Genetic Determinants of Neurobehavioral Responses to Caffeine Administration during Sleep Deprivation: A Randomized, Cross Over Study (NCT03859882)
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Testing Conditions
2.3. Caffeine Administration
2.4. Measurements
2.4.1. Psychomotor Vigilance Task (PVT) for Sustained Attention
2.4.2. Karolinska Sleeping Scale (KSS)
2.4.3. Visual Analogic Scales (VAS)
2.4.4. EEG Recording during PVT
EEG Procedure
EEG Analysis
2.4.5. Genotyping
2.5. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. Adverse Events (AEs)
3.3. Genotypes Repartition
3.4. Genetics and Inter-Individual Vulnerability to Caffeine Administration during Sleep Deprivation
3.4.1. Awakening (Total Sleep Deprivation—TSD) and Caffeine (Treatment—TRT) Effects
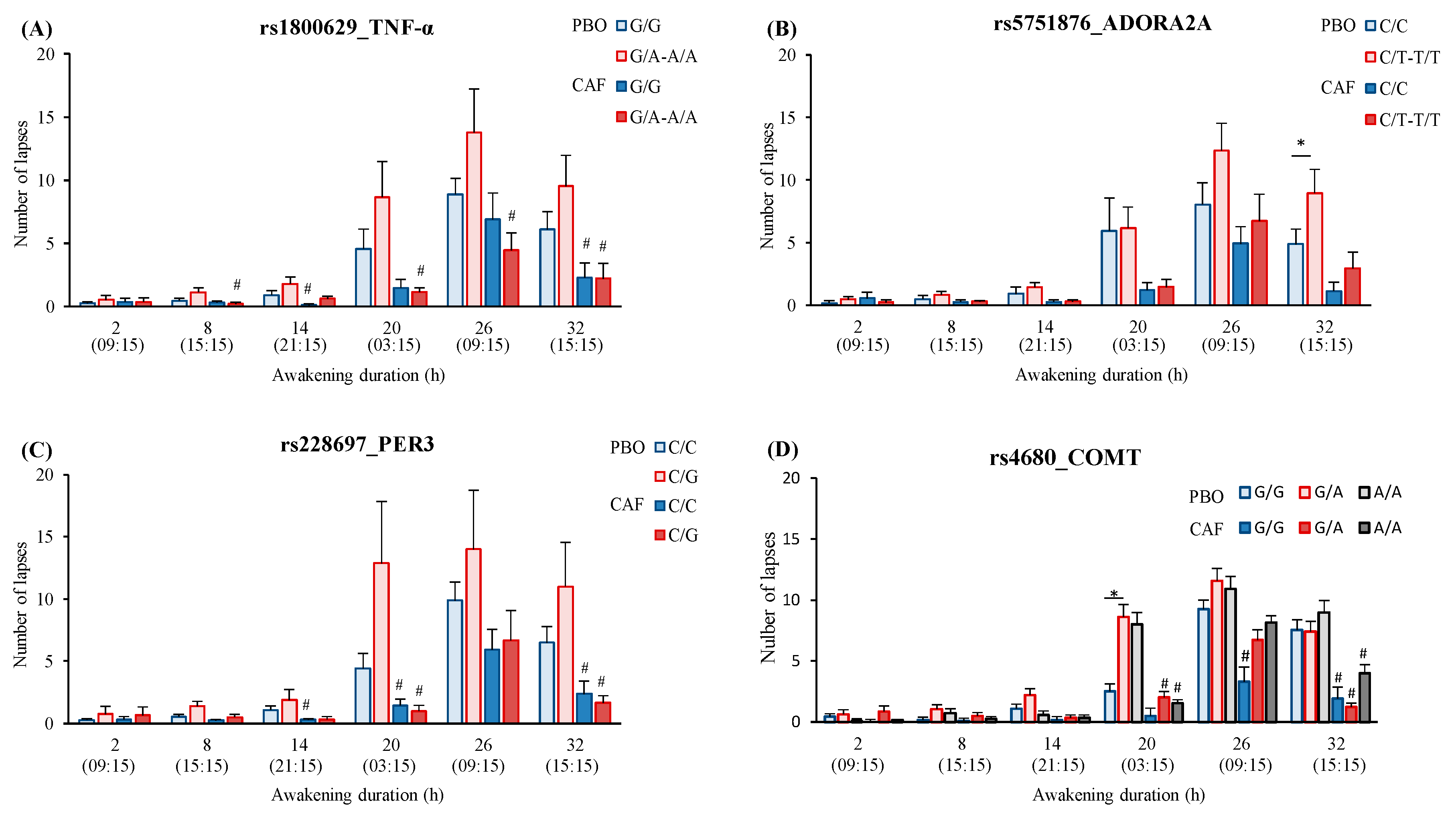
3.4.2. SNPs Effects
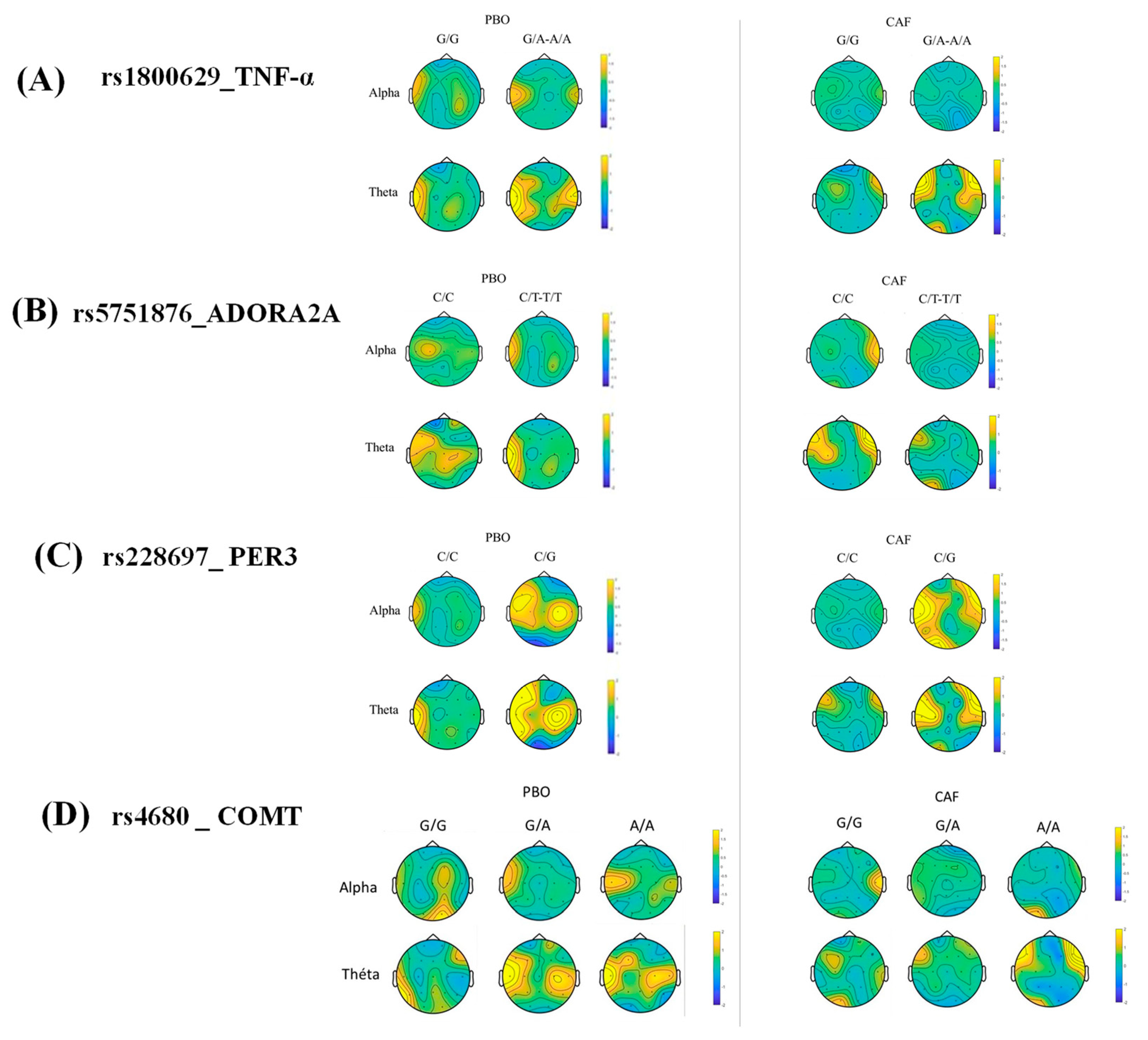
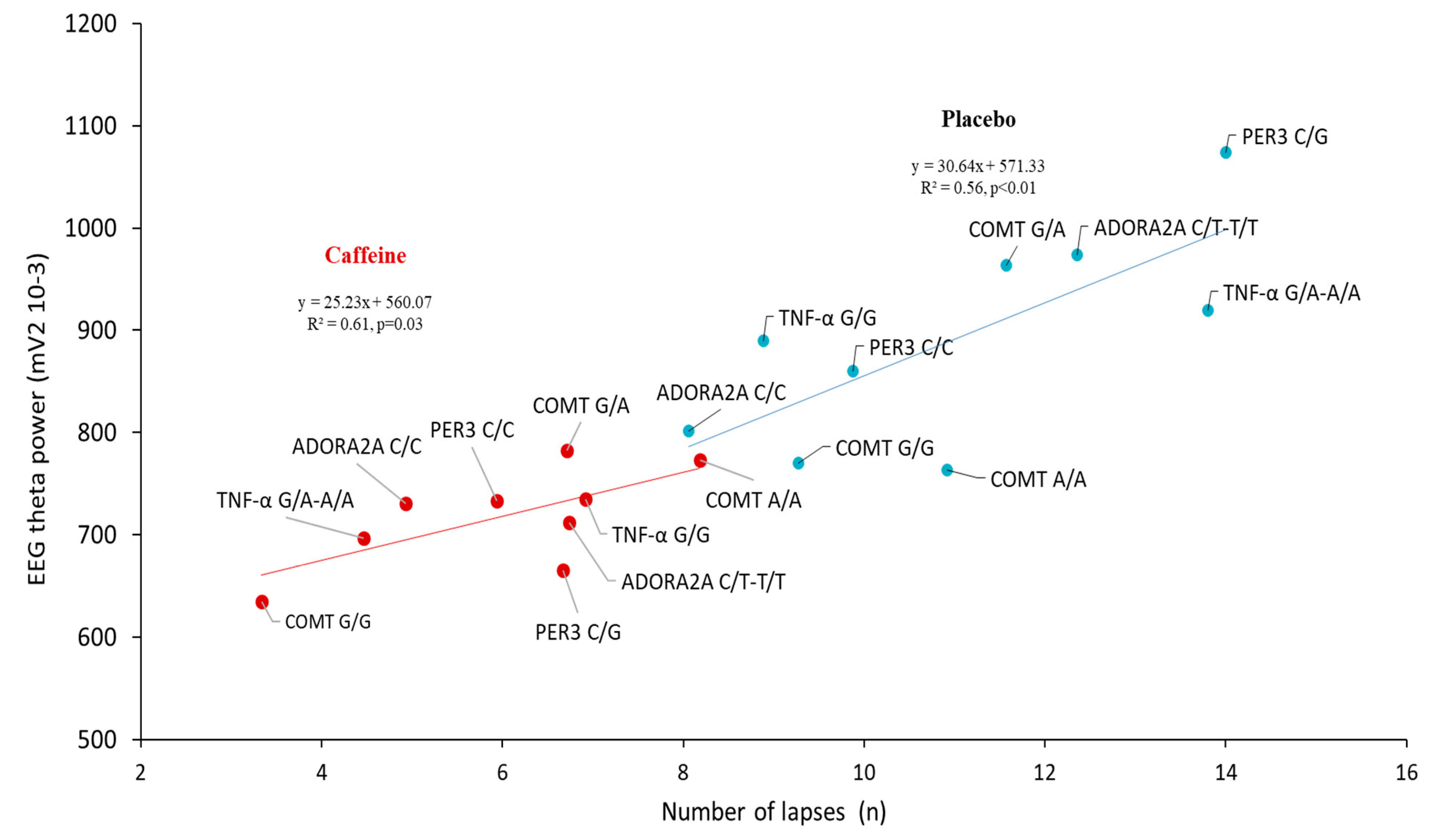
3.5. Correlation Analysis between the Lapses Number and EEG Alpha and Theta Power during the PVT Testing after Sleep Deprivation (D2 Day) in the Centro-Temporal Brain Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connor, J.; Norton, R.; Ameratunga, S.; Robinson, E.; Wigmore, B.; Jackson, R. Prevalence of driver sleepiness in a random population-based sample of car driving. Sleep 2001, 24, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Rao, H.; Durmer, J.S.; Dinges, D.F. Neurocognitive Consequences of Sleep Deprivation. Semin. Neurol. 2009, 29, 320–339. [Google Scholar] [CrossRef] [PubMed]
- Arnal, P.J.; Sauvet, F.; Leger, D.; van Beers, P.; Bayon, V.; Bougard, C.; Rabat, A.; Millet, G.Y.; Chennaoui, M. Benefits of Sleep Extension on Sustained Attention and Sleep Pressure Before and During Total Sleep Deprivation and Recovery. Sleep 2015, 38, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Basner, M.; Dinges, D.F. Maximizing Sensitivity of the Psychomotor Vigilance Test (PVT) to Sleep Loss. Sleep 2011, 34, 581–591. [Google Scholar] [CrossRef]
