Effects of N-Acetylcysteine on the Proliferation, Hormone Secretion Level, and Gene Expression Profiles of Goat Ovarian Granulosa Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Sampling
2.2. Cell Culture and Identification
2.3. CCK-8 Assay
2.4. EdU Assay
2.5. Estradiol and Progesterone Content by ELISA
2.6. Transcriptome Sequencing
2.6.1. Sample Preparation and mRNA Sequencing
2.6.2. Transcriptome Data Analysis
2.6.3. Quantitative Real-Time PCR
2.7. Construction and Cell Transfection of Overexpression Recombinant Plasmid
2.8. Expression Abundance Detection of Related Genes under RSPO2 Overexpression
2.9. Statistical Analysis
3. Results
3.1. GC Isolation and Characterization
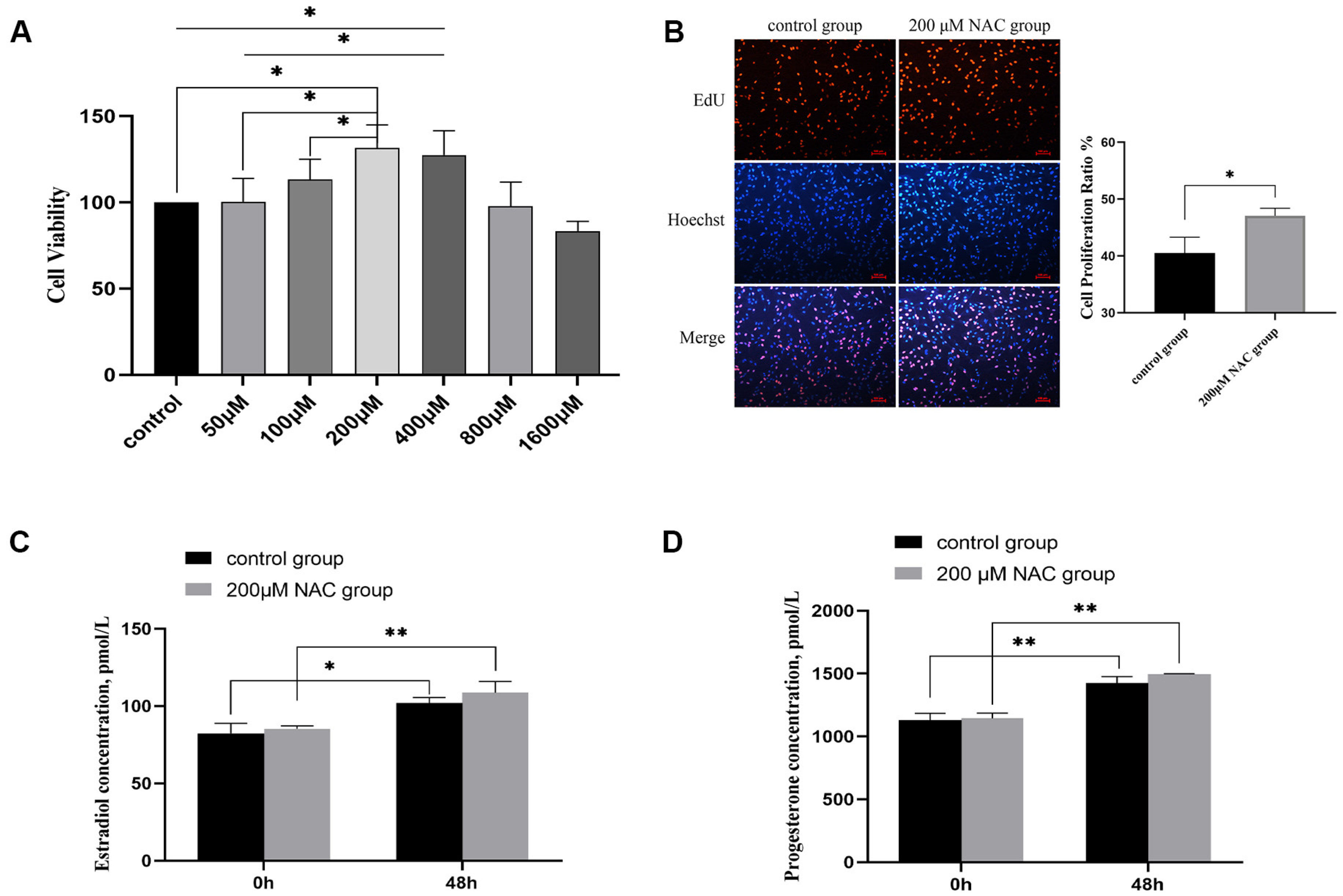
3.2. The Effect of NAC on Cell Proliferation
3.3. Estradiol and Progesterone Levels
3.4. Transcriptomic Analysis of Cell Samples of the 200 μM NAC and Control Groups
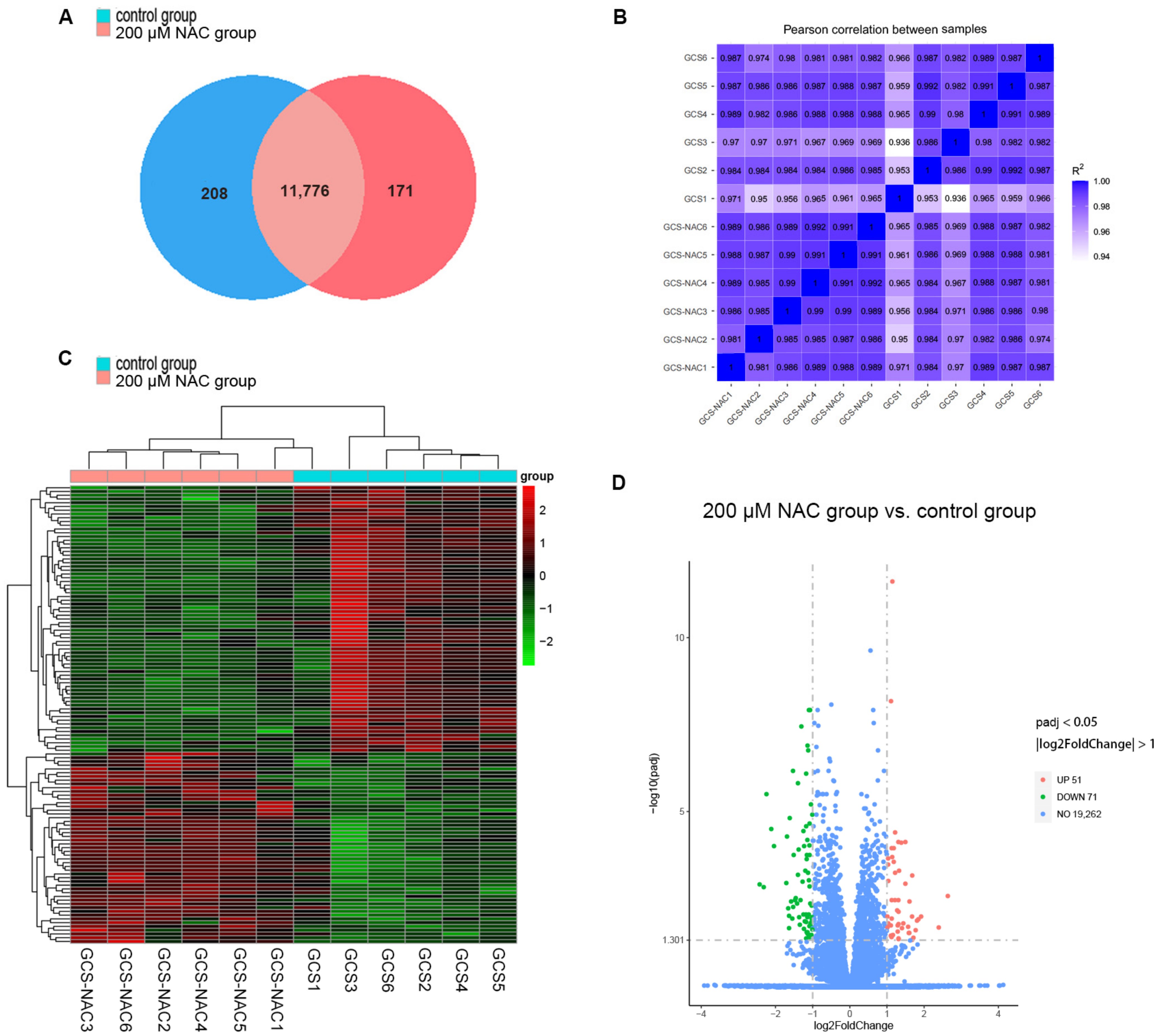
3.4.1. Total RNA Quality Detection and Data Quality Control Analysis
3.4.2. Gene Expression
3.4.3. GO Functional and KEGG Pathway Enrichment Analysis of DEGs
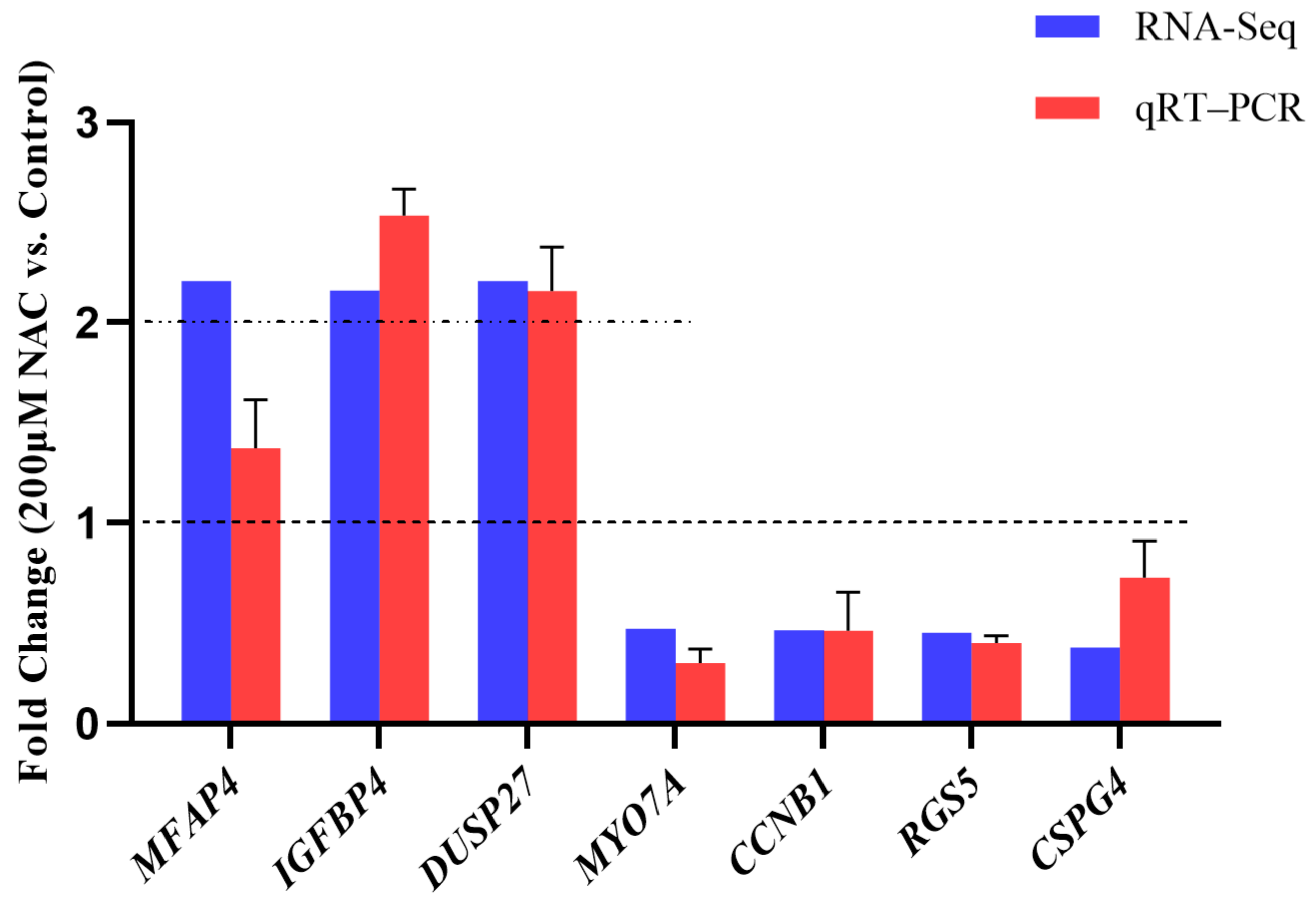
3.5. RNA-seq Data Validation
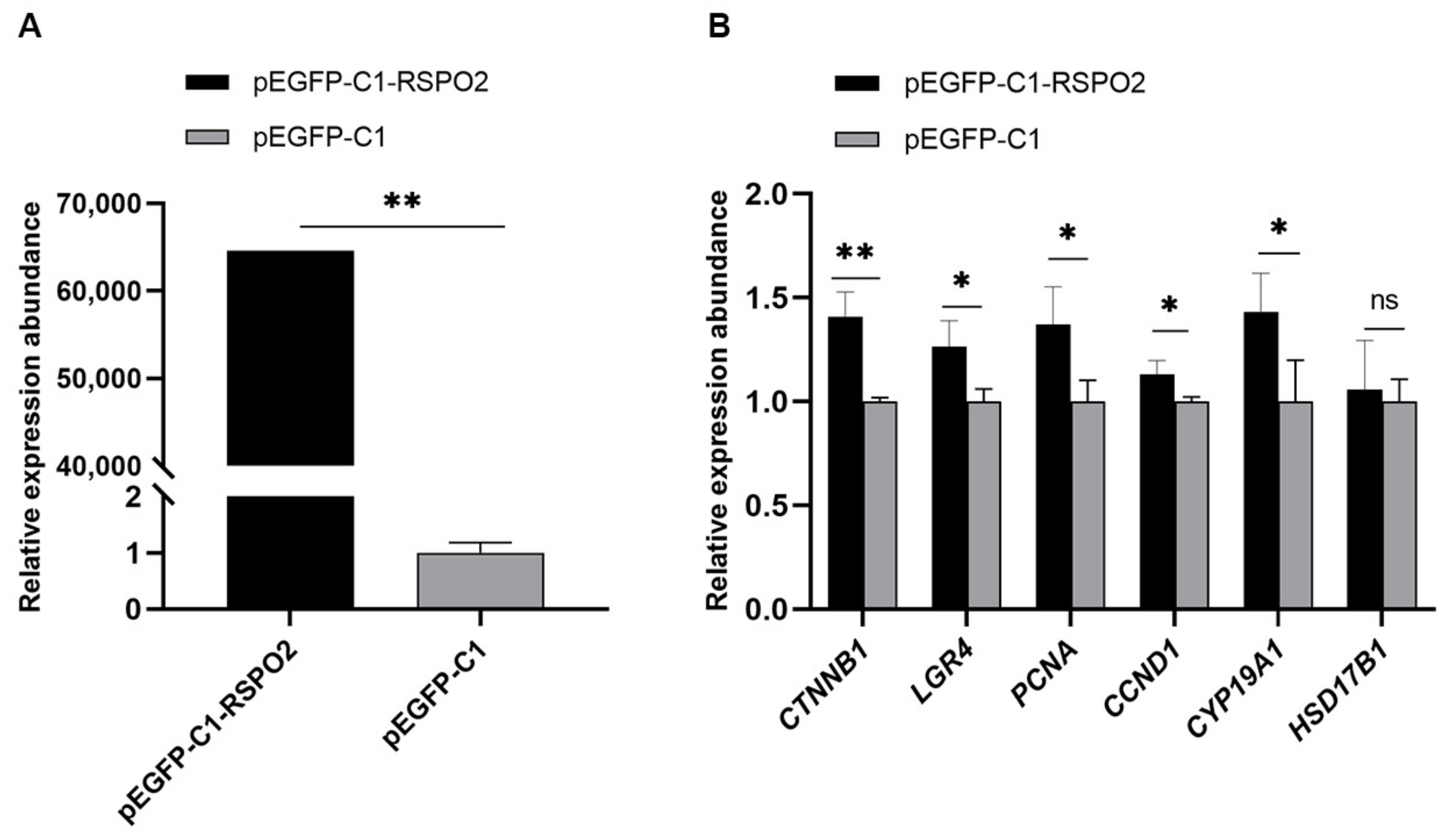
3.6. Regulation of Overexpression of RSPO2 on GCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, W.; Goldstein, G.; Adams, C.; Matthews, R.H.; Ercal, N. Separation and quantification of N-acetyl-l-cysteine and N-acetyl-cysteine-amide by HPLC with fluorescence detection. Biomed. Chromatogr. 2006, 20, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Slattery, J.; Kumar, N.; Delhey, L.; Berk, M.; Dean, O.; Spielholz, C.; Frye, R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015, 55, 294–321. [Google Scholar] [CrossRef]
