Karyotype Aberrations in Action: The Evolution of Cancer Genomes and the Tumor Microenvironment
Abstract
1. Introduction
2. Cellular Routes to Karyotype Change
3. Environmental Causes of Karyotype Change
4. Aneuploidy and Polyploidy Can Both Promote and Buffer Karyotypic Heterogeneity
5. The Role of Aneuploidy and Polyploidy in Tumor Niche Construction
5.1. Environment Remodeling by Aneuploid and Polyploid Cells: Home Is Where You Make It
5.1.1. The Transmission of ER Stress to Immune Cells Impairs Anti-Tumor Immunity
5.1.2. Changes in Metabolism and ROS Homeostasis May Contribute to Tumor Acidosis and Inflammation
5.1.3. CIN, Cell Death, and Senescence: Potent Forces in Tissue Niche Construction
5.1.4. Altered Centrosome Homeostasis Affects Tissue Organization, Invasiveness, and the Cellular Secretome
5.1.5. Aneuploid Stromal Cells May Also Alter the Tumor Microenvironment
5.1.6. Environmental Remodeling by Aneuploid and Polyploid Cells—Summary
5.2. Rigged Selection? Stress Conditions in the TME May Favor the Growth and Survival of Karyotypically Abnormal Cells
5.2.1. Karyotype Aberrations Can Confer Selective Advantage of Cancer Cells in Their Constructed Niches and in the Face of Cancer Therapeutics
5.2.2. Karyotypic, Genetic, and Epigenetic Changes Alter Selective Survival of Tumor Stromal Cells
5.2.3. Karyotype Aberrations and Immune Interactions: A Matter of Context
5.2.4. Increased Motility in Aneuploid and Polyploid Cells May Provide a Fitness Advantage in Some Contexts
5.2.5. Effects of the TME on Karyotypically Abnormal Cells—Summary
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Swanton, C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Navin, N.E. The first five years of single-cell cancer genomics and beyond. Genome Res. 2015, 25, 1499–1507. [Google Scholar] [CrossRef]
- Klughammer, J.; Kiesel, B.; Roetzer, T.; Fortelny, N.; Nemc, A.; Nenning, K.-H.; Furtner, J.; Sheffield, N.C.; Datlinger, P.; Peter, N. The DNA methylation landscape of glioblastoma disease progression shows extensive heterogeneity in time and space. Nat. Med. 2018, 24, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.L.; Hedley, D.W.; Taylor, I. Clinical and biological significance of aneuploidy in human tumours. J. Clin. Pathol. 1984, 37, 961–974. [Google Scholar] [CrossRef]
- Jamal-Hanjani, M.; Wilson, G.A.; McGranahan, N.; Birkbak, N.J.; Watkins, T.B.; Veeriah, S.; Shafi, S.; Johnson, D.H.; Mitter, R.; Rosenthal, R. Tracking the evolution of non–small-cell lung cancer. N. Engl. J. Med. 2017, 376, 2109–2121. [Google Scholar] [CrossRef]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 501, 338–345. [Google Scholar] [CrossRef]
- Lee, A.J.; Endesfelder, D.; Rowan, A.J.; Walther, A.; Birkbak, N.J.; Futreal, P.A.; Downward, J.; Szallasi, Z.; Tomlinson, I.P.; Howell, M. Chromosomal instability confers intrinsic multidrug resistance. Cancer Res. 2011, 71, 1858–1870. [Google Scholar] [CrossRef]
- Pribluda, A.; Cecile, C.; Jackson, E.L. Intratumoral heterogeneity: From diversity comes resistance. Clin. Cancer Res. 2015, 21, 2916–2923. [Google Scholar] [CrossRef]
- Reiter, J.G.; Baretti, M.; Gerold, J.M.; Makohon-Moore, A.P.; Daud, A.; Iacobuzio-Donahue, C.A.; Azad, N.S.; Kinzler, K.W.; Nowak, M.A.; Vogelstein, B. An analysis of genetic heterogeneity in untreated cancers. Nat. Rev. Cancer 2019, 19, 639–650. [Google Scholar] [CrossRef]
- Loeb, L.A.; Kohrn, B.F.; Loubet-Senear, K.J.; Dunn, Y.J.; Ahn, E.H.; O’Sullivan, J.N.; Salk, J.J.; Bronner, M.P.; Beckman, R.A. Extensive subclonal mutational diversity in human colorectal cancer and its significance. Proc. Natl. Acad. Sci. USA 2019, 116, 26863–26872. [Google Scholar] [CrossRef] [PubMed]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C. Intratumor heterogeneity: Evolution through space and time. Cancer Res. 2012, 72, 4875–4882. [Google Scholar] [CrossRef]
- Weigelt, B.; Reis-Filho, J.S. Histological and molecular types of breast cancer: Is there a unifying taxonomy? Nat. Rev. Clin. Oncol. 2009, 6, 718. [Google Scholar] [CrossRef] [PubMed]
- Almendro, V.; Marusyk, A.; Polyak, K. Cellular heterogeneity and molecular evolution in cancer. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2010, 1805, 105–117. [Google Scholar] [CrossRef]
- Guo, M.; Peng, Y.; Gao, A.; Du, C.; Herman, J.G. Epigenetic heterogeneity in cancer. Biomark. Res. 2019, 7, 23. [Google Scholar] [CrossRef]
- Landau, D.A.; Carter, S.L.; Stojanov, P.; McKenna, A.; Stevenson, K.; Lawrence, M.S.; Sougnez, C.; Stewart, C.; Sivachenko, A.; Wang, L. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell 2013, 152, 714–726. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Govindan, R.; Ding, L.; Griffith, M.; Subramanian, J.; Dees, N.D.; Kanchi, K.L.; Maher, C.A.; Fulton, R.; Fulton, L.; Wallis, J. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012, 150, 1121–1134. [Google Scholar] [CrossRef]
- Dalgliesh, G.L.; Furge, K.; Greenman, C.; Chen, L.; Bignell, G.; Butler, A.; Davies, H.; Edkins, S.; Hardy, C.; Latimer, C. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 2010, 463, 360–363. [Google Scholar] [CrossRef]
- de Matos, M.R.; Posa, I.; Carvalho, F.S.; Morais, V.A.; Grosso, A.R.; de Almeida, S.F. A systematic pan-cancer analysis of genetic heterogeneity reveals associations with epigenetic modifiers. Cancers 2019, 11, 391. [Google Scholar] [CrossRef]
- Hinohara, K.; Polyak, K. Intratumoral heterogeneity: More than just mutations. Trends Cell Biol. 2019, 29, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.J.; Esteller, M. Non-coding RNAs, epigenetics, and cancer: Tying it all together. Cancer Metastasis Rev. 2018, 37, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Mitelman, F.; Johansson, B.; Mertens, F. Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. Available online: http://cgap.nci.nih.gov/Chromosomes/Mitelman (accessed on 6 April 2021).
- Nicholson, J.M.; Cimini, D. How mitotic errors contribute to karyotypic diversity in cancer. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2011; Volume 112, pp. 43–75. [Google Scholar]
- Davoli, T.; de Lange, T. The causes and consequences of polyploidy in normal development and cancer. Annu. Rev. Cell Dev. Biol. 2011, 27, 585–610. [Google Scholar] [CrossRef] [PubMed]
- Geigl, J.B.; Obenauf, A.C.; Schwarzbraun, T.; Speicher, M.R. Defining ‘chromosomal instability’. Trends Genet. 2008, 24, 64–69. [Google Scholar] [CrossRef]
- Wei, W.; Cheng, Y.; Wang, B. Cancer and Genomic Instability. In Genome Stability; Elsevier: Amsterdam, The Netherlands, 2016; pp. 463–486. [Google Scholar]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Weaver, B.A.; Cleveland, D.W. Does aneuploidy cause cancer? Curr. Opin. Cell Biol. 2006, 18, 658–667. [Google Scholar] [CrossRef]
- Cimini, D. Merotelic kinetochore orientation, aneuploidy, and cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2008, 1786, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Shih, J.; Ha, G.; Gao, G.F.; Zhang, X.; Berger, A.C.; Schumacher, S.E.; Wang, C.; Hu, H.; Liu, J.; et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell 2018, 33, 676–689.e673. [Google Scholar] [CrossRef] [PubMed]
- Chunduri, N.K.; Storchova, Z. The diverse consequences of aneuploidy. Nat. Cell Biol. 2019, 21, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Bandi, M.; Nitta, M.; Ivanova, E.V.; Bronson, R.T.; Pellman, D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 2005, 437, 1043. [Google Scholar] [CrossRef] [PubMed]
- Stopsack, K.H.; Whittaker, C.A.; Gerke, T.A.; Loda, M.; Kantoff, P.W.; Mucci, L.A.; Amon, A. Aneuploidy drives lethal progression in prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11390–11395. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A.; Silk, A.D.; Montagna, C.; Verdier-Pinard, P.; Cleveland, D.W. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 2007, 11, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Sotillo, R.; Hernando, E.; Díaz-Rodríguez, E.; Teruya-Feldstein, J.; Cordón-Cardo, C.; Lowe, S.W.; Benezra, R. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 2007, 11, 9–23. [Google Scholar] [CrossRef]
- Baker, D.J.; Jin, F.; Jeganathan, K.B.; van Deursen, J.M. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell 2009, 16, 475–486. [Google Scholar] [CrossRef]
- Hanks, S.; Coleman, K.; Reid, S.; Plaja, A.; Firth, H.; FitzPatrick, D.; Kidd, A.; Méhes, K.; Nash, R.; Robin, N. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 2004, 36, 1159–1161. [Google Scholar] [CrossRef]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M. The landscape of somatic copy-number alteration across human cancers. Nature 2010, 463, 899. [Google Scholar] [CrossRef]
- Turajlic, S.; Sottoriva, A.; Graham, T.; Swanton, C. Resolving genetic heterogeneity in cancer. Nat. Rev. Genet. 2019, 20, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Mazor, T.; Pankov, A.; Song, J.S.; Costello, J.F. Intratumoral heterogeneity of the epigenome. Cancer Cell 2016, 29, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Amon, A. Context is everything: Aneuploidy in cancer. Nat. Rev. Genet. 2019, 21, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Jeganathan, K.B.; Cameron, J.D.; Thompson, M.; Juneja, S.; Kopecka, A.; Kumar, R.; Jenkins, R.B.; De Groen, P.C.; Roche, P. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 2004, 36, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Jeganathan, K.B.; Malureanu, L.; Perez-Terzic, C.; Terzic, A.; van Deursen, J.M. Early aging–associated phenotypes in Bub3/Rae1 haploinsufficient mice. J. Cell Biol. 2006, 172, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Melo Pereira, S.; Ribeiro, R.; Logarinho, E. Approaches towards longevity: Reprogramming, senolysis, and improved mitotic competence as anti-aging therapies. Int. J. Mol. Sci. 2019, 20, 938. [Google Scholar] [CrossRef]