- Van Dongen, H.P.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The Cumulative Cost of Additional Wakefulness: Dose-Response Effects on Neurobehavioral Functions and Sleep Physiology From Chronic Sleep Restriction and Total Sleep Deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Tharion, W.J.; Shukitt-Hale, B.; Speckman, K.L.; Tulley, R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Psychopharmacoly 2002, 164, 250–261. [Google Scholar] [CrossRef]
- Urry, E.; Landolt, H.P. Adenosine, Caffeine, and Performance: From Cognitive Neuroscience of Sleep to Sleep Pharmacogenetics. In Sleep, Neuronal Plasticity and Brain Function; Meerlo, P., Benca, R.M., Abel, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 25, pp. 331–366. [Google Scholar]
- Killgore, W.D.S.; Rupp, T.L.; Grugle, N.L.; Reichardt, R.M.; Lipizzi, E.L.; Balkin, T.J. Effects of dextroamphetamine, caffeine and modafinil on psychomotor vigilance test performance after 44 h of continuous wakefulness. J. Sleep Res. 2008, 17, 309–321. [Google Scholar] [CrossRef]
- Lanini, J.; Galduróz, J.C.F.; Pompéia, S. Acute personalized habitual caffeine doses improve attention and have selective effects when considering the fractionation of executive functions. Hum. Psychopharmacol. Clin. Exp. 2016, 31, 29–43. [Google Scholar] [CrossRef]
- Hansen, D.A.; Ramakrishnan, S.; Satterfield, B.C.; Wesensten, N.J.; Layton, M.E.; Reifman, J.; Van Dongen, H.P.A. Randomized, double-blind, placebo-controlled, crossover study of the effects of repeated-dose caffeine on neurobehavioral performance during 48 h of total sleep deprivation. Psychopharmacoly 2019, 236, 1313–1322. [Google Scholar] [CrossRef]
- Dager, S.R.; Layton, M.E.; Strauss, W.; Richards, T.L.; Heide, A.; Friedman, S.D.; Artru, A.A.; Hayes, C.E.; Posse, S. Human brain metabolic response to caffeine and the effects of tolerance. Am. J. Psychiatry 1999, 156, 229–237. [Google Scholar]
- Tkachenko, O.; Dinges, D.F. Interindividual variability in neurobehavioral response to sleep loss: A comprehensive review. Neurosci. Biobehav. Rev. 2018, 89, 29–48. [Google Scholar] [CrossRef]