- Whitaker, B.D.; Knight, J.W. Effects of N-acetyl-cysteine and N-acetyl-cysteine-amide supplementation on in vitro matured porcine oocytes. Reprod. Domest. Anim. 2010, 45, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yang, Y.; Wei, S.; Spicer, L.J.; Kenéz, Á.; Xu, W.; Liu, Y.; Feng, T. Influence of N-acetylcysteine on steroidogenesis and gene expression in porcine placental trophoblast cells. Theriogenology 2021, 161, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Ye, X.; Liu, K.; Huang, J.; Wang, L.; Ji, G.; Liu, N.; Tang, X.; Baltz, J.M.; et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum. Reprod. 2012, 27, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ao, Z.; Duan, Z.; Ao, Y.; Wei, S.; Chen, W.; Chen, X. Effects of N-Acetylcysteine on the reproductive performance, oxidative stress and RNA sequencing of Nubian goats. Vet. Med. Sci. 2021, 7, 156–163. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, K.Q.; Moura, F.A.; dos Santos, J.M.; de Araujo, O.R.; de Farias Santos, J.C.; Goulart, M.O. Oxidative stress and inflammation in hepatic diseases: Therapeutic possibilities of N-acetylcysteine. Int. J. Mol. Sci. 2015, 16, 30269–30308. [Google Scholar] [CrossRef]
- More, J.; Galusso, N.; Veloso, P.; Montecinos, L.; Finkelstein, J.P.; Sanchez, G.; Bull, R.; Valdés, J.L.; Hidalgo, C.; Paula-Lima, A. N-Acetylcysteine Prevents the Spatial Memory Deficits and the Redox-Dependent RyR2 Decrease Displayed by an Alzheimer’s Disease Rat Model. Front. Aging Neurosci. 2018, 10, 399. [Google Scholar] [CrossRef]
- Martacic, J.; Filipovic, M.K.; Borozan, S.; Cvetkovic, Z.; Popovic, T.; Arsic, A.; Takic, M.; Vucic, V.; Glibetic, M. N-acetyl-L-cysteine protects dental tissue stem cells against oxidative stress in vitro. Clin. Oral. Investig. 2018, 22, 2897–2903. [Google Scholar] [CrossRef]
- Gao, W.; Liang, J.X.; Ma, C.; Dong, J.Y.; Yan, Q. The Protective Effect of N-Acetylcysteine on Ionizing Radiation Induced Ovarian Failure and Loss of Ovarian Reserve in Female Mouse. Biomed. Res. Int. Erratum in Biomed. Res. Int. 2021, 2021, 9817842. 2017, 2017, 4176170. [Google Scholar] [CrossRef]