- Bissell, M.J. Context matters. Trends Cancer 2015, 1, 6–8. [Google Scholar]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320. [Google Scholar] [CrossRef]
- Yuan, Y. Spatial Heterogeneity in the Tumor Microenvironment. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Lee, H.O.; Davidson, J.M.; Duronio, R.J. Endoreplication: Polyploidy with purpose. Genes Dev. 2009, 23, 2461–2477. [Google Scholar] [CrossRef]
- Storchova, Z.; Pellman, D. From polyploidy to aneuploidy, genome instability and cancer. Nat. Rev. Mol. Cell Biol. 2004, 5, 45–54. [Google Scholar] [CrossRef]
- Orth, J.D.; Loewer, A.; Lahav, G.; Mitchison, T.J. Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Mol. Biol. Cell 2012, 23, 567–576. [Google Scholar] [CrossRef]
- Silkworth, W.T.; Nardi, I.K.; Scholl, L.M.; Cimini, D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE 2009, 4, e6564. [Google Scholar] [CrossRef]
- Ganem, N.J.; Godinho, S.A.; Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 2009, 460, 278–282. [Google Scholar] [CrossRef]
- Zack, T.I.; Schumacher, S.E.; Carter, S.L.; Cherniack, A.D.; Saksena, G.; Tabak, B.; Lawrence, M.S.; Zhang, C.-Z.; Wala, J.; Mermel, C.H. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 2013, 45, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, N.C.; Nicholson, J.M.; Soto, K.; Martin, O.; Chen, J.; Cimini, D. Asymmetric clustering of centrosomes defines the early evolution of tetraploid cells. Elife 2020, 9. [Google Scholar] [CrossRef]
- Thompson, S.L.; Compton, D.A. Chromosome missegregation in human cells arises through specific types of kinetochore–microtubule attachment errors. Proc. Natl. Acad. Sci. USA 2011, 108, 17974–17978. [Google Scholar] [CrossRef] [PubMed]
- Cimini, D.; Degrassi, F. Aneuploidy: A matter of bad connections. Trends Cell Biol. 2005, 15, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Torosantucci, L.; Puzzonia, M.D.S.; Cenciarelli, C.; Rens, W.; Degrassi, F. Aneuploidy in mitosis of PtK1 cells is generated by random loss and nondisjunction of individual chromosomes. J. Cell Sci. 2009, 122, 3455–3461. [Google Scholar] [CrossRef]
- Gregan, J.; Polakova, S.; Zhang, L.; Tolić-Nørrelykke, I.M.; Cimini, D. Merotelic kinetochore attachment: Causes and effects. Trends Cell Biol. 2011, 21, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Canman, J.C.; Sharma, N.; Straight, A.; Shannon, K.B.; Fang, G.; Salmon, E.D. Anaphase onset does not require the microtubule-dependent depletion of kinetochore and centromere-binding proteins. J. Cell Sci. 2002, 115, 3787–3795. [Google Scholar] [CrossRef][Green Version]
- Cimini, D.; Moree, B.; Canman, J.C.; Salmon, E. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 2003, 116, 4213–4225. [Google Scholar] [CrossRef] [PubMed]
- Cimini, D.; Fioravanti, D.; Salmon, E.D.; Degrassi, F. Merotelic kinetochore orientation versus chromosome mono-orientation in the origin of lagging chromosomes in human primary cells. J. Cell Sci. 2002, 115, 507–515. [Google Scholar]
- Terradas, M.; Martin, M.; Hernandez, L.; Tusell, L.; Genesca, A. Nuclear envelope defects impede a proper response to micronuclear DNA lesions. Mutat Res. 2012, 729, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA damage in micronuclei. Nature 2015, 522, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kwon, M.; Mannino, M.; Yang, N.; Renda, F.; Khodjakov, A.; Pellman, D. Nuclear envelope assembly defects link mitotic errors to chromothripsis. Nature 2018, 561, 551–555. [Google Scholar] [CrossRef]
- Hatch, E.M.; Fischer, A.H.; Deerinck, T.J.; Hetzer, M.W. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 2013, 154, 47–60. [Google Scholar] [CrossRef]
- Umbreit, N.T.; Zhang, C.-Z.; Lynch, L.D.; Blaine, L.J.; Cheng, A.M.; Tourdot, R.; Sun, L.; Almubarak, H.F.; Judge, K.; Mitchell, T.J. Mechanisms generating cancer genome complexity from a single cell division error. Science 2020, 368, eaba0712. [Google Scholar] [CrossRef]
- Ikeuchi, T.; Weinfeld, H.; Sandberg, A.A. Chromosome pulverization in micronuclei induced by tritiated thymidine. J. Cell Biol. 1972, 52, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ly, P.; Brunner, S.F.; Shoshani, O.; Kim, D.H.; Lan, W.; Pyntikova, T.; Flanagan, A.M.; Behjati, S.; Page, D.C.; Campbell, P.J. Chromosome segregation errors generate a diverse spectrum of simple and complex genomic rearrangements. Nat. Genet. 2019, 51, 705–715. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Gnawali, N.; Hinman, A.W.; Mattingly, A.J.; Osimani, A.; Cimini, D. Chromosomes missegregated into micronuclei contribute to chromosomal instability by missegregating at the next division. Oncotarget 2019, 10, 2660. [Google Scholar] [CrossRef] [PubMed]
- McClintock, B. The stability of broken ends of chromosomes in Zea mays. Genetics 1941, 26, 234. [Google Scholar] [PubMed]
- Cimini, D.; Antoccia, A.; Tanzarella, C.; Degrassi, F. Topoisomerase II inhibition in mitosis produces numerical and structural chromosomal aberrations in human fibroblasts. Cytogenet. Genome Res. 1997, 76, 61–67. [Google Scholar] [CrossRef]
- O’Sullivan, J.N.; Bronner, M.P.; Brentnall, T.A.; Finley, J.C.; Shen, W.-T.; Emerson, S.; Emond, M.J.; Gollahon, K.A.; Moskovitz, A.H.; Crispin, D.A. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat. Genet. 2002, 32, 280–284. [Google Scholar] [CrossRef]
- Stewénius, Y.; Gorunova, L.; Jonson, T.; Larsson, N.; Höglund, M.; Mandahl, N.; Mertens, F.; Mitelman, F.; Gisselsson, D. Structural and numerical chromosome changes in colon cancer develop through telomere-mediated anaphase bridges, not through mitotic multipolarity. Proc. Natl. Acad. Sci. USA 2005, 102, 5541–5546. [Google Scholar] [CrossRef]
- Pampalona, J.; Roscioli, E.; Silkworth, W.T.; Bowden, B.; Genescà, A.; Tusell, L.; Cimini, D. Chromosome bridges maintain kinetochore-microtubule attachment throughout mitosis and rarely break during anaphase. PLoS ONE 2016, 11, e0147420. [Google Scholar] [CrossRef]
- Pampalona, J.; Frías, C.; Genescà, A.; Tusell, L. Progressive telomere dysfunction causes cytokinesis failure and leads to the accumulation of polyploid cells. PLoS Genet. 2012, 8, e1002679. [Google Scholar] [CrossRef] [PubMed]
- Pampalona, J.; Soler, D.; Genescà, A.; Tusell, L. Whole chromosome loss is promoted by telomere dysfunction in primary cells. Geneschromosomes Cancer 2010, 49, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Degrassi, F. Molecular cytogenetics of the micronucleus: Still surprising. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2018, 836, 36–40. [Google Scholar] [CrossRef]
- Ye, C.J.; Sharpe, Z.; Alemara, S.; Mackenzie, S.; Liu, G.; Abdallah, B.; Horne, S.; Regan, S.; Heng, H.H. Micronuclei and genome chaos: Changing the system inheritance. Genes 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pacchierotti, F.; Cimini, D.; Ganem, N.J.; Genescà, A.; Natarajan, A.T.; Pavanello, S.; Valle, G.; Degrassi, F. Genomic instability: Crossing pathways at the origin of structural and numerical chromosome changes. Environ. Mol. Mutagenesis 2015, 56, 563–580. [Google Scholar] [CrossRef]
- Young, S.; Marshall, R.; Hill, R. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc. Natl. Acad. Sci. USA 1988, 85, 9533–9537. [Google Scholar] [CrossRef]
- Goncharova, E.I.; Nádas, A.; Rossman, T.G. Serum deprivation, but not inhibition of growth per se, induces a hypermutable state in Chinese hamster G12 cells. Cancer Res. 1996, 56, 752–756. [Google Scholar] [PubMed]
- Bindra, R.S.; Glazer, P.M. Genetic instability and the tumor microenvironment: Towards the concept of microenvironment-induced mutagenesis. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2005, 569, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Glazer, P.M. Mutagenesis induced by the tumor microenvironment. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1998, 400, 439–446. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Kabeche, L.; Murnane, J.P.; Zaki, B.I.; Compton, D.A. DNA-damage response during mitosis induces whole-chromosome missegregation. Cancer Discov. 2014, 4, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Gentric, G.; Maillet, V.; Paradis, V.; Couton, D.; L’Hermitte, A.; Panasyuk, G.; Fromenty, B.; Celton-Morizur, S.; Desdouets, C. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chan, Y.J.A.; Chua, Y.J.K.; Rutledge, S.D.; Pavelka, N.; Cimini, D.; Rancati, G. Environmental stresses induce karyotypic instability in colorectal cancer cells. Mol. Biol. Cell 2019, 30, 42–55. [Google Scholar] [CrossRef]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–226. [Google Scholar] [PubMed]
- Galipeau, P.C.; Cowan, D.S.; Sanchez, C.A.; Barrett, M.T.; Emond, M.J.; Levine, D.S.; Rabinovitch, P.S.; Reid, B.J. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett’s esophagus. Proc. Natl. Acad. Sci. USA 1996, 93, 7081–7084. [Google Scholar] [CrossRef]
- Lothschütz, D.; Jennewein, M.; Pahl, S.; Lausberg, H.; Eichler, A.; Mutschler, W.; Hanselmann, R.; Oberringer, M. Polyploidization and centrosome hyperamplification in inflammatory bronchi. Inflamm. Res. 2002, 51, 416–422. [Google Scholar] [CrossRef]