- Satterfield, B.C.; Hinson, J.M.; Whitney, P.; Schmidt, M.A.; Wisor, J.P.; Van Dongen, H.P. Catechol-O-methyltransferase (COMT) genotype affects cognitive control during total sleep deprivation. Cortex 2018, 99, 179–186. [Google Scholar] [CrossRef]
- Maire, M.; Reichert, C.; Gabel, V.; Viola, A.; Strobel, W.; Krebs, J.; Landolt, H.; Bachmann, V.; Cajochen, C.; Schmidt, C. Sleep ability mediates individual differences in the vulnerability to sleep loss: Evidence from a PER3 polymorphism. Cortex 2014, 52, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Groeger, J.A.; Santhi, N.; Arbon, E.L.; Lazar, A.S.; Hasan, S.; Von Schantz, M.; Archer, S.N.; Dijk, D.-J. Effects of Partial and Acute Total Sleep Deprivation on Performance across Cognitive Domains, Individuals and Circadian Phase. PLoS ONE 2012, 7, e45987. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, B.C.; Wisor, J.P.; Field, S.A.; Schmidt, M.A.; Van Dongen, H.P. TNFα G308A polymorphism is associated with resilience to sleep deprivation-induced psychomotor vigilance performance impairment in healthy young adults. Brain Behav. Immun. 2015, 47, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, B.C.; Wisor, J.P.; Schmidt, M.; Van Dongen, H.P.A. Time-on-Task Effect During Sleep Deprivation in Healthy Young Adults Is Modulated by Dopamine Transporter Genotype. Sleep 2017, 40, zsx167. [Google Scholar] [CrossRef] [PubMed]
- Valomon, A.; Holst, S.C.; Bachmann, V.; Viola, A.U.; Schmidt, C.; Zürcher, J.; Berger, W.; Cajochen, C.; Landolt, H.-P. Genetic polymorphisms of DAT1 and COMT differentially associate with actigraphy-derived sleep–wake cycles in young adults. Chronobiol. Int. 2014, 31, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Erblang, M.; Drogou, C.; Gomez-Merino, D.; Metlaine, A.; Boland, A.; Deleuze, J.F.; Thomas, C.; Sauvet, F.; Chennaoui, M. The Impact of Genetic Variations in ADORA2A in the Association between Caffeine Consumption and Sleep. Genes 2019, 10, 1021. [Google Scholar] [CrossRef]
