Bta-miR-106b Regulates Bovine Mammary Epithelial Cell Proliferation, Cell Cycle, and Milk Protein Synthesis by Targeting the CDKN1A Gene
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Gene Prediction and Luciferase Assays
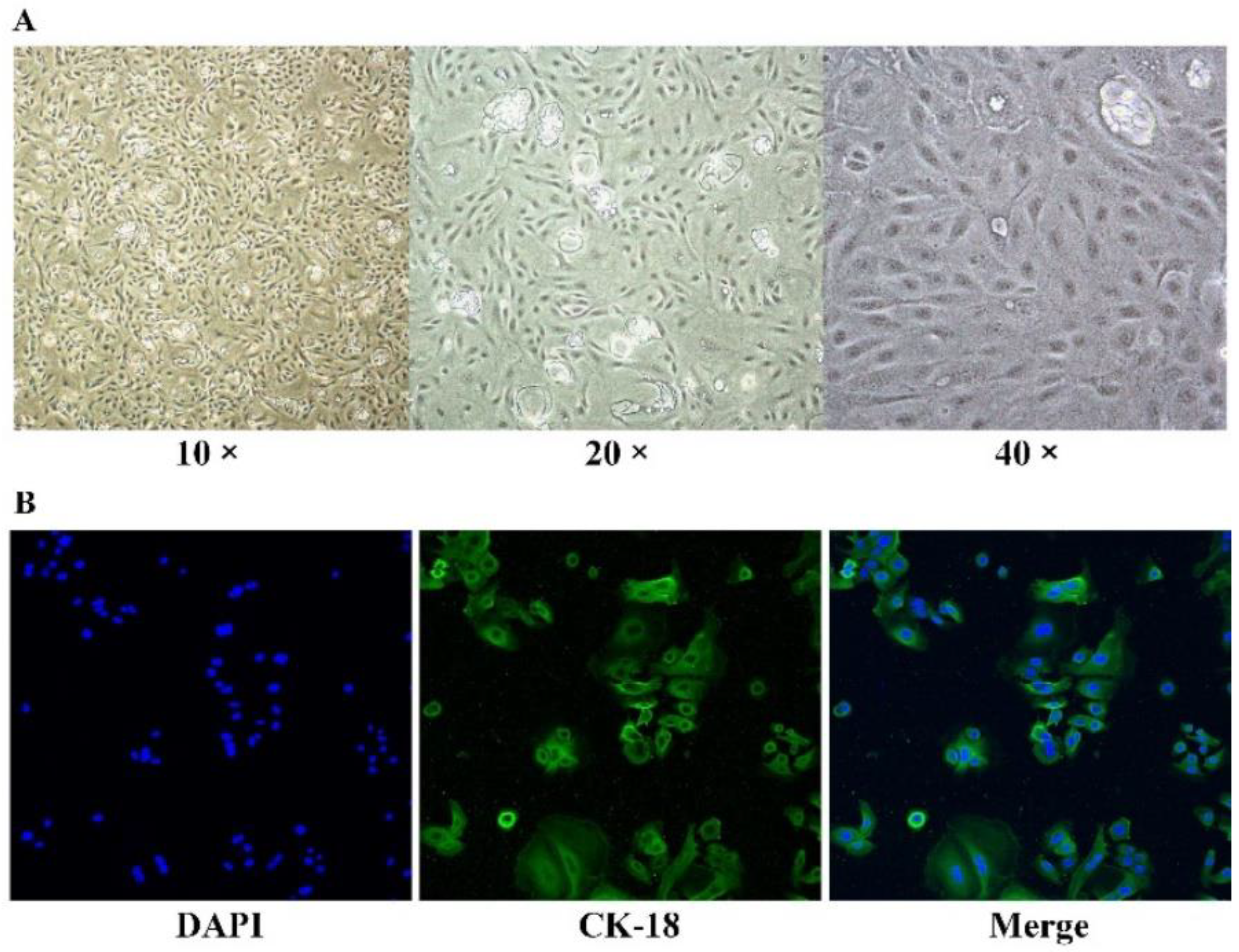
2.2. BMECs Isolation and Culture
2.3. Transfections of Bta-miR-106b to BMECs
2.4. Cell Proliferation Assay and Cell Cycle Analysis of BMECs
2.5. Over-Expression and RNA Interference (RNAi) Analysis of CDKN1A Gene in BMECs
2.6. Quantitative RT-PCR
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
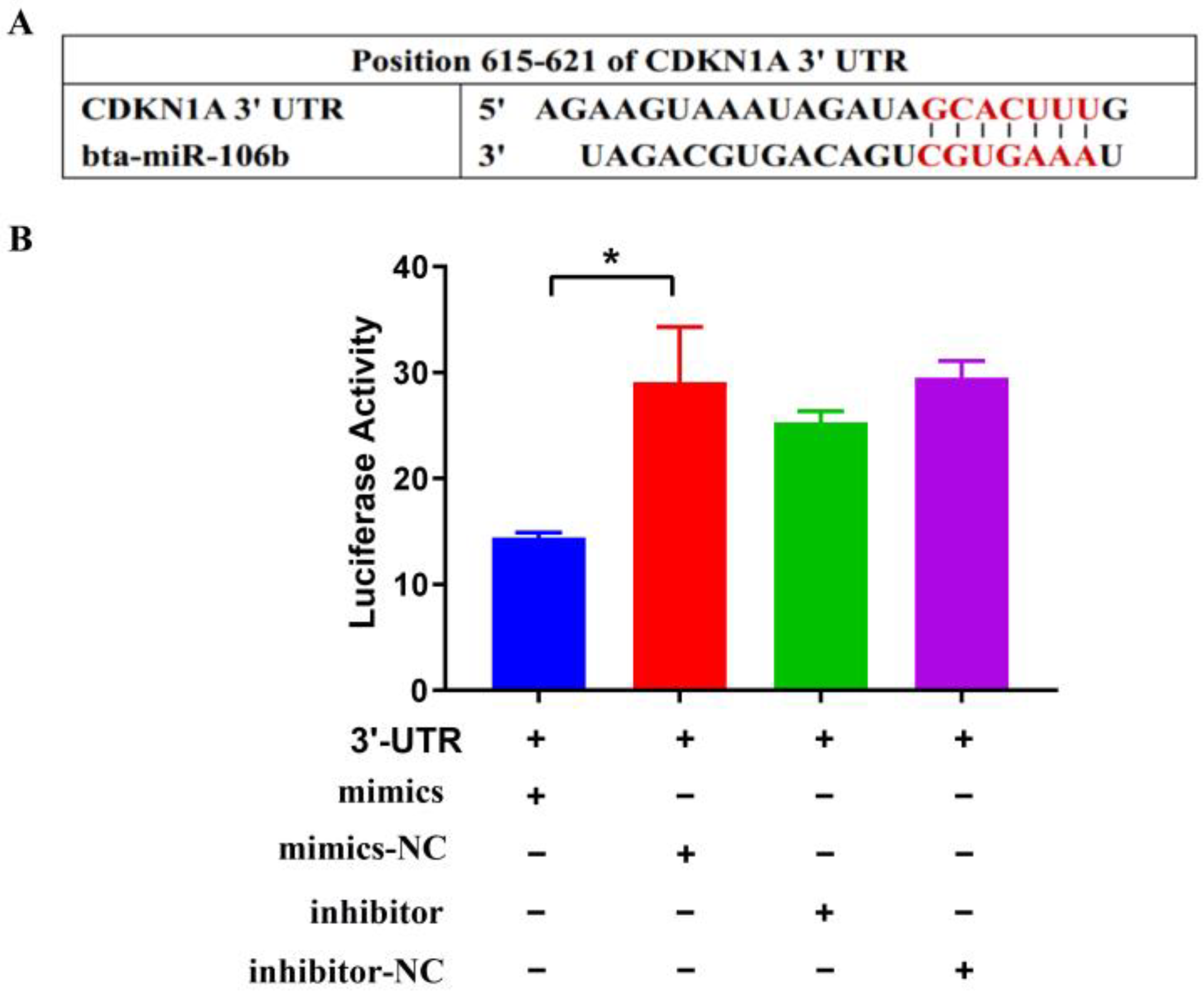
3.1. Bta-miR-106b Targeted the 3′UTR of the CDKN1A Gene
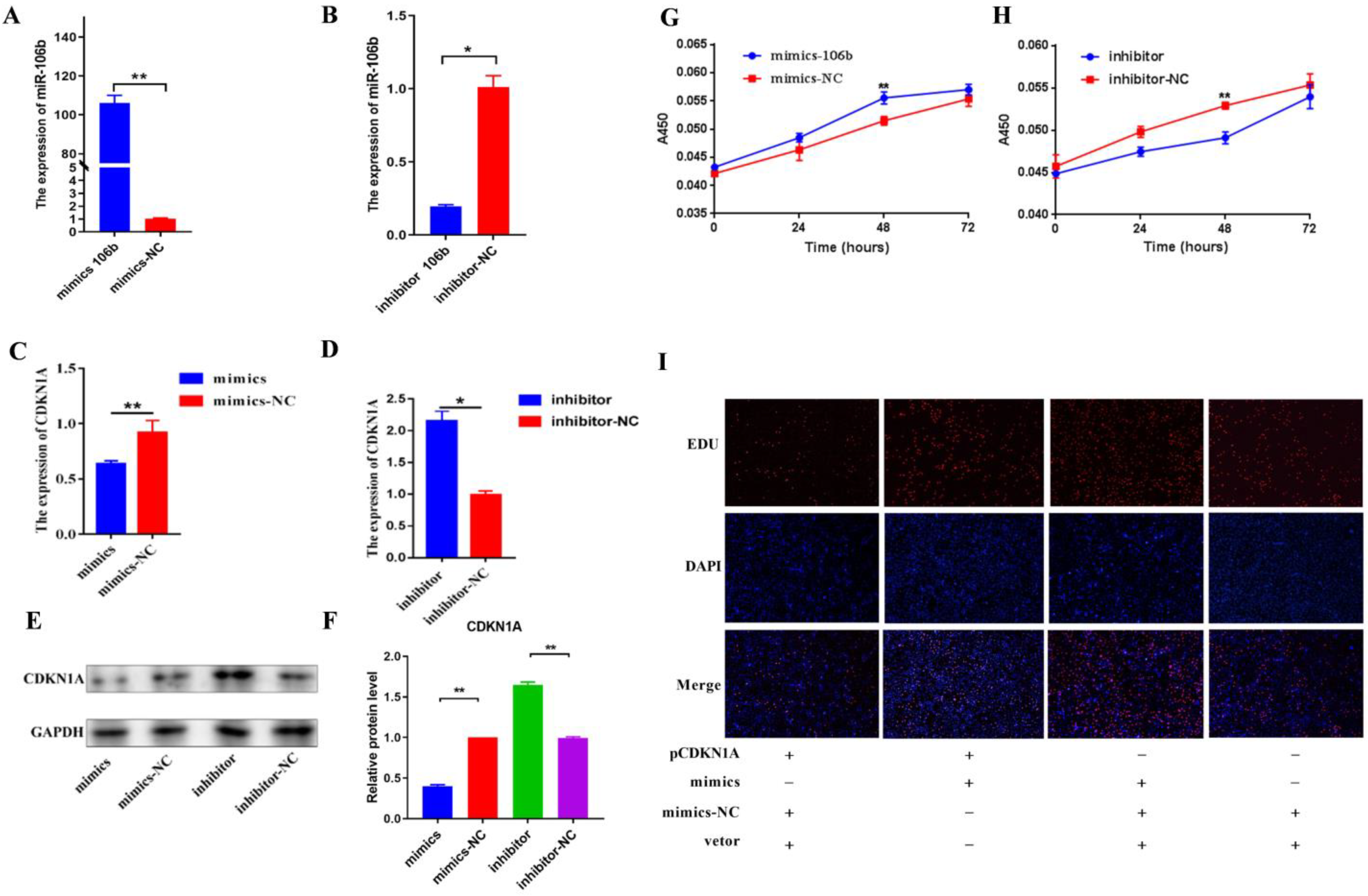
3.2. Regulation of Bta-miR-106b on CDKN1A Expression in BMECs
3.3. Bta-miR-106b Promoted the Proliferation and Cell Cycle of BMECs
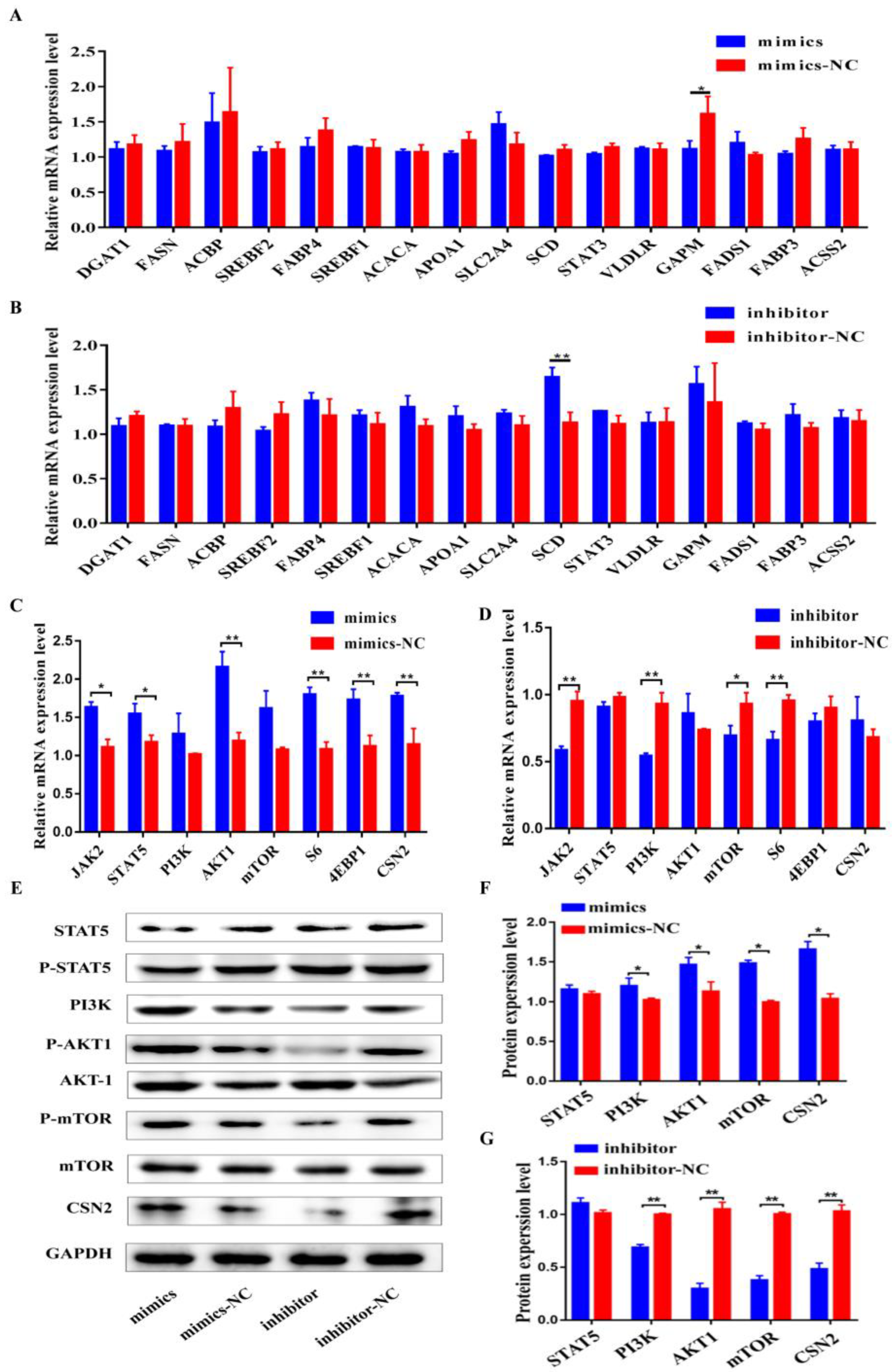
3.4. Bta-miR-106b Regulated Milk Protein Synthesis through the PI3K/AKT/mTOR Pathways
3.5. CDKN1A Regulated Cell Cycle and Milk Protein Synthesis through PI3K/AKT/mTOR Pathways of BMECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Jeurink, P.V.; van Bergenhenegouwen, J.; Jimenez, E.; Knippels, L.M.J.; Fernandez, L.; Garssen, J.; Knol, J.; Rodriguez, J.M.; Martin, R. Human milk: A source of more life than we imagine. Benef. Microbes 2013, 4, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.Q.; Li, H.H.; Liu, X.; Yan, Z.G.; Zhao, M.; Xu, Z.J.; Wang, Z.H.; Shi, K.R. MiR-24-3p regulates cell proliferation and milk protein synthesis of mammary epithelial cells through menin in dairy cows. J. Cell. Physiol. 2019, 234, 1522–1533. [Google Scholar]
- Wang, M.; Wang, Z.; Yang, C.; Liu, L.; Jiang, N. Protein 14-3-3epsilon Regulates Cell Proliferation and Casein Synthesis via PI3K-mTOR Pathway in Dairy Cow Mammary Epithelial Cells. J. Agric. Food Chem. 2018, 66, 12000–12008. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, X.; Jin, L.; Yu, G.; Li, Q.; Gao, X.; Ao, J.; Wang, C. MiR-139 suppresses β-casein synthesis and proliferation in bovine mammary epithelial cells by targeting the GHR and IGF1R signaling pathways. BMC Vet. Res. 2017, 13, 350. [Google Scholar] [CrossRef]