- Habermann, J.; Lenander, C.; Roblick, U.; Krüger, S.; Ludwig, D.; Alaiya, A.; Freitag, S.; Dümbgen, L.; Bruch, H.-P.; Stange, E. Ulcerative Colitis and Colorectal Carcinoma: DNA-Profile, Laminin-5? 2 Chain and Cyclin A Expression as Early Markers for Risk Assessment. Scand. J. Gastroenterol. 2001, 36, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Sloand, E.M.; Loeliger, K.; Pfannes, L.; Poon, A.; Calado, R.; Feng, X.; Padilla-Nash, H.; Chen, J.; Young, N.S. Does a Chronic Inflammatory Process Underlie Clonal Progression In Aplastic Anemia?—In Vitro and In Vivo Evidence That Inflammation Produces Aneuploidy for Chromosomes 7 and 8 In Replicating Cells; American Society of Hematology: Washington, DC, USA, 2010. [Google Scholar]
- Cianfarani, S.; Tedeschi, B.; Germani, D.; Prete, S.; Rossi, P.; Vernole, P.; Caporossi, D.; Boscherini, B. In vitro effects of growth hormone (GH) and insulin-like growth factor I and II (IGF-I and-II) on chromosome fragility and p53 protein expression in human lymphocytes. Eur. J. Clin. Investig. 1998, 28, 41–47. [Google Scholar] [CrossRef]
- Johansson, C.B.; Youssef, S.; Koleckar, K.; Holbrook, C.; Doyonnas, R.; Corbel, S.Y.; Steinman, L.; Rossi, F.M.; Blau, H.M. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat. Cell Biol. 2008, 10, 575–583. [Google Scholar] [CrossRef]
- Rosin, M.P.; Anwar, W.A.; Ward, A.J. Inflammation, chromosomal instability, and cancer: The schistosomiasis model. Cancer Res. 1994, 54, 1929s–1933s. [Google Scholar] [PubMed]
- Matsumoto, Y.; Marusawa, H.; Kinoshita, K.; Niwa, Y.; Sakai, Y.; Chiba, T. Up-regulation of activation-induced cytidine deaminase causes genetic aberrations at the CDKN2b-CDKN2a in gastric cancer. Gastroenterology 2010, 139, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Golubkov, V.S.; Boyd, S.; Savinov, A.Y.; Chekanov, A.V.; Osterman, A.L.; Remacle, A.; Rozanov, D.V.; Doxsey, S.J.; Strongin, A.Y. Membrane type-1 matrix metalloproteinase (MT1-MMP) exhibits an important intracellular cleavage function and causes chromosome instability. J. Biol. Chem. 2005, 280, 25079–25086. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Lochter, A.; Sympson, C.J.; Huey, B.; Rougier, J.-P.; Gray, J.W.; Pinkel, D.; Bissell, M.J.; Werb, Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999, 98, 137–146. [Google Scholar] [CrossRef]
- Lochter, A.; Srebrow, A.; Sympson, C.J.; Terracio, N.; Werb, Z.; Bissell, M.J. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J. Biol. Chem. 1997, 272, 5007–5015. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Carmona, M.; Lesage, J.; Cataldo, D.; Gilles, C. EMT and inflammation: Inseparable actors of cancer progression. Mol. Oncol. 2017, 11, 805–823. [Google Scholar] [CrossRef]
- Comaills, V.; Kabeche, L.; Morris, R.; Buisson, R.; Yu, M.; Madden, M.W.; LiCausi, J.A.; Boukhali, M.; Tajima, K.; Pan, S. Genomic instability is induced by persistent proliferation of cells undergoing epithelial-to-mesenchymal transition. Cell Rep. 2016, 17, 2632–2647. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Chiera, F.; Meccia, E.; Degan, P.; Aquilina, G.; Pietraforte, D.; Minetti, M.; Lambeth, D.; Bignami, M. Overexpression of human NOX1 complex induces genome instability in mammalian cells. Free Radic. Biol. Med. 2008, 44, 332–342. [Google Scholar] [CrossRef]
- Limoli, C.L.; Giedzinski, E. Induction of chromosomal instability by chronic oxidative stress. Neoplasia 2003, 5, 339–346. [Google Scholar] [CrossRef]
- Limoli, C.L.; Giedzinski, E.; Morgan, W.F.; Swarts, S.G.; Jones, G.D.; Hyun, W. Persistent oxidative stress in chromosomally unstable cells. Cancer Res. 2003, 63, 3107–3111. [Google Scholar]
- Samper, E.; Nicholls, D.; Melov, S. Mitochondrial oxidative stress causes chromosomal instability of mouse embryonic fibroblasts. Aging Cell 2003, 2, 277–285. [Google Scholar] [CrossRef]
- Mishra, P.K.; Raghuram, G.V.; Panwar, H.; Jain, D.; Pandey, H.; Maudar, K.K. Mitochondrial oxidative stress elicits chromosomal instability after exposure to isocyanates in human kidney epithelial cells. Free Radic. Res. 2009, 43, 718–728. [Google Scholar] [CrossRef]
- Houben, J.M.; Moonen, H.J.; van Schooten, F.J.; Hageman, G.J. Telomere length assessment: Biomarker of chronic oxidative stress? Free Radic. Biol. Med. 2008, 44, 235–246. [Google Scholar] [CrossRef]
- Chow, J.P.; Poon, R.Y. DNA damage and polyploidization. In Polyploidization and Cancer; Springer: Berlin/Heidelberg, Germany, 2010; pp. 57–71. [Google Scholar]
- Ivanov, A.; Cragg, M.S.; Erenpreisa, J.; Emzinsh, D.; Lukman, H.; Illidge, T.M. Endopolyploid cells produced after severe genotoxic damage have the potential to repair DNA double strand breaks. J. Cell Sci. 2003, 116, 4095–4106. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, P.R.; Lacroix, F.B.; Lohez, O.D.; Margolis, R.L. Neither p21WAF1 nor 14-3-3σ prevents G2 progression to mitotic catastrophe in human colon carcinoma cells after DNA damage, but p21WAF1 induces stable G1 arrest in resulting tetraploid cells. Cancer Res. 2001, 61, 7660–7668. [Google Scholar]
- Tan, Z.; Chan, Y.J.A.; Lu, Y.E.; Rancati, G. Mammalian cells undergo endoreduplication in response to lactic acidosis. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Machida, K.; Liu, J.-C.; McNamara, G.; Levine, A.; Duan, L.; Lai, M.M. Hepatitis C virus causes uncoupling of mitotic checkpoint and chromosomal polyploidy through the Rb pathway. J. Virol. 2009, 83, 12590–12600. [Google Scholar] [CrossRef]
- Bloomfield, M.; Duesberg, P. Karyotype alteration generates the neoplastic phenotypes of SV40-infected human and rodent cells. Mol. Cytogenet. 2015, 8, 79. [Google Scholar] [CrossRef]
- McCormack, A.; Fan, J.L.; Duesberg, M.; Bloomfield, M.; Fiala, C.; Duesberg, P. Individual karyotypes at the origins of cervical carcinomas. Mol. Cytogenet. 2013, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, M.; McCormack, A.; Mandrioli, D.; Fiala, C.; Aldaz, C.M.; Duesberg, P. Karyotypic evolutions of cancer species in rats during the long latent periods after injection of nitrosourea. Mol. Cytogenet. 2014, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Cortez, B.A.; Teixeira, P.R.; Redick, S.; Doxsey, S.; Machado-Santelli, G.M. Multipolar mitosis and aneuploidy after chrysotile treatment: A consequence of abscission failure and cytokinesis regression. Oncotarget 2016, 7, 8979. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef]
- Brechot, C.; Pourcel, C.; Louise, A.; Rain, B.; Tiollais, P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature 1980, 286, 533–535. [Google Scholar] [CrossRef]
- Perera, F.; Lin, C.-j.; Qu, L.; Tang, D. Shorter telomere length in cord blood associated with prenatal air pollution exposure: Benefits of intervention. Environ. Int. 2018, 113, 335–340. [Google Scholar] [CrossRef]
- Awada, Z.; Sleiman, F.; Mailhac, A.; Mouneimne, Y.; Tamim, H.; Zgheib, N. BPA exposure is associated with non-monotonic alteration in ESR1 promoter methylation in peripheral blood of men and shorter relative telomere length in peripheral blood of women. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Needham, B.L.; Blackburn, E.H.; Lin, J.; Park, S.K.; Rehkopf, D.H.; Epel, E.S. Associations of cadmium and lead exposure with leukocyte telomere length: Findings from National Health and Nutrition Examination Survey, 1999–2002. Am. J. Epidemiol. 2015, 181, 127–136. [Google Scholar] [CrossRef]
- Maser, R.S.; DePinho, R.A. Connecting chromosomes, crisis, and cancer. Science 2002, 297, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Davoli, T.; Denchi, E.L.; de Lange, T. Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell 2010, 141, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Knouse, K.A.; Lopez, K.E.; Bachofner, M.; Amon, A. Chromosome segregation fidelity in epithelia requires tissue architecture. Cell 2018, 175, 200–211.e213. [Google Scholar] [CrossRef] [PubMed]
- De Santis Puzzonia, M.; Gonzalez, L.; Ascenzi, S.; Cundari, E.; Degrassi, F. Tetraploid cells produced by absence of substrate adhesion during cytokinesis are limited in their proliferation and enter senescence after DNA replication. Cell Cycle 2016, 15, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Oberringer, M.; Lothschütz, D.; Jennewein, M.; Koschnick, M.; Mutschler, W.; Hanselmann, R.G. Centrosome multiplication accompanies a transient clustering of polyploid cells during tissue repair. Mol. Cell Biol. Res. Commun. 1999, 2, 190–196. [Google Scholar] [CrossRef]
- Lewis, J.M.; Truong, T.N.; Schwartz, M.A. Integrins regulate the apoptotic response to DNA damage through modulation of p53. Proc. Natl. Acad. Sci. USA 2002, 99, 3627–3632. [Google Scholar] [CrossRef]
- Truong, T.; Sun, G.; Doorly, M.; Wang, J.Y.; Schwartz, M.A. Modulation of DNA damage-induced apoptosis by cell adhesion is independently mediated by p53 and c-Abl. Proc. Natl. Acad. Sci. USA 2003, 100, 10281–10286. [Google Scholar] [CrossRef]
- Thompson, S.L.; Compton, D.A. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J. Cell Biol. 2010, 188, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, P.R.; Lohez, O.D.; Lacroix, F.B.; Margolis, R.L. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol. Biol. Cell 2001, 12, 1315–1328. [Google Scholar] [CrossRef]
- Newell, G.; Spitz, M.; Sider, J. Cancer and age. In Proceedings of Seminars in Oncology. Unpublished work.