- Rétey, J.V.; Adam, M.; Khatami, R.O.; Luhmann, U.F.; Jung, H.H.; Berger, W.; Landolt, H.-P. A Genetic Variation in the Adenosine A2A Receptor Gene (ADORA2A) Contributes to Individual Sensitivity to Caffeine Effects on Sleep. Clin. Pharmacol. Ther. 2007, 81, 692–698. [Google Scholar] [CrossRef]
- Childs, E.; Hohoff, C.; Deckert, J.; Xu, K.; Badner, J.; De Wit, H. Association between ADORA2A and DRD2 Polymorphisms and Caffeine-Induced Anxiety. Neuropsychopharmacoly 2008, 33, 2791–2800. [Google Scholar] [CrossRef]
- Rogers, P.J.; Hohoff, C.; Heatherley, S.V.; Mullings, E.L.; Maxfield, P.J.; Evershed, R.P.; Deckert, J.; Nutt, D.J. Association of the Anxiogenic and Alerting Effects of Caffeine with ADORA2A and ADORA1 Polymorphisms and Habitual Level of Caffeine Consumption. Neuropsychopharmacoly 2010, 35, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Bodenmann, S.; Hohoff, C.; Freitag, C.; Deckert, J.; Rétey, J.V.; Bachmann, V.; Landolt, H.-P. Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioural performance and sleep EEG after sleep deprivation. Br. J. Pharmacol. 2012, 165, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Skeiky, L.; Brager, A.J.; Satterfield, B.C.; Petrovick, M.; Balkin, T.J.; Capaldi, V.F.; Ratcliffe, R.H.; A. Van Dongen, H.P.; Hansen, D.A. TNFα G308A genotype, resilience to sleep deprivation, and the effect of caffeine on psychomotor vigilance performance in a randomized, double-blind, placebo-controlled, crossover study. Chrono-Int. 2020, 37, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Wyatt, J.; Czeisler, C.; Dijk, D. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience 2002, 114, 1047–1060. [Google Scholar] [CrossRef]
- Zigmond, A.; Philip Snaith, R. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Khitrov, M.Y.; Laxminarayan, S.; Thorsley, D.; Ramakrishnan, S.; Rajaraman, S.; Wesensten, N.J.; Reifman, J. PC-PVT: A platform for psychomotor vigilance task testing, analysis, and prediction. Behav. Res. Methods 2014, 46, 140–147. [Google Scholar] [CrossRef]