- Xu, L.; Shi, L.; Liu, L.; Liang, R.; Li, Q.; Li, J.; Han, B.; Sun, D. Analysis of Liver Proteome and Identification of Critical Proteins Affecting Milk Fat, Protein, and Lactose Metabolism in Dariy Cattle with iTRAQ. Proteomics 2019, 19, e1800387. [Google Scholar] [CrossRef]
- Liang, R.; Han, B.; Li, Q.; Yuan, Y.; Li, J.; Sun, D. Using RNA sequencing to identify putative competing endogenous RNAs (ceRNAs) potentially regulating fat metabolism in bovine liver. Sci. Rep. 2017, 7, 6396. [Google Scholar] [CrossRef]
- Li, Q.; Liang, R.; Li, Y.; Gao, Y.; Li, Q.; Sun, D.; Li, J. Identification of candidate genes for milk production traits by RNA sequencing on bovine liver at different lactation stages. BMC Genet. 2020, 21, 72. [Google Scholar] [CrossRef]
- Amalfitano, N.; Macedo Mota, L.F.; Rosa, G.M.; Cecchinato, A.; Bittante, G. Role of CSN2, CSN3, and BLG genes and the polygenic background in the cattle milk protein profile. J. Dairy Sci. 2022, 105, 6001–6020. [Google Scholar] [CrossRef]
- Wang, M.; Bissonnette, N.; Dudemaine, P.L.; Zhao, X.; Ibeagha-Awemu, E.M. Whole Genome DNA Methylation Variations in Mammary Gland Tissues from Holstein Cattle Producing Milk with Various Fat and Protein Contents. Genes 2021, 12, 1727. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Martin, E.C.; Qureshi, A.T.; Dasa, V.; Freitas, M.A.; Gimble, J.M.; Davis, T.A. MicroRNA regulation of stem cell differentiation and diseases of the bone and adipose tissue: Perspectives on miRNA biogenesis and cellular transcriptome. Biochimie 2016, 124, 98–111. [Google Scholar] [CrossRef]
- Gu, Z.; Eleswarapu, S.; Jiang, H. Identification and characterization of microRNAs from the bovine adipose tissue and mammary gland. FEBS Lett. 2007, 581, 981–988. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q. Identification of differentially expressed microRNAs during the development of Chinese murine mammary gland. J. Genet. Genom. 2007, 34, 966–973. [Google Scholar] [CrossRef]