- Rubin, H. Cancer as a dynamic developmental disorder. Cancer Res. 1985, 45, 2935–2942. [Google Scholar] [PubMed]
- Rehen, S.K.; McConnell, M.J.; Kaushal, D.; Kingsbury, M.A.; Yang, A.H.; Chun, J. Chromosomal variation in neurons of the developing and adult mammalian nervous system. Proc. Natl. Acad. Sci. USA 2001, 98, 13361–13366. [Google Scholar] [CrossRef]
- Baker, D.J.; Dawlaty, M.M.; Wijshake, T.; Jeganathan, K.B.; Malureanu, L.; Van Ree, J.H.; Crespo-Diaz, R.; Reyes, S.; Seaburg, L.; Shapiro, V. Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat. Cell Biol. 2013, 15, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Goto, H.; Inoko, A.; Makihara, H.; Enomoto, A.; Horimoto, K.; Matsuyama, M.; Kurita, K.; Izawa, I.; Inagaki, M. Cytokinetic failure-induced tetraploidy develops into aneuploidy, triggering skin aging in phosphovimentin-deficient mice. J. Biol. Chem. 2015, 290, 12984–12998. [Google Scholar] [CrossRef]
- Tanaka, K.; Goto, H.; Nishimura, Y.; Kasahara, K.; Mizoguchi, A.; Inagaki, M. Tetraploidy in cancer and its possible link to aging. Cancer Sci. 2018, 109, 2632–2640. [Google Scholar] [CrossRef]
- Knouse, K.A.; Wu, J.; Whittaker, C.A.; Amon, A. Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 13409–13414. [Google Scholar] [CrossRef] [PubMed]
- Duesberg, P.; Li, R.; Sachs, R.; Fabarius, A.; Upender, M.B.; Hehlmann, R. Cancer drug resistance: The central role of the karyotype. Drug Resist. Updates 2007, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, S.; Huang, X.; Li, L.; Zhang, C.; Pan, Q.; Ren, Z.; Zhou, R.; Ren, Y.; Zi, J. Drug resistance in colorectal cancer cell lines is partially associated with aneuploidy status in light of profiling gene expression. J. Proteome Res. 2016, 15, 4047–4059. [Google Scholar] [CrossRef] [PubMed]
- Ouillette, P.; Saiya-Cork, K.; Seymour, E.; Li, C.; Shedden, K.; Malek, S.N. Clonal evolution, genomic drivers, and effects of therapy in chronic lymphocytic leukemia. Clin. Cancer Res. 2013, 19, 2893–2904. [Google Scholar] [CrossRef]
- McCormick, F. New-age drug meets resistance. Nature 2001, 412, 281. [Google Scholar] [CrossRef] [PubMed]
- Duesberg, P.; Stindl, R.; Hehlmann, R. Explaining the high mutation rates of cancer cells to drug and multidrug resistance by chromosome reassortments that are catalyzed by aneuploidy. Proc. Natl. Acad. Sci. USA 2000, 97, 14295–14300. [Google Scholar] [CrossRef]
- Swanton, C.; Nicke, B.; Schuett, M.; Eklund, A.C.; Ng, C.; Li, Q.; Hardcastle, T.; Lee, A.; Roy, R.; East, P.; et al. Chromosomal instability determines taxane response. Proc. Natl. Acad. Sci. USA 2009, 106, 8671–8676. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, J.; Derksen, P.W. Modeling metastatic breast cancer in mice. J. Mammary Gland Biol. Neoplasia 2007, 12, 191–203. [Google Scholar] [CrossRef]
- Frankfurt, O.S.; Chin, J.L.; Englander, L.S.; Greco, W.R.; Pontes, J.E.; Rustum, Y.M. Relationship between DNA ploidy, glandular differentiation, and tumor spread in human prostate cancer. Cancer Res. 1985, 45, 1418–1423. [Google Scholar] [PubMed]
- Korabiowska, M.; Brinck, U.; Kotthaus, I.; Berger, H.; Droese, M. Analysis of the DNA content in the progression of recurrent and metastatic melanomas. Anticancer Res. 2000, 20, 2791–2794. [Google Scholar] [PubMed]
- Laubert, T.; Bente, V.; Freitag-Wolf, S.; Voulgaris, H.; Oberländer, M.; Schillo, K.; Kleemann, M.; Bürk, C.; Bruch, H.-P.; Roblick, U.J. Aneuploidy and elevated CEA indicate an increased risk for metachronous metastasis in colorectal cancer. Int. J. Colorectal Dis. 2013, 28, 767–775. [Google Scholar] [CrossRef]
- Li, L.; Mu, K.; Zhou, G.; Lan, L.; Auer, G.; Zetterberg, A. Genomic instability and proliferative activity as risk factors for distant metastases in breast cancer. Br. J. Cancer 2008, 99, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Chambers, T.; Lopez, J.I.; Nicol, D.; O’Brien, T.; Larkin, J.; Horswell, S. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell 2018, 173, 581–594.e512. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef]
- Tijhuis, A.E.; Johnson, S.C.; McClelland, S.E. The emerging links between chromosomal instability (CIN), metastasis, inflammation and tumour immunity. Mol. Cytogenet 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Fallenius, A.G.; Auer, G.U.; Carstensen, J.M. Prognostic significance of DNA measurements in 409 consecutive breast cancer patients. Cancer 1988, 62, 331–341. [Google Scholar] [CrossRef]
- Fallenius, A.G.; Auer, G.U.; Franzén, S.A. Predictive value of nuclear DNA content in breast cancer in relation to clinical and morphologic factors. A retrospective study of 227 consecutive cases. Cancer 1988, 62, 521–530. [Google Scholar] [CrossRef]
- Sheffer, M.; Bacolod, M.D.; Zuk, O.; Giardina, S.F.; Pincas, H.; Barany, F.; Paty, P.B.; Gerald, W.L.; Notterman, D.A.; Domany, E. Association of survival and disease progression with chromosomal instability: A genomic exploration of colorectal cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 7131–7136. [Google Scholar] [CrossRef]
- Walther, A.; Houlston, R.; Tomlinson, I. Association between chromosomal instability and prognosis in colorectal cancer: A meta-analysis. Gut 2008, 57, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.L.; Eklund, A.C.; Kohane, I.S.; Harris, L.N.; Szallasi, Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat. Genet. 2006, 38, 1043. [Google Scholar] [CrossRef] [PubMed]
- Silk, A.D.; Zasadil, L.M.; Holland, A.J.; Vitre, B.; Cleveland, D.W.; Weaver, B.A. Chromosome missegregation rate predicts whether aneuploidy will promote or suppress tumors. Proc. Natl. Acad. Sci. USA 2013, 110, E4134–E4141. [Google Scholar] [CrossRef]
- Bolhaqueiro, A.C.; Ponsioen, B.; Bakker, B.; Klaasen, S.J.; Kucukkose, E.; van Jaarsveld, R.H.; Vivié, J.; Verlaan-Klink, I.; Hami, N.; Spierings, D.C. Ongoing chromosomal instability and karyotype evolution in human colorectal cancer organoids. Nat. Genet. 2019, 51, 824–834. [Google Scholar] [CrossRef]
- Nicholson, J.M.; Cimini, D. Link between aneuploidy and chromosome instability. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 315, pp. 299–317. [Google Scholar]
- Nicholson, J.M.; Cimini, D. Cancer karyotypes: Survival of the fittest. Front. Oncol. 2013, 3, 148. [Google Scholar] [CrossRef]
- Thompson, S.L.; Compton, D.A. Examining the link between chromosomal instability and aneuploidy in human cells. J. Cell Biol. 2008, 180, 665–672. [Google Scholar] [CrossRef]
- Valind, A.; Jin, Y.; Gisselsson, D. Elevated tolerance to aneuploidy in cancer cells: Estimating the fitness effects of chromosome number alterations by in silico modelling of somatic genome evolution. PLoS ONE 2013, 8, e70445. [Google Scholar] [CrossRef]
- Bloomfield, M.; Duesberg, P. Inherent variability of cancer-specific aneuploidy generates metastases. Mol. Cytogenet. 2016, 9, 90. [Google Scholar] [CrossRef]
- Zasadil, L.M.; Britigan, E.M.; Weaver, B.A. 2n or not 2n: Aneuploidy, polyploidy and chromosomal instability in primary and tumor cells. Semin. Cell Dev. Biol. 2013, 24, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.Y.; Seget, K.; Moeller, G.K.; de Pagter, M.S.; de Roos, J.A.; Dürrbaum, M.; Kuffer, C.; Müller, S.; Zaman, G.J.; Kloosterman, W.P. Chromosomal instability, tolerance of mitotic errors and multidrug resistance are promoted by tetraploidization in human cells. Cell Cycle 2015, 14, 2810–2820. [Google Scholar] [CrossRef]
- Ganem, N.J.; Storchova, Z.; Pellman, D. Tetraploidy, aneuploidy and cancer. Curr. Opin. Genet. Dev. 2007, 17, 157–162. [Google Scholar] [CrossRef]
- Laughney, A.M.; Elizalde, S.; Genovese, G.; Bakhoum, S.F. Dynamics of tumor heterogeneity derived from clonal karyotypic evolution. Cell Rep. 2015, 12, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Storchova, Z.; Kuffer, C. The consequences of tetraploidy and aneuploidy. J. Cell Sci. 2008, 121, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, S.M.; McGranahan, N.; Burrell, R.A.; Rowan, A.J.; Gronroos, E.; Endesfelder, D.; Joshi, T.; Mouradov, D.; Gibbs, P.; Ward, R.L.; et al. Tolerance of whole-genome doubling propagates chromosomal instability and accelerates cancer genome evolution. Cancer Discov. 2014, 4, 175–185. [Google Scholar] [CrossRef]
- Ly, P.; Eskiocak, U.; Kim, S.B.; Roig, A.I.; Hight, S.K.; Lulla, D.R.; Zou, Y.S.; Batten, K.; Wright, W.E.; Shay, J.W. Characterization of aneuploid populations with trisomy 7 and 20 derived from diploid human colonic epithelial cells. Neoplasia 2011, 13, 348. [Google Scholar] [CrossRef]
- Duncan, A.W.; Newell, A.E.H.; Bi, W.; Finegold, M.J.; Olson, S.B.; Beaudet, A.L.; Grompe, M. Aneuploidy as a mechanism for stress-induced liver adaptation. J. Clin. Investig. 2012, 122, 3307–3315. [Google Scholar] [CrossRef]
- Rutledge, S.D.; Douglas, T.A.; Nicholson, J.M.; Vila-Casadesús, M.; Kantzler, C.L.; Wangsa, D.; Barroso-Vilares, M.; Kale, S.D.; Logarinho, E.; Cimini, D. Selective advantage of trisomic human cells cultured in non-standard conditions. Sci. Rep. 2016, 6, 22828. [Google Scholar] [CrossRef]