- Åkerstedt, T.; Anund, A.; Axelsson, J.; Kecklund, G. Subjective sleepiness is a sensitive indicator of insufficient sleep and impaired waking function. J. Sleep Res. 2014, 23, 242–254. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2010, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Drogou, C.; Sauvet, F.; Erblang, M.; Detemmerman, L.; Derbois, C.; Erkel, M.C.; Boland, A.; Deleuze, J.F.; Gomez-Merino, D.; Chennaoui, M. Genotyping on blood and buccal cells using loop-mediated isothermal amplification in healthy humans. Biotechnol. Rep. 2020, 26, e00468. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, R.W.; Biver, C.J.; North, D.M. Z Score EEG Biofeedback: Technical Foundations; Applied Neuroscience, Inc.: USA, 2004–2007; Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.495.4418&rep=rep1&type=pdf (accessed on 9 April 2021).
- Landolt, H.-P.; Rétey, J.V.; Tönz, K.; Gottselig, J.M.; Khatami, R.; Buckelmüller, I.; Peter Achermann, P. Caffeine Attenuates Waking and Sleep Electroencephalographic Markers of Sleep Homeostasis in Humans. Neuropsychopharmacoly 2004, 29, 1933–1939. [Google Scholar] [CrossRef]
- Rétey, J.V.; Adam, M.; Gottselig, J.M.; Khatami, R.; Dürr, R.; Achermann, P.; Landolt, H.-P. Adenosinergic Mechanisms Contribute to Individual Differences in Sleep Deprivation-Induced Changes in Neurobehavioral Function and Brain Rhythmic Activity. J. Neurosci. 2006, 26, 10472–10479. [Google Scholar] [CrossRef]
- Gorgoni, M.; Ferlazzo, F.; Ferrara, M.; Moroni, F.; D’Atri, A.; Fanelli, S.; Torriglia, I.G.; Lauri, G.; Marzano, C.; Rossini, P.M.; et al. Topographic electroencephalogram changes associated with psychomotor vigilance task performance after sleep deprivation. Sleep Med. 2014, 15, 1132–1139. [Google Scholar] [CrossRef]
- Holst, S.C.; Bersagliere, A.; Bachmann, V.; Berger, W.; Achermann, P.; Landolt, H.-P. Dopaminergic Role in Regulating Neurophysiological Markers of Sleep Homeostasis in Humans. J. Neurosci. 2014, 34, 566–573. [Google Scholar] [CrossRef]