- Wang, M.; Moisa, S.; Khan, M.J.; Wang, J.; Bu, D.; Loor, J.J. MicroRNA expression patterns in the bovine mammary gland are affected by stage of lactation. J. Dairy Sci. 2012, 95, 6529–6535. [Google Scholar] [CrossRef]
- Wicik, Z.; Gajewska, M.; Majewska, A.; Walkiewicz, D.; Osinska, E.; Motyl, T. Characterization of microRNA profile in mammary tissue of dairy and beef breed heifers. J. Anim. Breed. Genet. 2016, 133, 31–42. [Google Scholar] [CrossRef]
- Li, H.M.; Wang, C.M.; Li, Q.Z.; Gao, X.J. MiR-15a decreases bovine mammary epithelial cell viability and lactation and regulates growth hormone receptor expression. Molecules 2012, 17, 12037–12048. [Google Scholar] [CrossRef]
- Lian, S.; Guo, J.R.; Nan, X.M.; Ma, L.; Loor, J.J.; Bu, D.P. MicroRNA Bta-miR-181a regulates the biosynthesis of bovine milk fat by targeting ACSL1. J. Dairy Sci. 2016, 99, 3916–3924. [Google Scholar] [CrossRef]
- Wang, J.; Song, C.; Cao, X.; Li, H.; Cai, H.; Ma, Y.; Huang, Y.; Lan, X.; Lei, C.; Ma, Y.; et al. MiR-208b regulates cell cycle and promotes skeletal muscle cell proliferation by targeting CDKN1A. J. Cell. Physiol. 2019, 234, 3720–3729. [Google Scholar] [CrossRef]
- Jiao, B.L.; Zhang, X.L.; Wang, S.H.; Wang, L.X.; Luo, Z.X.; Zhao, H.B.; Khatib, H.; Wang, X. MicroRNA-221 regulates proliferation of bovine mammary gland epithelial cells by targeting the STAT5a and IRS1 genes. J. Dairy Sci. 2019, 102, 426–435. [Google Scholar] [CrossRef]
- Appuhamy, J.A.; Nayananjalie, W.A.; England, E.M.; Gerrard, D.E.; Akers, R.M.; Hanigan, M.D. Effects of AMP-activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. J. Dairy Sci. 2014, 97, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Burgos, S.A.; Dai, M.; Cant, J.P. Nutrient availability and lactogenic hormones regulate mammary protein synthesis through the mammalian target of rapamycin signaling pathway. J. Dairy Sci. 2010, 93, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, C.; Farberman, A.; Rideout, T.C.; de Lange, C.F.; France, J.; Fan, M.Z. The mammalian target of rapamycin-signaling pathway in regulating metabolism and growth. J. Anim. Sci. 2008, 86, E36–E50. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.A.; Nones, K.; Roy, N.C.; McNabb, W.C.; Mackenzie, D.S.; Pacheco, D.; McCoard, S. Initiation and elongation steps of mRNA translation are involved in the increase in milk protein yield caused by growth hormone administration during lactation. J. Dairy Sci. 2009, 92, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Toerien, C.A.; Trout, D.R.; Cant, J.P. Nutritional Stimulation of Milk Protein Yield of Cows Is Associated with Changes in Phosphorylation of Mammary Eukaryotic Initiation Factor 2 and Ribosomal S6 Kinase 1. J. Nutr. 2010, 140, 285–292. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, S.; Zhang, M.; Gao, Y.; Wang, J.; Hanigan, M.D.; Zheng, N. SESN2 negatively regulates cell proliferation and casein synthesis by inhibition the amino acid-mediated mTORC1 pathway in cow mammary epithelial cells. Sci. Rep. 2018, 8, 3912. [Google Scholar] [CrossRef]
- Gao, H.N.; Hu, H.; Zheng, N.; Wang, J.Q. Leucine and histidine independently regulate milk protein synthesis in bovine mammary epithelial cells via mTOR signaling pathway. J. Zhejiang Univ. Sci. B 2015, 16, 560–572. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, F.; Si, Y.; Huang, Y.; Yu, C.; Luo, C.; Zhang, N.; Li, Q.; Gao, X. GSK3beta regulates milk synthesis in and proliferation of dairy cow mammary epithelial cells via the mTOR/S6K1 signaling pathway. Molecules 2014, 19, 9435–9452. [Google Scholar] [CrossRef]
- Cui, X.; Hou, Y.; Yang, S.; Xie, Y.; Zhang, S.; Zhang, Y.; Zhang, Q.; Lu, X.; Liu, G.E.; Sun, D. Transcriptional profiling of mammary gland in Holstein cows with extremely different milk protein and fat percentage using RNA sequencing. BMC Genom. 2014, 15, 226. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, S.; Zhang, Q.; Guo, X.; Wu, C.; Yao, M.; Sun, D. Comprehensive MicroRNA Expression Profile of the Mammary Gland in Lactating Dairy Cows With Extremely Different Milk Protein and Fat Percentages. Front. Genet. 2020, 11, 548268. [Google Scholar] [CrossRef]