- Sheltzer, J.M.; Ko, J.H.; Replogle, J.M.; Burgos, N.C.H.; Chung, E.S.; Meehl, C.M.; Sayles, N.M.; Passerini, V.; Storchova, Z.; Amon, A. Single-chromosome gains commonly function as tumor suppressors. Cancer Cell 2017, 31, 240–255. [Google Scholar] [CrossRef]
- Williams, B.R.; Prabhu, V.R.; Hunter, K.E.; Glazier, C.M.; Whittaker, C.A.; Housman, D.E.; Amon, A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science 2008, 322, 703–709. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Au, B.; Kulkarni, M.; Shen, Y.; Lim, K.J.; Maimaiti, J.; Wong, C.K.; Luijten, M.N.; Chong, H.C.; Lim, E.H. Chromosomal instability-induced senescence potentiates cell non-autonomous tumourigenic effects. Oncogenesis 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Andriani, G.A.; Almeida, V.P.; Faggioli, F.; Mauro, M.; Tsai, W.L.; Santambrogio, L.; Maslov, A.; Gadina, M.; Campisi, J.; Vijg, J. Whole Chromosome Instability induces senescence and promotes SASP. Sci. Rep. 2016, 6, 35218. [Google Scholar] [CrossRef]
- Elizalde, S.; Laughney, A.M.; Bakhoum, S.F. A Markov chain for numerical chromosomal instability in clonally expanding populations. PLoS Comput. Biol. 2018, 14, e1006447. [Google Scholar] [CrossRef]
- Habermann, J.K.; Doering, J.; Hautaniemi, S.; Roblick, U.J.; Bündgen, N.K.; Nicorici, D.; Kronenwett, U.; Rathnagiriswaran, S.; Mettu, R.K.; Ma, Y. The gene expression signature of genomic instability in breast cancer is an independent predictor of clinical outcome. Int. J. Cancer 2009, 124, 1552–1564. [Google Scholar] [CrossRef]
- Kronenwett, U.; Ploner, A.; Zetterberg, A.; Bergh, J.; Hall, P.; Auer, G.; Pawitan, Y. Genomic instability and prognosis in breast carcinomas. Cancer Epidemiol. Prev. Biomark. 2006, 15, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; A’hern, R.; Birkbak, N.; Gorman, P.; Grönroos, E.; Ngang, S.; Nicola, P.; Rahman, L.; Thanopoulou, E.; Kelly, G. Extreme chromosomal instability forecasts improved outcome in ER-negative breast cancer: A prospective validation cohort study from the TACT trial. Ann. Oncol. 2015, 26, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Roylance, R.; Endesfelder, D.; Gorman, P.; Burrell, R.A.; Sander, J.; Tomlinson, I.; Hanby, A.M.; Speirs, V.; Richardson, A.L.; Birkbak, N.J. Relationship of extreme chromosomal instability with long-term survival in a retrospective analysis of primary breast cancer. Cancer Epidemiol. Prev. Biomark. 2011, 20, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Birkbak, N.J.; Eklund, A.C.; Li, Q.; McClelland, S.E.; Endesfelder, D.; Tan, P.; Tan, I.B.; Richardson, A.L.; Szallasi, Z.; Swanton, C. Paradoxical relationship between chromosomal instability and survival outcome in cancer. Cancer Res. 2011, 71, 3447–3452. [Google Scholar] [CrossRef]
- Sansregret, L.; Vanhaesebroeck, B.; Swanton, C. Determinants and clinical implications of chromosomal instability in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 139. [Google Scholar] [CrossRef]
- Janssen, A.; Kops, G.J.; Medema, R.H. Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19108–19113. [Google Scholar] [CrossRef] [PubMed]
- Lukow, D.A.; Sausville, E.L.; Suri, P.; Chunduri, N.K.; Leu, J.; Kendall, J.; Wang, Z.; Storchova, Z.; Sheltzer, J.M. Chromosomal instability accelerates the evolution of resistance to anti-cancer therapies. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ippolito, M.R.; Martis, V.; Hong, C.; Wardenaar, R.; Zerbib, J.; Spierings, D.C.; Ben-David, U.; Foijer, F.; Santaguida, S. Aneuploidy-driven genome instability triggers resistance to chemotherapy. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bergman, A.; Gligorijevic, B. Niche construction game cancer cells play. Eur. Phys. J. Plus 2015, 130, 203. [Google Scholar] [CrossRef]
- Day, R.L.; Laland, K.N.; Odling-Smee, F.J. Rethinking adaptation: The niche-construction perspective. Perspect. Biol. Med. 2003, 46, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Hashim, A.; Gillies, R.J.; Brown, J.S.; Gatenby, R.A. Coevolution of tumor cells and their microenvironment:“niche construction in cancer”. In Ecology and Evolution of Cancer; Elsevier: Amsterdam, The Netherlands, 2017; pp. 111–117. [Google Scholar]
- Han, X.; Hui, C. Niche construction on environmental gradients: The formation of fitness valley and stratified genotypic distributions. PLoS ONE 2014, 9, e99775. [Google Scholar] [CrossRef]
- Matthews, B.; De Meester, L.; Jones, C.G.; Ibelings, B.W.; Bouma, T.J.; Nuutinen, V.; Van De Koppel, J.; Odling-Smee, J. Under niche construction: An operational bridge between ecology, evolution, and ecosystem science. Ecol. Monogr. 2014, 84, 245–263. [Google Scholar] [CrossRef]
- Hui, C.; Li, Z.; Yue, D.-X. Metapopulation dynamics and distribution, and environmental heterogeneity induced by niche construction. Ecol. Model. 2004, 177, 107–118. [Google Scholar] [CrossRef]
- Han, X.; Chen, B.; Hui, C. Symmetry breaking in cyclic competition by niche construction. Appl. Math. Comput. 2016, 284, 66–78. [Google Scholar] [CrossRef]
- Merlo, L.M.; Pepper, J.W.; Reid, B.J.; Maley, C.C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 2006, 6, 924–935. [Google Scholar] [CrossRef]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306. [Google Scholar] [CrossRef]
- Rutledge, S.D.; Cimini, D. Consequences of aneuploidy in sickness and in health. Curr. Opin. Cell Biol. 2016, 40, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, S.; Amon, A. Short-and long-term effects of chromosome mis-segregation and aneuploidy. Nat. Rev. Mol. Cell Biol. 2015, 16, 473. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tsai, H.-J.; Gordon, M.R.; Li, R. Cellular stress associated with aneuploidy. Dev. Cell 2018, 44, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.A. Coevolution in the tumor microenvironment. Nat. Genet. 2008, 40, 494. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, N.; Passerini, V.; Durrbaum, M.; Stingele, S.; Storchova, Z. HSF1 deficiency and impaired HSP90-dependent protein folding are hallmarks of aneuploid human cells. EMBO J. 2014, 33, 2374–2387. [Google Scholar] [CrossRef]
- Donnelly, N.; Storchova, Z. Aneuploidy and proteotoxic stress in cancer. Mol. Cell Oncol. 2015, 2, e976491. [Google Scholar] [CrossRef] [PubMed]
- Oromendia, A.B.; Dodgson, S.E.; Amon, A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 2012, 26, 2696–2708. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Silberman, P.C.; Rutkowski, M.R.; Chopra, S.; Perales-Puchalt, A.; Song, M.; Zhang, S.; Bettigole, S.E.; Gupta, D.; Holcomb, K. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 2015, 161, 1527–1538. [Google Scholar] [CrossRef]
- Mahadevan, N.R.; Rodvold, J.; Sepulveda, H.; Rossi, S.; Drew, A.F.; Zanetti, M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc. Natl. Acad. Sci. USA 2011, 108, 6561–6566. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, N.R.; Anufreichik, V.; Rodvold, J.J.; Chiu, K.T.; Sepulveda, H.; Zanetti, M. Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8+ T cell priming. PLoS ONE 2012, 7, e51845. [Google Scholar] [CrossRef]
- Xian, S.; Searles, S.; Sahani, P.; Waller, T.C.; Jepsen, K.; Carter, H.; Zanetti, M. The unfolded protein response links tumor aneuploidy to local immune dysregulation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Newman, D.L.; Gregory, S.L. Co-operation between aneuploidy and metabolic changes in driving tumorigenesis. Int. J. Mol. Sci. 2019, 20, 4611. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fang, X.; Baker, D.J.; Guo, L.; Gao, X.; Wei, Z.; Han, S.; Van Deursen, J.M.; Zhang, P. The ATM–p53 pathway suppresses aneuploidy-induced tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 14188–14193. [Google Scholar] [CrossRef]
- Stingele, S.; Stoehr, G.; Peplowska, K.; Cox, J.; Mann, M.; Storchova, Z. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol. 2012, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-C.; Yuwen, H.; Wang, K.; Bruno, P.M.; Bullock, K.; Deik, A.; Santaguida, S.; Trakala, M.; Pfau, S.J.; Zhong, N. Aneuploid cell survival relies upon sphingolipid homeostasis. Cancer Res. 2017, 77, 5272–5286. [Google Scholar] [CrossRef]
- Biczowa, B.; Kieler, J.; Moore, J. Comparative studies of a near-tetraploid and a near-diploid line of Ehrlich’s ascites tumor propagated in vivo and in vitro I. Metabolism and growth. Eur. J. Cancer (1965) 1968, 4, 67–79. [Google Scholar] [CrossRef]
- Anatskaya, O.V.; Vinogradov, A.E. Genome multiplication as adaptation to tissue survival: Evidence from gene expression in mammalian heart and liver. Genomics 2007, 89, 70–80. [Google Scholar] [CrossRef]
- Sheltzer, J.M. A transcriptional and metabolic signature of primary aneuploidy is present in chromosomally unstable cancer cells and informs clinical prognosis. Cancer Res. 2013, 73, 6401–6412. [Google Scholar] [CrossRef]