- Sharma, G.T.; Dubey, P.K.; Kumar, G.S. Localization and expression of follicle-stimulating hormone receptor gene in buffalo (Bubalus bubalis) pre-antral follicles. Reprod. Domest. Anim. 2011, 46, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, X.; Zhou, Z.; Tang, W.; Zhang, Y.; Hui, M. The Effect of N-acetylcysteine on proliferation and related gene expression of goat endometrial stromal cells in vitro. Acta Vet. Et Zootech. Sin. 2022, 53, 141–150. (In Chinese) [Google Scholar]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H. Control of Mammalian Oocyte Development by Interactions with the Maternal Follicular Environment. Results Probl. Cell Differ. 2017, 63, 17–41. [Google Scholar] [CrossRef]
- Eppig, J.J. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays 1991, 13, 569–574. [Google Scholar] [CrossRef]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef]
- An, X.; Ma, H.; Liu, Y.; Li, F.; Song, Y.; Li, G.; Bai, Y.; Cao, B. Effects of miR-101-3p on goat granulosa cells in vitro and ovarian development in vivo via STC1. J. Anim. Sci. Biotechnol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Hoque, S.A.M.; Umehara, T.; Kawai, T.; Shimada, M. Adverse effect of superoxide-induced mitochondrial damage in granulosa cells on follicular development in mouse ovaries. Free Radic. Biol. Med. 2021, 163, 344–355. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, X.; Zhu, M.; Wang, W.; Ao, Z.; Zhao, J.; Tang, W.; Hong, L. Cathepsin D knockdown regulates biological behaviors of granulosa cells and affects litter size traits in goats. J. Zhejiang Univ. Sci. B 2021, 22, 893–905. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, G.; Guo, Y.; Ei-Samahy, M.; Wang, S.; Wan, Y.; Han, L.; Liu, Z.; Wang, F.; Zhang, Y. Vitamin D receptor expression and potential role of vitamin D on cell proliferation and steroidogenesis in goat ovarian granulosa cells. Theriogenology 2017, 102, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Wan, W.; Zhu, K.; Pan, M.; Zhao, X.; Ma, B.; Wei, Q. Effects of 4-vinylcyclohexene diepoxide on the cell cycle, apoptosis, and steroid hormone secretion of goat ovarian granulosa cells. In Vitro Cell Dev. Biol. Anim. 2022, 58, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, T.; Gong, Z.; Yu, Y.; Zhang, Y.; Zhao, S.; Qin, Y. Novel FSHR mutations in Han Chinese women with sporadic premature ovarian insufficiency. Mol. Cell Endocrinol. 