- Han, B.; Liang, W.; Liu, L.; Li, Y.; Sun, D. Determination of genetic effects of ATF3 and CDKN1A genes on milk yield and compositions in Chinese Holstein population. BMC Genet. 2017, 18, 47. [Google Scholar] [CrossRef]
- Xiong, Y.; Hannon, G.J.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. P21 Is a Universal Inhibitor Of Cyclin Kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef]
- Ivanovska, I.; Ball, A.S.; Diaz, R.L.; Magnus, J.F.; Kibukawa, M.; Schelter, J.M.; Kobayashi, S.V.; Lim, L.; Burchard, J.; Jackson, A.L.; et al. MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote cell cycle progression. Mol. Cell. Biol. 2008, 28, 2167–2174. [Google Scholar] [CrossRef]
- He, C.; Chen, H.; Liu, Y.; Li, X.; Zhang, C.; Qin, Q.; Pang, Q. miR-106b-5p promotes cell proliferation and cell cycle progression by directly targeting CDKN1A in osteosarcoma. Exp. Ther. Med. 2020, 19, 3203–3210. [Google Scholar] [CrossRef]
- Mendell, J.T. miRiad roles for the miR-17-92 cluster in development and disease. Cell 2008, 133, 217–222. [Google Scholar] [CrossRef]
- Zhang, J.X.; Song, W.; Chen, Z.H.; Wei, J.H.; Liao, Y.J.; Lei, J.; Hu, M.; Chen, G.Z.; Liao, B.; Lu, J.; et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: A microRNA expression analysis. Lancet Oncol. 2013, 14, 1295–1306. [Google Scholar] [CrossRef]
- Xia, X.; Lu, H.; Li, C.; Huang, Y.; Wang, Y.; Yang, X.; Zheng, J.C. miR-106b regulates the proliferation and differentiation of neural stem/progenitor cells through Tp53inp1-Tp53-Cdkn1a axis. Stem Cell Res. Ther. 2019, 10, 282. [Google Scholar] [CrossRef]
- Petrocca, F.; Visone, R.; Onelli, M.R.; Shah, M.H.; Nicoloso, M.S.; de Martino, I.; Iliopoulos, D.; Pilozzi, E.; Liu, C.G.; Negrini, M.; et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell 2008, 13, 272–286. [Google Scholar] [CrossRef]
- Gong, C.; Qu, S.; Lv, X.B.; Liu, B.; Tan, W.; Nie, Y.; Su, F.; Liu, Q.; Yao, H.; Song, E. BRMS1L suppresses breast cancer metastasis by inducing epigenetic silence of FZD10. Nat. Commun. 2014, 5, 5406. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Bu, D.; Wei, H.; Zhou, L.; Li, F.; Loor, J.J. In Vitro culture and characterization of a mammary epithelial cell line from Chinese Holstein dairy cow. PLoS ONE 2009, 4, e7636. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, L.; Shen, J.; Wang, C.; Jiang, Z. The antiproliferative effect of sildenafil on pulmonary artery smooth muscle cells is mediated via upregulation of mitogen-activated protein kinase phosphatase-1 and degradation of extracellular signal-regulated kinase 1/2 phosphorylation. Anesth. Analg. 2007, 105, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Mather, I.H.; Keenan, T.W. Origin and secretion of milk lipids. J. Mammary Gland. Biol. Neoplasia 1998, 3, 259–273. [Google Scholar] [CrossRef] [PubMed]
- McManaman, J.L.; Neville, M.C. Mammary physiology and milk secretion. Adv. Drug Deliv. Rev. 2003, 55, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Jia, H.; Tai, Q.; Li, Y.; Chen, D. miR-106b downregulates adenomatous polyposis coli and promotes cell proliferation in human hepatocellular carcinoma. Carcinogenesis 2012, 34, 211–219. [Google Scholar] [CrossRef]
- Wei, K.; Pan, C.; Yao, G.; Liu, B.; Ma, T.; Xia, Y.; Jiang, W.; Chen, L.; Chen, Y. MiR-106b-5p Promotes Proliferation and Inhibits Apoptosis by Regulating BTG3 in Non-Small Cell Lung Cancer. Cell. Physiol. Biochem. 2017, 44, 1545–1558. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Q.; Zhang, B.; Wu, W.; Yuan, F.; Zhu, Z. miR-106b Promotes Metastasis of Early Gastric Cancer by Targeting ALEX1 In Vitro and In Vivo. Cell. Physiol. Biochem. 2019, 52, 606–616. [Google Scholar]
- Hawke, T.J.; Meeson, A.P.; Jiang, N.; Graham, S.; Hutcheson, K.; DiMaio, J.M.; Garry, D.J. p21 is essential for normal myogenic progenitor cell function in regenerating skeletal muscle. Am. J. Physiol.-Cell Physiol. 2003, 285, C1019–C1027. [Google Scholar] [CrossRef]
- Li, C.; Lyu, J.; Meng, Q.H. MiR-93 Promotes Tumorigenesis and Metastasis of Non-Small Cell Lung Cancer Cells by Activating the PI3K/Akt Pathway via Inhibition of LKB1/PTEN/CDKN1A. J. Cancer 2017, 8, 870–879. [Google Scholar] [CrossRef]