- Torres, E.M.; Sokolsky, T.; Tucker, C.M.; Chan, L.Y.; Boselli, M.; Dunham, M.J.; Amon, A. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 2007, 317, 916–924. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef]
- Beaty, B.T.; Wang, Y.; Bravo-Cordero, J.J.; Sharma, V.P.; Miskolci, V.; Hodgson, L.; Condeelis, J. Talin regulates moesin-NHE-1 recruitment to invadopodia and promotes mammary tumor metastasis. J. Cell Biol. 2014, 205, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Huber, V.; Camisaschi, C.; Berzi, A.; Ferro, S.; Lugini, L.; Triulzi, T.; Tuccitto, A.; Tagliabue, E.; Castelli, C.; Rivoltini, L. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. 2017, 43, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.R.; Danai, L.V.; Lewis, C.A.; Chan, S.H.; Gui, D.Y.; Kunchok, T.; Dennstedt, E.A.; Vander Heiden, M.G.; Muir, A. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife 2019, 8, e44235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Zhou, Y.; He, Q.-Y. Jolkinolide B induces apoptosis of colorectal carcinoma through ROS-ER stress-Ca2+-mitochondria dependent pathway. Oncotarget 2017, 8, 91223. [Google Scholar] [CrossRef]
- Newman, D.L.; Thurgood, L.A.; Gregory, S.L. The impact of aneuploidy on cellular homeostasis. Free Radic. Res. 2019, 53, 705–713. [Google Scholar] [CrossRef]
- Dephoure, N.; Hwang, S.; O’Sullivan, C.; Dodgson, S.E.; Gygi, S.P.; Amon, A.; Torres, E.M. Quantitative proteomic analysis reveals posttranslational responses to aneuploidy in yeast. Elife 2014, 3, e03023. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, Z.; Liu, D.; Choo, A.; Hussain, R.; O’Keefe, L.; Richards, R.; Saint, R.; Gregory, S. Chromosomal instability causes sensitivity to metabolic stress. Oncogene 2015, 34, 4044–4055. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive oxygen species in the tumor microenvironment: An overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef]
- Liao, Z.; Chua, D.; Tan, N.S. Reactive oxygen species: A volatile driver of field cancerization and metastasis. Mol. Cancer 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-G.; Ahn, J.-H.; Choi, H.-S.; Lee, J.-H. DNA double-strand breaks and Aurora B mislocalization induced by exposure of early mitotic cells to H2O2 appear to increase chromatin bridges and resultant cytokinesis failure. Free Radic. Biol. Med. 2017, 108, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Balliet, R.M.; Rivadeneira, D.; Chiavarina, B.; Pavlides, S.; Wang, C.; Whitaker-Menezes, D.; Daumer, K.; Lin, Z.; Witkiewicz, A. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 2010, 9, 3276–3296. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Schoppmann, S.F.; Birner, P.; Stöckl, J.; Kalt, R.; Ullrich, R.; Caucig, C.; Kriehuber, E.; Nagy, K.; Alitalo, K.; Kerjaschki, D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002, 161, 947–956. [Google Scholar] [CrossRef]
- Zimmerman, M.A.; Huang, Q.; Li, F.; Liu, X.; Li, C.-Y. Cell death–stimulated cell proliferation: A tissue regeneration mechanism usurped by tumors during radiotherapy. Semin. Radiat. Oncol. 2013, 23, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Garijo, A.; Steller, H. Spreading the word: Non-autonomous effects of apoptosis during development, regeneration and disease. Development 2015, 142, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 99–126. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, G.; Zhang, P.; Fan, J. Programmed cell death and its role in inflammation. Mil. Med. Res. 2015, 2, 12. [Google Scholar] [CrossRef]
- Liu, S.; Edgerton, S.M.; Moore, D.H.; Thor, A.D. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin. Cancer Res. 2001, 7, 1716–1723. [Google Scholar] [PubMed]
- Soini, Y.; Pääkkö, P.; Lehto, V. Histopathological evaluation of apoptosis in cancer. Am. J. Pathol. 1998, 153, 1041. [Google Scholar] [CrossRef]
- Dou, Z.; Ghosh, K.; Vizioli, M.G.; Zhu, J.; Sen, P.; Wangensteen, K.J.; Simithy, J.; Lan, Y.; Lin, Y.; Zhou, Z. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 2017, 550, 402–406. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Freund, A.; Orjalo, A.V.; Desprez, P.-Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, Y.W.; Lee, J.; Soh, E.Y.; Kim, J.-H.; Park, T.J. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Wangsa, D.; Quintanilla, I.; Torabi, K.; Vila-Casadesús, M.; Ercilla, A.; Klus, G.; Yuce, Z.; Galofré, C.; Cuatrecasas, M.; Lozano, J.J. Near-tetraploid cancer cells show chromosome instability triggered by replication stress and exhibit enhanced invasiveness. Faseb J. 2018, 32, 3502–3517. [Google Scholar] [CrossRef]
- Mackenzie, K.J.; Carroll, P.; Martin, C.-A.; Murina, O.; Fluteau, A.; Simpson, D.J.; Olova, N.; Sutcliffe, H.; Rainger, J.K.; Leitch, A. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017, 548, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Xia, T.; Konno, H.; Konno, K.; Ruiz, P.; Barber, G.N. Inflammation-driven carcinogenesis is mediated through STING. Nat. Commun. 2014, 5, 5166. [Google Scholar] [CrossRef] [PubMed]
- Rathje, L.-S.Z.; Nordgren, N.; Pettersson, T.; Rönnlund, D.; Widengren, J.; Aspenström, P.; Gad, A.K. Oncogenes induce a vimentin filament collapse mediated by HDAC6 that is linked to cell stiffness. Proc. Natl. Acad. Sci. USA 2014, 111, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Strouhalova, K.; Přechová, M.; Gandalovičová, A.; Brábek, J.; Gregor, M.; Rosel, D. Vimentin intermediate filaments as potential target for cancer treatment. Cancers 2020, 12, 184. [Google Scholar] [CrossRef]
- Northey, J.J.; Przybyla, L.; Weaver, V.M. Tissue force programs cell fate and tumor aggression. Cancer Discov. 2017, 7, 1224–1237. [Google Scholar] [CrossRef]
- Vasudevan, A.; Baruah, P.S.; Smith, J.C.; Wang, Z.; Sayles, N.M.; Andrews, P.; Kendall, J.; Leu, J.; Chunduri, N.K.; Levy, D. Single-chromosomal gains can function as metastasis suppressors and promoters in colon cancer. Dev. Cell 2020, 52, 413–428.e416. [Google Scholar] [CrossRef] [PubMed]
- Flynn, P.J.; Koch, P.D.; Mitchison, T.J. Chromatin Bridges, not Micronuclei, Activate cGAS after Drug-induced Mitotic Errors in Human Cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ghadimi, B.M.; Sackett, D.L.; Difilippantonio, M.J.; Schrock, E.; Neumann, T.; Jauho, A.; Auer, G.; Ried, T. Centrosome amplification and instability occurs exclusively in aneuploid, but not in diploid colorectal cancer cell lines, and correlates with numerical chromosomal aberrations. Genes Chromosomes Cancer 2000, 27, 183–190. [Google Scholar] [CrossRef]
- Passerini, V.; Ozeri-Galai, E.; De Pagter, M.S.; Donnelly, N.; Schmalbrock, S.; Kloosterman, W.P.; Kerem, B.; Storchová, Z. The presence of extra chromosomes leads to genomic instability. Nat. Commun. 2016, 7, 10754. [Google Scholar] [CrossRef]
- Duesberg, P. Are centrosomes or aneuploidy the key to cancer? Science 1999, 284, 2091–2092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chan, J.Y. A clinical overview of centrosome amplification in human cancers. Int. J. Biol. Sci 2011, 7, 1122–1144. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.S.; Bakker, B.; Boeckx, B.; Moyett, J.; Lu, J.; Vitre, B.; Spierings, D.C.; Lansdorp, P.M.; Cleveland, D.W.; Lambrechts, D.; et al. Centrosome Amplification Is Sufficient to Promote Spontaneous Tumorigenesis in Mammals. Dev. Cell 2017, 40, 313–322.e315. [Google Scholar] [CrossRef]
- Sercin, O.; Larsimont, J.C.; Karambelas, A.E.; Marthiens, V.; Moers, V.; Boeckx, B.; Le Mercier, M.; Lambrechts, D.; Basto, R.; Blanpain, C. Transient PLK4 overexpression accelerates tumorigenesis in p53-deficient epidermis. Nat. Cell Biol. 2016, 18, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Ganier, O.; Schnerch, D.; Oertle, P.; Lim, R.Y.; Plodinec, M.; Nigg, E.A. Structural centrosome aberrations promote non-cell-autonomous invasiveness. EMBO J. 2018, 37, e98576. [Google Scholar] [CrossRef]
- Godinho, S.A.; Picone, R.; Burute, M.; Dagher, R.; Su, Y.; Leung, C.T.; Polyak, K.; Brugge, J.S.; Thery, M.; Pellman, D. Oncogene-like induction of cellular invasion from centrosome amplification. Nature 2014, 510, 167–171. [Google Scholar] [CrossRef]
- Arnandis, T.; Monteiro, P.; Adams, S.D.; Bridgeman, V.L.; Rajeeve, V.; Gadaleta, E.; Marzec, J.; Chelala, C.; Malanchi, I.; Cutillas, P.R. Oxidative stress in cells with extra centrosomes drives non-cell-autonomous invasion. Dev. Cell 2018, 47, 409–424.e409. [Google Scholar] [CrossRef]
- Potapova, T.A.; Seidel, C.W.; Box, A.C.; Rancati, G.; Li, R. Transcriptome analysis of tetraploid cells identifies cyclin D2 as a facilitator of adaptation to genome doubling in the presence of p53. Mol. Biol. Cell 2016, 27, 3065–3084. [Google Scholar] [CrossRef]
- Akino, T.; Hida, K.; Hida, Y.; Tsuchiya, K.; Freedman, D.; Muraki, C.; Ohga, N.; Matsuda, K.; Akiyama, K.; Harabayashi, T. Cytogenetic abnormalities of tumor-associated endothelial cells in human malignant tumors. Am. J. Pathol. 2009, 175, 2657–2667. [Google Scholar] [CrossRef]