2019, 492, 110446. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Ren, F.; Li, Y.; Xian, M.; Wang, L.; Hu, J. FSH inhibits autophagy and lysosomal biogenesis to regulate protein degradation in cultured goat Sertoli cells. Mol. Cell. Endocrinol. 2022, 540, 111505. [Google Scholar] [CrossRef]
- Tian, X.; Wang, X.; Li, J.; Luo, Q.; Ban, C.; Lu, Q. The effects of selenium on rumen fermentation parameters and microbial metagenome in goats. Fermentation 2022, 8, 240. [Google Scholar] [CrossRef]
- Gong, T.; Wang, W.; Xu, H.; Yang, Y.; Chen, X.; Meng, L.; Xu, Y.; Li, Z.; Wan, S.; Mu, Q. Longitudinal expression of testicular TAS1R3 from prepuberty to sexual maturity in Congjiang Xiang Pigs. Animals 2021, 11, 437. [Google Scholar] [CrossRef]
- Bensalel, J.; Xu, H.; Lu, M.L.; Capobianco, E.; Wei, J. RNA-seq analysis reveals significant transcriptome changes in huntingtin-null human neuroblastoma cells. BMC Med. Genom. 2021, 14, 176. [Google Scholar] [CrossRef]
- Deng, M.; Chen, B.; Liu, Z.; Cai, Y.; Wan, Y.; Zhang, G.; Fan, Y.; Zhang, Y.; Wang, F. YTHDF2 Regulates Maternal Transcriptome Degradation and Embryo Development in Goat. Front. Cell Dev. Biol. 2020, 8, 580367. [Google Scholar] [CrossRef]
- Li, X.; Kim, J.; Wu, J.; Ahamed, A.I.; Wang, Y.; Martins-Green, M. N-Acetyl-cysteine and Mechanisms Involved in Resolution of Chronic Wound Biofilm. J. Diabetes Res. 2020, 2020, 9589507. [Google Scholar] [CrossRef]
- Nynca, A.; Jablonska, O.; Slomczynska, M.; Petroff, B.K.; Ciereszko, R.E. Effects of phytoestrogen daidzein and estradiol on steroidogenesis and expression of estrogen receptors in porcine luteinized granulosa cells from large follicles. J. Physiol. Pharmacol. 2009, 60, 95–105. [Google Scholar]
