] |
| ISO-induced cognitive impairment in rats + quercetin | ↑ miR-138-5p, ↓ LCN2, ↓ TNF-α, ↓ IL-1β, ↓ IL-6 | ↓ cognitive impairment, ↓ neuroinflammation | Neuroprotective, Epigenetic Modulator | In vivo | [97] |
| PC12 cells + H2O2 + quercetin | 297 lncRNAs, 194 miRNAs, and 14 mRNAs dysregulated | ↓ oxidative damage, regulation of ceRNA network | Antioxidant, Epigenetic Modulator | In vitro | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez-Garzón, V.R.; Velázquez-Enríquez, J.M.; Santos-Álvarez, J.C.; Ramírez-Hernández, A.A.; Arellanes-Robledo, J.; Jiménez-Martínez, C.; Baltiérrez-Hoyos, R. Quercetin in Idiopathic Pulmonary Fibrosis and Its Comorbidities: Gene Regulatory Mechanisms and Therapeutic Implications. Genes 2025, 16, 856. https://doi.org/10.3390/genes16080856
Vásquez-Garzón VR, Velázquez-Enríquez JM, Santos-Álvarez JC, Ramírez-Hernández AA, Arellanes-Robledo J, Jiménez-Martínez C, Baltiérrez-Hoyos R. Quercetin in Idiopathic Pulmonary Fibrosis and Its Comorbidities: Gene Regulatory Mechanisms and Therapeutic Implications. Genes. 2025; 16(8):856. https://doi.org/10.3390/genes16080856
Chicago/Turabian StyleVásquez-Garzón, Verónica Rocío, Juan Manuel Velázquez-Enríquez, Jovito Cesar Santos-Álvarez, Alma Aurora Ramírez-Hernández, Jaime Arellanes-Robledo, Cristian Jiménez-Martínez, and Rafael Baltiérrez-Hoyos. 2025. "Quercetin in Idiopathic Pulmonary Fibrosis and Its Comorbidities: Gene Regulatory Mechanisms and Therapeutic Implications" Genes 16, no. 8: 856. https://doi.org/10.3390/genes16080856
APA StyleVásquez-Garzón, V. R., Velázquez-Enríquez, J. M., Santos-Álvarez, J. C., Ramírez-Hernández, A. A., Arellanes-Robledo, J., Jiménez-Martínez, C., & Baltiérrez-Hoyos, R. (2025). Quercetin in Idiopathic Pulmonary Fibrosis and Its Comorbidities: Gene Regulatory Mechanisms and Therapeutic Implications. Genes, 16(8), 856. https://doi.org/10.3390/genes16080856