- Hida, K.; Hida, Y.; Amin, D.N.; Flint, A.F.; Panigrahy, D.; Morton, C.C.; Klagsbrun, M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004, 64, 8249–8255. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, M.; Ohga, N.; Akiyama, K.; Hida, Y.; Maishi, N.; Towfik, A.M.; Inoue, N.; Shindoh, M.; Hida, K. Hypoxia-induced reactive oxygen species cause chromosomal abnormalities in endothelial cells in the tumor microenvironment. PLoS ONE 2013, 8, e80349. [Google Scholar] [CrossRef]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.M.; Baluk, P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002, 62, 5381–5385. [Google Scholar] [PubMed]
- Corver, W.E.; ter Haar, N.T.; Fleuren, G.J.; Oosting, J. Cervical carcinoma-associated fibroblasts are DNA diploid and do not show evidence for somatic genetic alterations. Cell. Oncol. 2011, 34, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, Y.; Zhou, H.; Chen, Q.; Li, B. Analysis of chromosome karyotype of oral carcinoma-associated Fibroblasts. West. China J. Stomatol. 2005, 23, 159–160. [Google Scholar]
- Dudley, A.C.; Shih, S.-C.; Cliffe, A.R.; Hida, K.; Klagsbrun, M. Attenuated p53 activation in tumour-associated stromal cells accompanies decreased sensitivity to etoposide and vincristine. Br. J. Cancer 2008, 99, 118–125. [Google Scholar] [CrossRef]
- Pelham, R.J.; Rodgers, L.; Hall, I.; Lucito, R.; Nguyen, K.C.; Navin, N.; Hicks, J.; Mu, D.; Powers, S.; Wigler, M. Identification of alterations in DNA copy number in host stromal cells during tumor progression. Proc. Natl. Acad. Sci. USA 2006, 103, 19848–19853. [Google Scholar] [CrossRef]
- Tuhkanen, H.; Anttila, M.; Kosma, V.M.; Heinonen, S.; Juhola, M.; Helisalmi, S.; Kataja, V.; Mannermaa, A. Frequent gene dosage alterations in stromal cells of epithelial ovarian carcinomas. Int. J. Cancer 2006, 119, 1345–1353. [Google Scholar] [CrossRef]
- Fukino, K.; Shen, L.; Matsumoto, S.; Morrison, C.D.; Mutter, G.L.; Eng, C. Combined total genome loss of heterozygosity scan of breast cancer stroma and epithelium reveals multiplicity of stromal targets. Cancer Res. 2004, 64, 7231–7236. [Google Scholar] [CrossRef]
- Fukino, K.; Shen, L.; Patocs, A.; Mutter, G.L.; Eng, C. Genomic instability within tumor stroma and clinicopathological characteristics of sporadic primary invasive breast carcinoma. JAMA 2007, 297, 2103–2111. [Google Scholar] [CrossRef]
- Groß, O.; Brummer, T.; Zeiser, R. Immune modulatory effects of oncogenic KRAS in cancer. Nat. Commun. 2020, 11, 5439. [Google Scholar]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Yang, T.-H.O.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A. The immune landscape of cancer. Immunity 2018, 48, 812–830.e814. [Google Scholar] [CrossRef]
- Ohashi, A.; Ohori, M.; Iwai, K.; Nakayama, Y.; Nambu, T.; Morishita, D.; Kawamoto, T.; Miyamoto, M.; Hirayama, T.; Okaniwa, M. Aneuploidy generates proteotoxic stress and DNA damage concurrently with p53-mediated post-mitotic apoptosis in SAC-impaired cells. Nat. Commun. 2015, 6, 7668. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Richardson, A.; Diaz-Meco, M.T. Nutrient stress revamps cancer cell metabolism. Cell Res. 2015, 25, 537–538. [Google Scholar] [CrossRef][Green Version]
- Choi, S.Y.C.; Collins, C.C.; Gout, P.W.; Wang, Y. Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? J. Pathol. 2013, 230, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Kumari, G.; Ulrich, T.; Krause, M.; Finkernagel, F.; Gaubatz, S. Induction of p21CIP1 protein and cell cycle arrest after inhibition of Aurora B kinase is attributed to aneuploidy and reactive oxygen species. J. Biol. Chem. 2014, 289, 16072–16084. [Google Scholar] [CrossRef]
- Odling-Smee, J.; Erwin, D.H.; Palkovacs, E.P.; Feldman, M.W.; Laland, K.N. Niche construction theory: A practical guide for ecologists. Q. Rev. Biol. 2013, 88, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, N.; Rancati, G.; Zhu, J.; Bradford, W.D.; Saraf, A.; Florens, L.; Sanderson, B.W.; Hattem, G.L.; Li, R. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature 2010, 468, 321. [Google Scholar] [CrossRef]
- Rancati, G.; Pavelka, N. Karyotypic changes as drivers and catalyzers of cellular evolvability: A perspective from non-pathogenic yeasts. Semin. Cell Dev. Biol. 2013, 24, 332–338. [Google Scholar] [CrossRef]
- Chang, S.-L.; Lai, H.-Y.; Tung, S.-Y.; Leu, J.-Y. Dynamic large-scale chromosomal rearrangements fuel rapid adaptation in yeast populations. PLoS Genet. 2013, 9, e1003232. [Google Scholar] [CrossRef] [PubMed]
- Selmecki, A.; Forche, A.; Berman, J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 2006, 313, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Yona, A.H.; Manor, Y.S.; Herbst, R.H.; Romano, G.H.; Mitchell, A.; Kupiec, M.; Pilpel, Y.; Dahan, O. Chromosomal duplication is a transient evolutionary solution to stress. Proc. Natl. Acad. Sci. USA 2012, 109, 21010–21015. [Google Scholar] [CrossRef]
- Chen, G.; Bradford, W.D.; Seidel, C.W.; Li, R. Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy. Nature 2012, 482, 246. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Crowther, J.; Pastor, T.; Asbagh, L.A.; Baietti, M.F.; De Troyer, M.; Vazquez, I.; Talebi, A.; Renzi, F.; Dehairs, J. Loss of chromosome 8p governs tumor progression and drug response by altering lipid metabolism. Cancer Cell 2016, 29, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Kitzing, T.; Roessler, S.; Zuber, J.; Krasnitz, A.; Schultz, N.; Revill, K.; Weissmueller, S.; Rappaport, A.R.; Simon, J. A cluster of cooperating tumor-suppressor gene candidates in chromosomal deletions. Proc. Natl. Acad. Sci. USA 2012, 109, 8212–8217. [Google Scholar] [CrossRef]
- Dai, C.; Sun, F.; Zhu, C.; Hu, X. Tumor environmental factors glucose deprivation and lactic acidosis induce mitotic chromosomal instability–an implication in aneuploid human tumors. PLoS ONE 2013, 8, e63054. [Google Scholar] [CrossRef] [PubMed]
- Ried, T.; Knutzen, R.; Steinbeck, R.; Blegen, H.; Schröck, E.; Heselmeyer, K.; du Manoir, S.; Auer, G. Comparative genomic hybridization reveals a specific pattern of chromosomal gains and losses during the genesis of colorectal tumors. Geneschromosomes Cancer 1996, 15, 234–245. [Google Scholar] [CrossRef]
- Weihua, Z.; Tsan, R.; Huang, W.-C.; Wu, Q.; Chiu, C.-H.; Fidler, I.J.; Hung, M.-C. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 2008, 13, 385–393. [Google Scholar] [CrossRef]
- Shukla, A.; Nguyen, T.H.; Moka, S.B.; Ellis, J.J.; Grady, J.P.; Oey, H.; Cristino, A.S.; Khanna, K.K.; Kroese, D.P.; Krause, L. Chromosome arm aneuploidies shape tumour evolution and drug response. Nat. Commun. 2020, 11, 449. [Google Scholar] [CrossRef]
- Ravichandran, M.C.; Fink, S.; Clarke, M.N.; Hofer, F.C.; Campbell, C.S. Genetic interactions between specific chromosome copy number alterations dictate complex aneuploidy patterns. Genes Dev. 2018, 32, 1485–1498. [Google Scholar] [CrossRef]
- Raftopoulou, C.; Roumelioti, F.-M.; Dragona, E.; Gimelli, S.; Sloan-Béna, F.; Gorgoulis, V.; Antonarakis, S.E.; Gagos, S. Karyotypic Flexibility of the Complex Cancer Genome and the Role of Polyploidization in Maintenance of Structural Integrity of Cancer Chromosomes. Cancers 2020, 12, 591. [Google Scholar] [CrossRef] [PubMed]
- Anatskaya, O.V.; Vinogradov, A.E. Somatic polyploidy promotes cell function under stress and energy depletion: Evidence from tissue-specific mammal transcriptome. Funct. Integr. Genom. 2010, 10, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Schoenfelder, K.P.; Fox, D.T. The expanding implications of polyploidy. J. Cell Biol. 2015, 209, 485–491. [Google Scholar] [CrossRef]
- Galofré, C.; Gönül Geyik, Ö.; Asensio, E.; Wangsa, D.; Hirsch, D.; Parra, C.; Saez, J.; Mollà, M.; Yüce, Z.; Castells, A. Tetraploidy-associated genetic heterogeneity confers chemo-radiotherapy resistance to colorectal cancer cells. Cancers 2020, 12, 1118. [Google Scholar] [CrossRef]
- Coward, J.; Harding, A. Size does matter: Why polyploid tumor cells are critical drug targets in the war on cancer. Front. Oncol. 2014, 4, 123. [Google Scholar] [CrossRef] [PubMed]
- Illidge, T.M.; Cragg, M.S.; Fringes, B.; Olive, P.; Erenpreisa, J.A. Polyploid giant cells provide a survival mechanism for p53 mutant cells after DNA damage. Cell Biol. Int. 2000, 24, 621–633. [Google Scholar] [CrossRef]
- Donovan, P.; Cato, K.; Legaie, R.; Jayalath, R.; Olsson, G.; Hall, B.; Olson, S.; Boros, S.; Reynolds, B.A.; Harding, A. Hyperdiploid tumor cells increase phenotypic heterogeneity within Glioblastoma tumors. Mol. Biosyst. 2014, 10, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Deleyrolle, L.P.; Harding, A.; Cato, K.; Siebzehnrubl, F.A.; Rahman, M.; Azari, H.; Olson, S.; Gabrielli, B.; Osborne, G.; Vescovi, A. Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain 2011, 134, 1331–1343. [Google Scholar] [CrossRef]
- Sundaram, M.; Guernsey, D.L.; Rajaraman, M.M.; Rajaraman, R. Neosis: A novel type of cell division in cancer. Cancer Biol. Ther. 2004, 3, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Puig, P.E.; Guilly, M.N.; Bouchot, A.; Droin, N.; Cathelin, D.; Bouyer, F.; Favier, L.; Ghiringhelli, F.; Kroemer, G.; Solary, E. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol. Int. 2008, 32, 1031–1043. [Google Scholar] [CrossRef]