- Inoue, S.; Yang, R.; Tantry, A.; Davis, C.H.; Yang, T.; Knoedler, J.R.; Wei, Y.; Adams, E.L.; Thombare, S.; Golf, S.R.; et al. Periodic Remodeling in a Neural Circuit Governs Timing of Female Sexual Behavior. Cell 2019, 179, 1393–1408. [Google Scholar] [CrossRef]
- Li, S. Effects of Melatonin and N-Acetyl Cysteine on the Proliferation of Sheep Follicle Cells. Master’s Thesis, Northwest Minzu University, Lanzhou, China, 2022. (In Chinese) [Google Scholar] [CrossRef]
- Prior, J.C. Progesterone for the prevention and treatment of osteoporosis in women. Climacteric 2018, 21, 366–374. [Google Scholar] [CrossRef]
- Peluso, J.J.; Pru, J.K. Non-canonical progesterone signaling in granulosa cell function. Reproduction 2014, 147, R169–R178. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.; Figueiredo, J.R.; van den Hurk, R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009, 71, 1193–1208. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Penny, D.; Monget, P.; Arraztoa, J.A.; Fogelson, L.J.; Bondy, C.A. Insulin-like growth factor binding protein 4 expression parallels luteinizing hormone receptor expression and follicular luteinization in the primate ovary. Biol. Reprod. 2003, 69, 22–29. [Google Scholar] [CrossRef]
- Liu, J.; Koenigsfeld, A.T.; Cantley, T.C.; Boyd, C.K.; Kobayashi, Y.; Lucy, M.C. Growth and the initiation of steroidogenesis in porcine follicles are associated with unique patterns of gene expression for individual componentsof the ovarian insulin-like growth factor system. Biol. Reprod. 2000, 63, 942–952. [Google Scholar] [CrossRef]
- Fantone, S.; Giannubilo, S.R.; Marzioni, D.; Tossetta, G. HTRA family proteins in pregnancy outcome. Tissue Cell 2021, 72, 101549. [Google Scholar] [CrossRef]
- Tang, W. Studies on NAC Regulation of IGF1 and IGFBP1 Genes That Influence Lambing Traits of Goat. Master’s Thesis, Guizhou University, Guiyang, China, 2021. (In Chinese) [Google Scholar] [CrossRef]
- Holst, N.; Jacobsen, M.B.; Haug, E.; Tanbo, T.; Abyholm, T. Somatostatin in physiological concentrations inhibits basal and enhances luteinizing hormone-stimulated progesterone release from human granulosa-luteal cells. Hum. Reprod. 1995, 10, 1363–1366. [Google Scholar] [CrossRef]