- Wilson, A.G.; Symons, J.A.; McDowell, T.L.; McDevitt, H.O.; Duff, G.W. Effects of a polymorphism in the human tumor necrosis factor promoter on transcriptional activation. Proc. Natl. Acad. Sci. USA 1997, 94, 3195–3199. [Google Scholar] [CrossRef]
- Louis, E.; Franchimont, D.; Piron, A.; Gevaert, Y.; Schaaf-Lafontaine, N.; Roland, S.; Mahieu, P.; Malaise, M.; De Groote, D.; Belaiche, J. Tumour necrosis factor (TNF) gene polymorphism influences TNF-α production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans. Clin. Exp. Immunol. 1998, 113, 401–406. [Google Scholar] [CrossRef]
- Jewett, K.A.; Krueger, J.M. Humoral Sleep Regulation; Interleukin-1 and Tumor Necrosis Factor. Vitam. Horm. 2012, 89, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Chennaoui, M.; Sauvet, F.; Drogou, C.; Van Beers, P.; Langrume, C.; Guillard, M.; Gourby, B.; Bourrilhon, C.; Florence, G.; Gomez-Merino, D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine 2011, 56, 318–324. [Google Scholar] [CrossRef]
- Rogers, P.J.; Heatherley, S.V.; Mullings, E.L.; Smith, J.E. Faster but not smarter: Effects of caffeine and caffeine withdrawal on alertness and performance. Psychopharmacoly 2013, 226, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Auton, A.; Salcedo, T. The 1000 Genomes Project. In Assessing Rare Variation in Complex Traits; Metzler, J.B., Ed.; Springer: New York, NY, USA, 2015; pp. 71–85. [Google Scholar]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Caffeine suppresses TNF-α production via activation of the cyclic AMP/protein kinase A pathway. Int. Immunopharmacol. 2004, 4, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.C.; Simhadri, V.L.; Iandoli, M.; Sauna, Z.E.; Kimchi-Sarfaty, C. Exposing synonymous mutations. Trends Genet. 2014, 30, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Chennaoui, M.; Arnal, P.J.; Drogou, C.; Leger, D.; Sauvet, F.; Gomez-Merino, D. Leukocyte Expression of Type 1 and Type 2 Purinergic Receptors and Pro-Inflammatory Cytokines during Total Sleep Deprivation and/or Sleep Extension in Healthy Subjects. Front. Neurosci. 2017, 11, 240. [Google Scholar] [CrossRef]