- Zhang, S.; Mercado-Uribe, I.; Xing, Z.; Sun, B.; Kuang, J.; Liu, J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene 2014, 33, 116–128. [Google Scholar] [CrossRef]
- Quinton, R.J.; DiDomizio, A.; Vittoria, M.A.; Kotýnková, K.; Ticas, C.J.; Patel, S.; Koga, Y.; Vakhshoorzadeh, J.; Hermance, N.; Kuroda, T.S. Whole-genome doubling confers unique genetic vulnerabilities on tumour cells. Nature 2021, 590, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Sharir, Y.; McFarland, J.M.; Abdusamad, M.; Marquis, C.; Bernhard, S.V.; Kazachkova, M.; Tang, H.; Ippolito, M.R.; Laue, K.; Zerbib, J. Aneuploidy renders cancer cells vulnerable to mitotic checkpoint inhibition. Nature 2021, 590, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Ohga, N.; Ishikawa, S.; Maishi, N.; Akiyama, K.; Hida, Y.; Kawamoto, T.; Sadamoto, Y.; Osawa, T.; Yamamoto, K.; Kondoh, M. Heterogeneity of tumor endothelial cells: Comparison between tumor endothelial cells isolated from high-and low-metastatic tumors. Am. J. Pathol. 2012, 180, 1294–1307. [Google Scholar] [CrossRef]
- Hida, K.; Hida, Y.; Shindoh, M. Understanding tumor endothelial cell abnormalities to develop ideal anti-angiogenic therapies. Cancer Sci. 2008, 99, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Ohga, N.; Akiyama, K.; Maishi, N.; Hida, Y. Heterogeneity of tumor endothelial cells. Cancer Sci. 2013, 104, 1391–1395. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, N.; Yu, X.; Zhang, X.; Li, S.; Lei, Z.; Hu, R.; Li, H.; Mao, Y.; Wang, X. Tumacrophage: Macrophages transformed into tumor stem-like cells by virulent genetic material from tumor cells. Oncotarget 2017, 8, 82326. [Google Scholar] [CrossRef] [PubMed]
- Clawson, G.A.; Matters, G.L.; Xin, P.; Imamura-Kawasawa, Y.; Du, Z.; Thiboutot, D.M.; Helm, K.F.; Neves, R.I.; Abraham, T. Macrophage-tumor cell fusions from peripheral blood of melanoma patients. PLoS ONE 2015, 10, e0134320. [Google Scholar] [CrossRef]
- Bottoni, G.; Katarkar, A.; Tassone, B.; Ghosh, S.; Clocchiatti, A.; Goruppi, S.; Bordignon, P.; Jafari, P.; Tordini, F.; Lunardi, T. CSL controls telomere maintenance and genome stability in human dermal fibroblasts. Nat. Commun. 2019, 10, 3884. [Google Scholar] [CrossRef] [PubMed]
- Katarkar, A.; Bottoni, G.; Clocchiatti, A.; Goruppi, S.; Bordignon, P.; Lazzaroni, F.; Gregnanin, I.; Ostano, P.; Neel, V.; Dotto, G.P. NOTCH1 gene amplification promotes expansion of Cancer Associated Fibroblast populations in human skin. Nat. Commun. 2020, 11, 5126. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, S.; Richardson, A.; Iyer, D.R.; M’Saad, O.; Zasadil, L.; Knouse, K.A.; Wong, Y.L.; Rhind, N.; Desai, A.; Amon, A. Chromosome Mis-segregation Generates Cell-Cycle-Arrested Cells with Complex Karyotypes that Are Eliminated by the Immune System. Dev. Cell 2017, 41, 638–651.e5. [Google Scholar] [CrossRef] [PubMed]
- Senovilla, L.; Vitale, I.; Martins, I.; Tailler, M.; Pailleret, C.; Michaud, M.; Galluzzi, L.; Adjemian, S.; Kepp, O.; Niso-Santano, M.; et al. An immunosurveillance mechanism controls cancer cell ploidy. Science 2012, 337, 1678–1684. [Google Scholar] [CrossRef]
- Watson, E.V.; Elledge, S.J. Aneuploidy Police Detect Chromosomal Imbalance Triggering Immune Crackdown! Trends Genet. 2017, 33, 662–664. [Google Scholar] [CrossRef]
- Davoli, T.; Uno, H.; Wooten, E.C.; Elledge, S.J. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 2017, 355. [Google Scholar] [CrossRef]
- Wang, R.W.; Viganò, S.; Ben-David, U.; Amon, A.; Santaguida, S. Aneuploid cells activate NF-κB to promote their immune clearance by NK cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- Granados, D.P.; Tanguay, P.L.; Hardy, M.P.; Caron, E.; de Verteuil, D.; Meloche, S.; Perreault, C. ER stress affects processing of MHC class I-associated peptides. Bmc Immunol. 2009, 10, 10. [Google Scholar] [CrossRef]
- Zanetti, M.; Rodvold, J.J.; Mahadevan, N.R. The evolving paradigm of cell-nonautonomous UPR-based regulation of immunity by cancer cells. Oncogene 2016, 35, 269–278. [Google Scholar] [CrossRef]
- Tripathi, R.; Modur, V.; Senovilla, L.; Kroemer, G.; Komurov, K. Suppression of tumor antigen presentation during aneuploid tumor evolution contributes to immune evasion. OncoImmunology 2019, 8, 1657374. [Google Scholar] [CrossRef]
- McGranahan, N.; Rosenthal, R.; Hiley, C.T.; Rowan, A.J.; Watkins, T.B.; Wilson, G.A.; Birkbak, N.J.; Veeriah, S.; Van Loo, P.; Herrero, J. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 2017, 171, 1259–1271.e1211. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Cadieux, E.L.; Salgado, R.; Al Bakir, M.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanić, M.; Reading, J.L.; Joshi, K. Neoantigen-directed immune escape in lung cancer evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Milo, I.; Bedora-Faure, M.; Garcia, Z.; Thibaut, R.; Périé, L.; Shakhar, G.; Deriano, L.; Bousso, P. The immune system profoundly restricts intratumor genetic heterogeneity. Sci. Immunol. 2018, 3, eaat1435. [Google Scholar] [CrossRef]
- Hatzikirou, H.; Basanta, D.; Simon, M.; Schaller, K.; Deutsch, A. ‘Go or grow’: The key to the emergence of invasion in tumour progression? Math. Med. Biol. A J. Ima 2012, 29, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Daoust, S.P.; Fahrig, L.; Martin, A.E.; Thomas, F. From forest and agro-ecosystems to the microecosystems of the human body: What can landscape ecology tell us about tumor growth, metastasis, and treatment options? Evol. Appl. 2013, 6, 82–91. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Nash, H.M.; McNeil, N.E.; Yi, M.; Nguyen, Q.T.; Hu, Y.; Wangsa, D.; Mack, D.L.; Hummon, A.B.; Case, C.; Cardin, E.; et al. Aneuploidy, oncogene amplification and epithelial to mesenchymal transition define spontaneous transformation of murine epithelial cells. Carcinogenesis 2013, 34, 1929–1939. [Google Scholar] [CrossRef]
- Gao, C.; Su, Y.; Koeman, J.; Haak, E.; Dykema, K.; Essenberg, C.; Hudson, E.; Petillo, D.; Khoo, S.K.; Vande Woude, G.F. Chromosome instability drives phenotypic switching to metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, 14793–14798. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Cantley, L.C. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell 2018, 174, 1347–1360. [Google Scholar] [CrossRef]
- Gebhart, E.; Liehr, T. Patterns of genomic imbalances in human solid tumors. Int. J. Oncol. 2000, 16, 383–482. [Google Scholar] [CrossRef] [PubMed]
- Auslander, N.; Heselmeyer-Haddad, K.; Patkar, S.; Hirsch, D.; Camps, J.; Brown, M.; Bronder, D.; Chen, W.-D.; Lokanga, R.; Wangsa, D. Cancer-type specific aneuploidies hard-wire chromosome-wide gene expression patterns of their tissue of origin. BioRxiv 2019, 563858. [Google Scholar] [CrossRef]
- Foley, J.W.; Zhu, C.; Jolivet, P.; Zhu, S.X.; Lu, P.; Meaney, M.J.; West, R.B. Gene expression profiling of single cells from archival tissue with laser-capture microdissection and Smart-3SEQ. Genome Res. 2019, 29, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Rozenblatt-Rosen, O.; Regev, A.; Oberdoerffer, P.; Nawy, T.; Hupalowska, A.; Rood, J.E.; Ashenberg, O.; Cerami, E.; Coffey, R.J.; Demir, E. The Human Tumor Atlas Network: Charting Tumor Transitions across Space and Time at Single-Cell Resolution. Cell 2020, 181, 236–249. [Google Scholar] [CrossRef]
- Natrajan, R.; Sailem, H.; Mardakheh, F.K.; Garcia, M.A.; Tape, C.J.; Dowsett, M.; Bakal, C.; Yuan, Y. Microenvironmental heterogeneity parallels breast cancer progression: A histology–genomic integration analysis. PLoS Med. 2016, 13, e1001961. [Google Scholar] [CrossRef] [PubMed]
- Meadows, D.H. Leverage Points: Places to Intervene in a System; Sustainability Institute: Hartland, VT, USA, 1999. [Google Scholar]
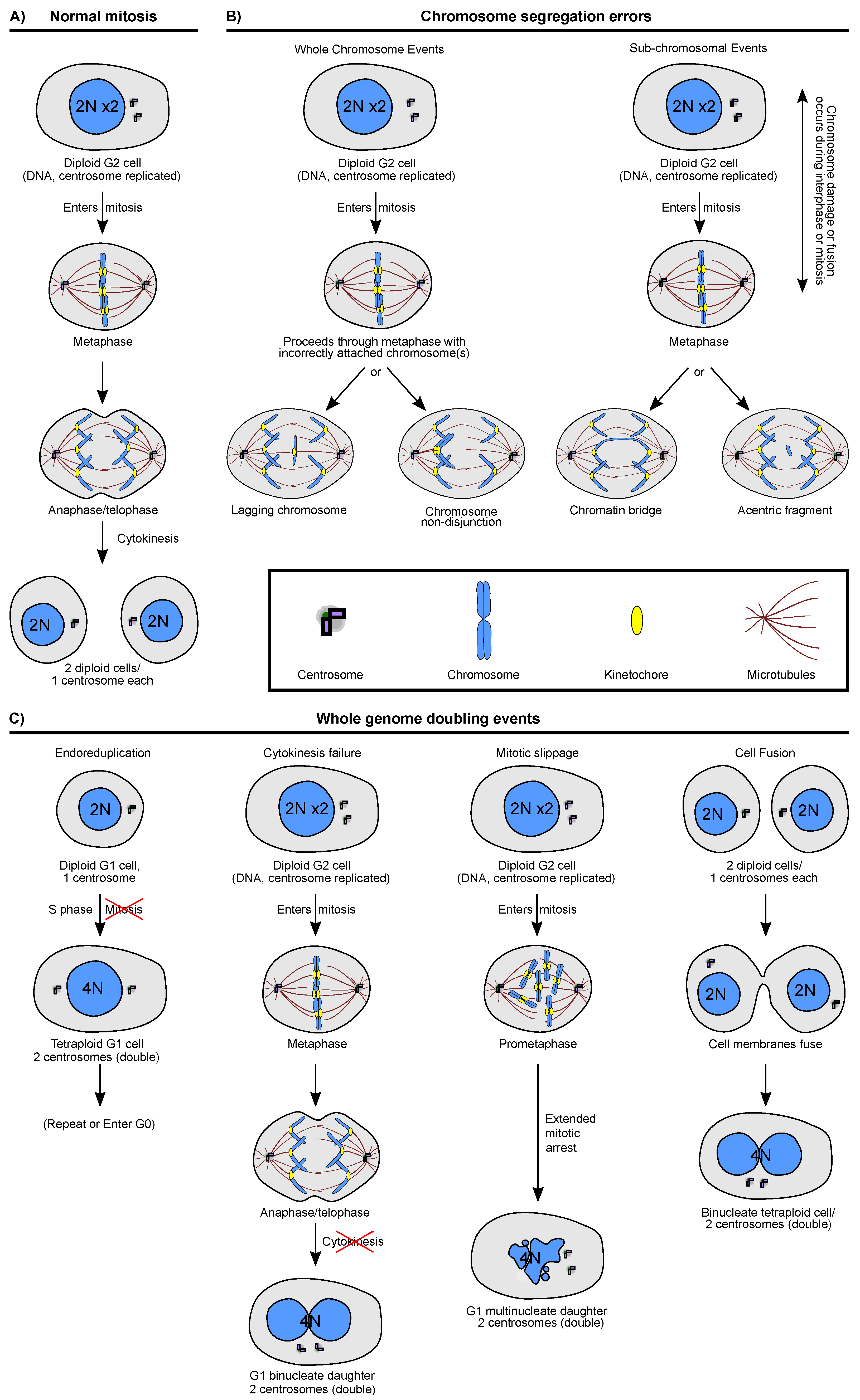
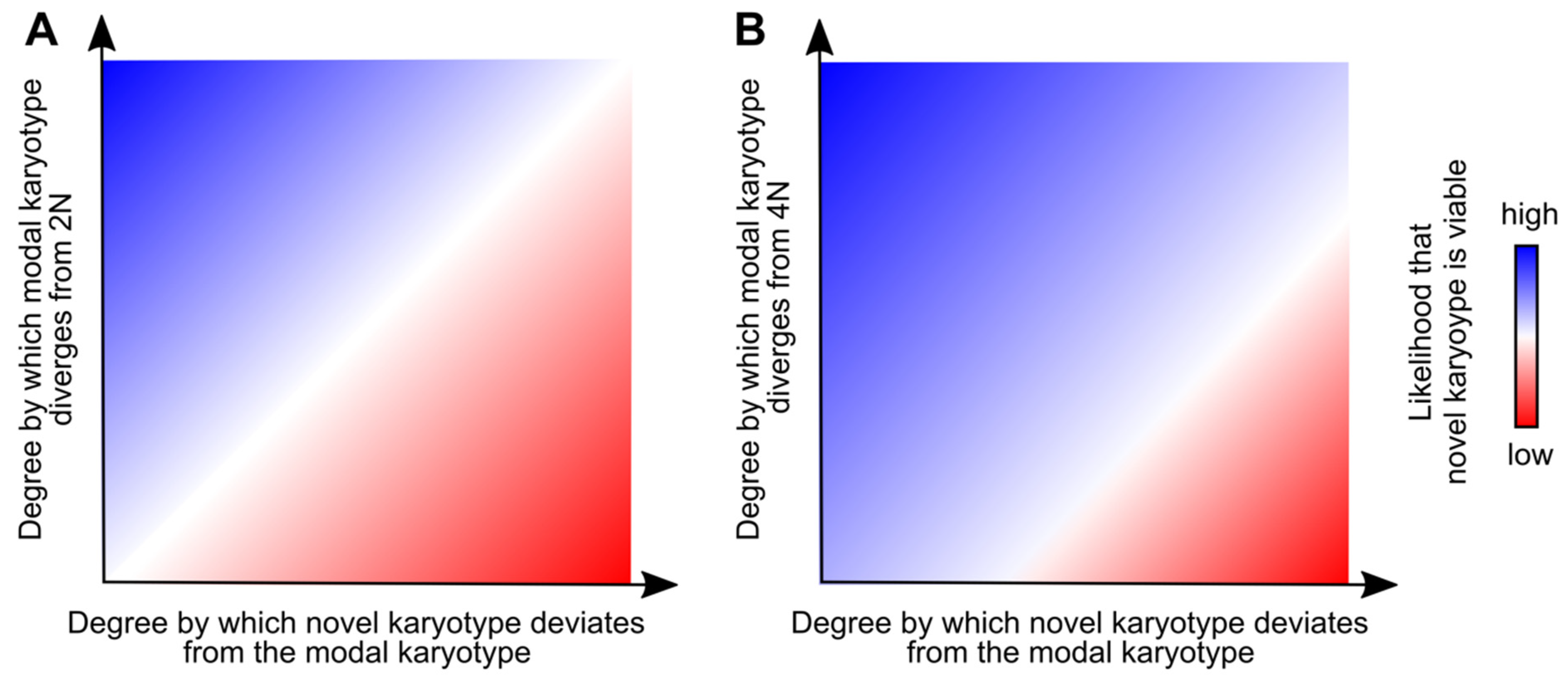

| Experimental System | Cellular Effect(s) | Influence of the Cellular Effect(s) on the TME |
|---|---|---|
| ||
| ||
| ||
| ||
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudoin, N.C.; Bloomfield, M. Karyotype Aberrations in Action: The Evolution of Cancer Genomes and the Tumor Microenvironment. Genes 2021, 12, 558. https://doi.org/10.3390/genes12040558
Baudoin NC, Bloomfield M. Karyotype Aberrations in Action: The Evolution of Cancer Genomes and the Tumor Microenvironment. Genes. 2021; 12(4):558. https://doi.org/10.3390/genes12040558
Chicago/Turabian StyleBaudoin, Nicolaas C., and Mathew Bloomfield. 2021. "Karyotype Aberrations in Action: The Evolution of Cancer Genomes and the Tumor Microenvironment" Genes 12, no. 4: 558. https://doi.org/10.3390/genes12040558
APA StyleBaudoin, N. C., & Bloomfield, M. (2021). Karyotype Aberrations in Action: The Evolution of Cancer Genomes and the Tumor Microenvironment. Genes, 12(4), 558. https://doi.org/10.3390/genes12040558