- Mitsogiannis, I.C.; Skolarikos, A.; Deliveliotis, C. Somatostatin analog lanreotide in the treatment of castration-resistant prostate cancer (CRPC). Expert Opin. Pharm. 2009, 10, 493–501. [Google Scholar] [CrossRef]
- Nakamura, E.; Otsuka, F.; Inagaki, K.; Tsukamoto, N.; Ogura-Ochi, K.; Miyoshi, T.; Toma, K.; Takeda, M.; Makino, H. Involvement of bone morphogenetic protein activity in somatostatin actions on ovarian steroidogenesis. J. Steroid. Biochem. Mol. Biol. 2013, 134, 67–74. [Google Scholar] [CrossRef]
- Moaeen-ud-Din, M.; Malik, N.; Yang, L.G. Somatostatin can alter fertility genes expression, oocytes maturation, and embryo development in cattle. Anim. Biotechnol. 2009, 20, 144–150. [Google Scholar] [CrossRef]
- Wang, S.; Chong, Z.Z.; Shang, Y.C.; Maiese, K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr. Neurovasc. Res. 2012, 9, 20–31. [Google Scholar] [CrossRef] [PubMed]
- De Alwis, N.; Beard, S.; Binder, N.K.; Pritchard, N.; Kaitu’u-Lino, T.J.; Walker, S.P.; Stock, O.; Groom, K.; Petersen, S.; Henry, A.; et al. DAAM2 is elevated in the circulation and placenta in pregnancies complicated by fetal growth restriction and is regulated by hypoxia. Sci. Rep. 2021, 11, 5540. [Google Scholar] [CrossRef] [PubMed]
- Hull, K.L.; Harvey, S. Growth hormone and reproduction: A review of endocrine and autocrine/paracrine interactions. Int. J. Endocrinol. 2014, 2014, 234014. [Google Scholar] [CrossRef] [PubMed]
- Bouilly, J.; Beau, I.; Barraud, S.; Bernard, V.; Delemer, B.; Young, J.; Binart, N. R-spondin2, a novel target of NOBOX: Identification of variants in a cohort of women with primary ovarian insufficiency. J. Ovarian. Res. 2017, 10, 51. [Google Scholar] [CrossRef]
- Kocer, A.; Pinheiro, I.; Pannetier, M.; Renault, L.; Parma, P.; Radi, O.; Kim, K.A.; Camerino, G.; Pailhoux, E. R-spondin1 and FOXL2 act into two distinct cellular types during goat ovarian differentiation. BMC Dev. Biol. 2008, 8, 36. [Google Scholar] [CrossRef]