- Carswell, A.T.; Howland, K.; Martinez-Gonzalez, B.; Baron, P.; Davison, G. The effect of caffeine on cognitive performance is influenced by CYP1A2 but not ADORA2A genotype, yet neither genotype affects exercise performance in healthy adults. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 120, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.N.; Schmidt, C.; Vandewalle, G.; Dijk, D.-J. Phenotyping of PER3 variants reveals widespread effects on circadian preference, sleep regulation, and health. Sleep Med. Rev. 2018, 40, 109–126. [Google Scholar] [CrossRef]
- Liberman, A.R.; Halitjaha, L.; Ay, A.; Ingram, K.K. Modeling Strengthens Molecular Link between Circadian Polymorphisms and Major Mood Disorders. J. Biol. Rhythm. 2018, 33, 318–336. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.U.; Archer, S.N.; James, L.M.; Groeger, J.A.; Lo, J.C.; Skene, D.J.; von Schantz, M.; Dijk, D.-J. PER3 Polymorphism Predicts Sleep Structure and Waking Performance. Curr. Biol. 2007, 17, 613–618. [Google Scholar] [CrossRef]
- Vandewalle, G.; Archer, S.N.; Wuillaume, C.; Balteau, E.; Degueldre, C.; Luxen, A.; Maquet, P.; Dijk, D.-J. Functional Magnetic Resonance Imaging-Assessed Brain Responses during an Executive Task Depend on Interaction of Sleep Homeostasis, Circadian Phase, and PER3 Genotype. J. Neurosci. 2009, 29, 7948–7956. [Google Scholar] [CrossRef]
- Ebisawa, T.; Uchiyama, M.; Kajimura, N.; Mishima, K.; Kamei, Y.; Katoh, M.; Watanabe, T.; Sekimoto, M.; Shibui, K.; Kim, K.; et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001, 2, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.; Biscontin, A.; Corrias, M.; Caccin, L.; Bano, M.; Chiaromanni, F.; Salamanca, M.; Mattei, D.; Salvoro, C.; Mazzotta, G.; et al. Diurnal Preference, Mood and the Response to Morning Light in Relation to Polymorphisms in the Human Clock Gene PER3. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gamble, K.L.; Motsinger-Reif, A.A.; Hida, A.; Borsetti, H.M.; Servick, S.V.; Ciarleglio, C.M.; Robbins, S.; Hicks, J.; Carver, K.; Hamilton, N.; et al. Shift Work in Nurses: Contribution of Phenotypes and Genotypes to Adaptation. PLoS ONE 2011, 6, e18395. [Google Scholar] [CrossRef]