- Li, D.; Dai, C.; Yang, X.; Wang, F.; Yu, X.; Xiao, X.; Tang, S. Critical role of p21 on olaquindox-induced mitochondrial apoptosis and S-phase arrest involves activation of PI3K/AKT and inhibition of Nrf2/HO-1pathway. Food Chem. Toxicol. 2017, 108, 148–160. [Google Scholar] [CrossRef]
- Mao, K.; Kobayashi, S.; Jaffer, Z.M.; Huang, Y.; Volden, P.; Chernoff, J.; Liang, Q. Regulation of Akt/PKB activity by P21-activated kinase in cardiomyocytes. J. Mol. Cell. Cardiol. 2008, 44, 429–434. [Google Scholar] [CrossRef]
- Anderson, S.M.; Rudolph, M.C.; McManaman, J.L.; Neville, M.C. Key stages in mammary gland development. Secretory activation in the mammary gland: It’s not just about milk protein synthesis! Breast Cancer Res. 2007, 9, 204. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinform. Biol. Insights 2011, 5, 83–98. [Google Scholar] [CrossRef]


| siRNAs | siRNA Sequences (5′-3′) | Target Gene Sequence (5′-3′) |
|---|---|---|
| siRNA-122 | CCAGGGACGCGCAUCAAAUTT | CCAGGGACGCGCATCAAAT |
| AUUUGAUGCGCGUCCCUGGTT | ||
| siRNA-563 | GCAGACUGAUCUGCUCCAATT | GCAGACTGATCTGCTCCAA |
| UUGGAGCAGAUCAGUCUGCTT | ||
| siRNA-669 | CCUUCAGUUUGUGCGUCUUTT | CCTTCAGTTTGTGCGTCTT |
| AAGACGCACAAACUGAAGGTT |
| Genes | Forward Primers | Reverse Primers |
|---|---|---|
| CSN2 | AGTGAGGAACAGCAGCAAACAG | AGCAGAGGCAGAGGAAGGTG |
| JAK2 | ACAGGGGCTGGCGTTCA | TATTGGTAACCAACAGCTCAAGG |
| STAT5 | GTCCCTTCCCGTGGTTGT | CGGCCTTGAATTTCATGTTG |
| mTOR | ATGCTGTCCCTGGTCCTTATG | GGGTCAGAGAGTGGCCTTCAA |
| S6K1 | CTGGGGAAGAGGTGCTTCAG | GTGCTCTGGTCGTTTGGAGA |
| AKT1 | CCTGCCCTTCTACAACCAGG | GTCTTGGTCAGGTGGCGTAA |
| PI3K | AGCGCTGAGCAGTGTATCTT | GGGTATGGAGCCATCAGACG |
| 4EBP1 | CTGGGGACTACAGCACCAC | AGGTGATTCTGCCTGGCTTC |
| CDKN1A | GAGACCCCCAGAAGAGCCAC | AAAGTCGAAGTTCCACCGCT |
| CDK2 | TTTGCTGAGATGGTGACCCG | TAACTCCTGGCCAAACCACC |
| Cyclin D | ATGAAGGAGACCATCCCCCT | CGCCAGGTTCCACTTGAGTT |
| Cyclin E | CGATGTCTCTGTTCGCTCCA | CCACACTGGCTTCTCACAGT |
| CDK4 | GGCGAGGGTCTTCTCTGGT | CAGACGTCCATAAGCCTGACA |
| CDK6 | GGCTCTTACCTCAGTGGTCG | TCGACATCTGAACTTCCACGA |
| DGATI | CCACTGGGACCTGAGGTGTC | GCATCACCACACACCAATTCA |
| FASN | ACCTCGTGAAGGCTGTGACTCA | TGAGTCGAGGCCAAGGTCTGAA |