- De Cian, M.C.; Gregoire, E.P.; Le Rolle, M.; Lachambre, S.; Mondin, M.; Bell, S.; Guigon, C.J.; Chassot, A.A.; Chaboissier, M.C. R-spondin2 signaling is required for oocyte-driven intercellular communication and follicular growth. Cell Death Differ. 2020, 27, 2856–2871. [Google Scholar] [CrossRef]
- Zhou, X.; He, Y.; Li, N.; Bai, G.; Pan, X.; Zhang, Z.; Zhang, H.; Li, J.; Yuan, X. DNA methylation mediated RSPO2 to promote follicular development in mammals. Cell Death Dis. 2021, 12, 653. [Google Scholar] [CrossRef]


| Gene Name | Login ID | Oligonucleotide Primers (5′-3′) | Product Size (bp) |
|---|---|---|---|
| MFAP4 | XM_018064747.1 | F: GGGGCCCAGCACATAACTC R: ACGACTTCTCCAGAGCATCTCC | 187 |
| IGFBP4 | NM_001285678.1 | F: GGCCAAAATTCGAGACCGGA R: TAAAGGTCTTCGTGGGTGCG | 162 |
| DUSP27 | XM_018046440.1 | F: TGGAGGACCTGTACAACCGT R: TTGTTCACAGCCACACTCTTCT | 178 |
| MYO7A | XM_018059307.1 | F: CTGTCAGGGATGGAGTCGTG R: AGTGCACTTGTGGTAGCCTC | 158 |
| CCNB1 | XM_005694606.3 | F: GCTGTTGGCCTGTGGTTGAT R: GCATTAATCTTCGTGTTCCTGGT | 130 |
| RGS5 | XM_005677115.3 | F: CCAAGACCCAGAAACCCTCG R: TTTGCCGTCTCAGCCATCTT | 199 |
| CSPG4 | XM_018066268.1 | F: TATGAGCACGAGATGCCCTC R: TCGGAGACCCAGAGACCTTT | 179 |
| β-actin | XM_018039831.1 | F: CCGTGACATCAAGGAGAAGC R: CCGTGTTGGCGTAAAGGT | 266 |
| Gene Name | Login ID | Oligonucleotide Primers (5′-3′) | Product Size (bp) |
|---|---|---|---|
| RSPO2 | XM_005689159.3 | F: ACCATGTCCGACCATTGCTGAATC R: GTGTTGCTCCTGGGCTCTTTCTATC | 142 |
| CTNNB1 | XM_018066894.1 | F: CGGCAATCAAGAAAGCAAGCTCATC R: CACAGACAGCACCTTCAGCACTC | 130 |
| LGR4 | XM_018059305.1 | F: GCAGTCCGCCCACTCTGATTATG R: CTCTTCCGCAAGCCTGAACCTG | 83 |
| PCNA | XM_005688167.3 | F: GAAGAAAGTGCTGGAGGC R: TCGGAGCGAAGGGTTA | 129 |
| CCND1 | XM_018043271.1 | F: GCTGCGAGATGGAAAC R: AAGTAGGACACCGAGGG | 115 |
| CYP19A1 | NM_001285747.1 | F: CCCAAGGCATTACAATGT R: TAAGGGTTTCCTCTCCAC | 266 |
| HSD17B1 | XM_018065125.1 | F: GGAGCATAGGCGGCTTGAT R: TGGCGACAGTAGCGGTAGAA | 241 |