| ACBP | AGGCTGATTTTGACAAGGCG | GATCTAACAGTGCTGGACACTCAATATC |
| ACACA | CATCTTGTCCGAAACGTCGAT | CCCTTCGAACATACACCTCCA |
| SREBF1 | CCAGCTGACAGCTCCATTGA | TGCGCGCCACAAGGA |
| SREBF2 | AGGTCTCTGGGCACCATGC | CATCACCGCAACCCCAAG |
| FABP4 | TCCAGTGTGATGCGGTGTGTA | TGGATAGTGCAGCCAGTGTGA |
| APOA1 | CGGCGGCTTCTCTTGTATAGC | TTCAAGCGTGAGCTGAAACG |
| SLC2A4 | AAGCAAGTTGCCCATCCTCA | AAACTGTGGCTCCAATTTCGA |
| STAT3 | TGACCGAGGTTGGAGGTTTG | TGGTCCACCTGATCATTCTGG |
| FABP3 | GAACTCGACTCCCAGCTTGAA | AAGCCTACCACAATCATCGAAG |
| ACSS2 | GGCGAATGCCTCTACTGCTT | GGCCAATCTTTTCTCTAATCTGCTT |
| SCD | TCCTGTTGTTGTGCTTCATCC | GGCATAACGGAATAAGGTGGC |
| GAPM | GCAGGTTTATCCAGTATGGCATT | GGACTGATATCTTCCTGATCATCTTG |
| VLDLR | GCCCAGAACAGTGCCATATGA | TTTTCACCATCACACCGCC |
| FADS1 | GGTGGACTTGGCCTGGATG | TGACCATGAAGACAAGCCCC |
| GAPDH | GCTGCTTTTAATTCTGGC | CTTTCCATTGATGACGAG |
| MARVELD1 | GCAGAAGUAUGGGGAAGCCTT | TCTGATCACAGACAGAGCACCAT |
| ITGB4BP | GAGGGCTGGTACATCCCAAG | CTCGCTGCCTCGGTTCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Huang, J.; Liu, Y.; Li, H.; Han, B.; Sun, D. Bta-miR-106b Regulates Bovine Mammary Epithelial Cell Proliferation, Cell Cycle, and Milk Protein Synthesis by Targeting the CDKN1A Gene. Genes 2022, 13, 2308. https://doi.org/10.3390/genes13122308
Wu X, Huang J, Liu Y, Li H, Han B, Sun D. Bta-miR-106b Regulates Bovine Mammary Epithelial Cell Proliferation, Cell Cycle, and Milk Protein Synthesis by Targeting the CDKN1A Gene. Genes. 2022; 13(12):2308. https://doi.org/10.3390/genes13122308
Chicago/Turabian StyleWu, Xin, Jinfeng Huang, Yanan Liu, Houcheng Li, Bo Han, and Dongxiao Sun. 2022. "Bta-miR-106b Regulates Bovine Mammary Epithelial Cell Proliferation, Cell Cycle, and Milk Protein Synthesis by Targeting the CDKN1A Gene" Genes 13, no. 12: 2308. https://doi.org/10.3390/genes13122308
APA StyleWu, X., Huang, J., Liu, Y., Li, H., Han, B., & Sun, D. (2022). Bta-miR-106b Regulates Bovine Mammary Epithelial Cell Proliferation, Cell Cycle, and Milk Protein Synthesis by Targeting the CDKN1A Gene. Genes, 13(12), 2308. https://doi.org/10.3390/genes13122308