| Samples | RIN | Clean Reads | GC Content/% | Reads Quality ≥ Q30/% | Mapped Reads/% | Unique Mapped Reads/% |
|---|---|---|---|---|---|---|
| GCS-NAC1 | 8.80 | 42,795,496 | 51.96 | 94.14 | 95.80 | 90.45 |
| GCS-NAC2 | 9.50 | 47,220,802 | 52.37 | 95.04 | 97.38 | 92.12 |
| GCS-NAC3 | 9.40 | 40,030,012 | 52.94 | 94.54 | 96.48 | 91.51 |
| GCS-NAC4 | 9.00 | 41,594,026 | 52.45 | 94.62 | 96.50 | 91.26 |
| GCS-NAC5 | 8.90 | 39,502,108 | 52.76 | 94.73 | 96.54 | 91.62 |
| GCS-NAC6 | 9.90 | 45,110,510 | 52.21 | 93.91 | 96.72 | 91.50 |
| GCS1 | 9.10 | 45,609,488 | 49.60 | 93.32 | 93.17 | 87.78 |
| GCS2 | 10.00 | 42,688,270 | 52.84 | 94.72 | 96.33 | 91.15 |
| GCS3 | 10.00 | 41,268,492 | 52.99 | 94.27 | 96.16 | 90.82 |
| GCS4 | 10.00 | 40,585,386 | 52.38 | 94.65 | 96.16 | 90.85 |
| GCS5 | 9.40 | 41,043,756 | 52.52 | 94.81 | 96.40 | 91.38 |
| GCS6 | 8.90 | 40,193,144 | 51.78 | 94.78 | 95.98 | 90.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, T.; Chen, X.; Zhang, Y.; Fu, K.; Zou, Y.; Wang, W.; Zhao, J. Effects of N-Acetylcysteine on the Proliferation, Hormone Secretion Level, and Gene Expression Profiles of Goat Ovarian Granulosa Cells. Genes 2022, 13, 2306. https://doi.org/10.3390/genes13122306
Ji T, Chen X, Zhang Y, Fu K, Zou Y, Wang W, Zhao J. Effects of N-Acetylcysteine on the Proliferation, Hormone Secretion Level, and Gene Expression Profiles of Goat Ovarian Granulosa Cells. Genes. 2022; 13(12):2306. https://doi.org/10.3390/genes13122306
Chicago/Turabian StyleJi, Taotao, Xiang Chen, Yan Zhang, Kaibin Fu, Yue Zou, Weiwei Wang, and Jiafu Zhao. 2022. "Effects of N-Acetylcysteine on the Proliferation, Hormone Secretion Level, and Gene Expression Profiles of Goat Ovarian Granulosa Cells" Genes 13, no. 12: 2306. https://doi.org/10.3390/genes13122306
APA StyleJi, T., Chen, X., Zhang, Y., Fu, K., Zou, Y., Wang, W., & Zhao, J. (2022). Effects of N-Acetylcysteine on the Proliferation, Hormone Secretion Level, and Gene Expression Profiles of Goat Ovarian Granulosa Cells. Genes, 13(12), 2306. https://doi.org/10.3390/genes13122306