- Bodenmann, S.; Rusterholz, T.; Dürr, R.; Stoll, C.; Bachmann, V.; Geissler, E.; Jaggi-Schwarz, K.; Landolt, H.-P. The Functional Val158Met Polymorphism of COMT Predicts Interindividual Differences in Brain Oscillations in Young Men. J. Neurosci. 2009, 29, 10855–10862. [Google Scholar] [CrossRef]
- Tunbridge, E.M.; Narajos, M.; Harrison, C.H.; Beresford, C.; Cipriani, A.; Harrison, P.J. Which Dopamine Polymorphisms Are Functional? Systematic Review and Meta-analysis of COMT, DAT, DBH, DDC, DRD1–5, MAOA, MAOB, TH, VMAT1, and VMAT2. Biol. Psychiatry 2019, 86, 608–620. [Google Scholar] [CrossRef]
- Gabryelska, A.; Feige, B.; Riemann, D.; Spiegelhalder, K.; Johann, A.; Białasiewicz, P.; Hertenstein, E. Can spectral power predict subjective sleep quality in healthy individuals? J. Sleep Res. 2019, 28, e12848. [Google Scholar] [CrossRef]
- Hertenstein, E.; Gabryelska, A.; Spiegelhalder, K.; Nissen, C.; Johann, A.F.; Umarova, R.; Riemann, D.; Baglioni, C.; Feige, B. Reference Data for Polysomnography-Measured and Subjective Sleep in Healthy Adults. J. Clin. Sleep Med. 2018, 14, 523–532. [Google Scholar] [CrossRef]
- Santhi, N.; Lazar, A.S.; McCabe, P.J.; Lo, J.C.; Groeger, J.A.; Dijk, D.-J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E2730–E2739. [Google Scholar] [CrossRef]
- Adam, M.; Rétey, J.V.; Khatami, R.; Landolt, H.-P. Age-Related Changes in the Time Course of Vigilant Attention During 40 Hours Without Sleep in Men. Sleep 2006, 29, 55–57. [Google Scholar] [CrossRef] [PubMed]
 VAS, KSS, PVT;
VAS, KSS, PVT;  Placebo or caffeine (2.5 mg/kg) treatment (at D1 and D2: 08:30, 14:30).
Placebo or caffeine (2.5 mg/kg) treatment (at D1 and D2: 08:30, 14:30).
 VAS, KSS, PVT;
VAS, KSS, PVT;  Placebo or caffeine (2.5 mg/kg) treatment (at D1 and D2: 08:30, 14:30).
Placebo or caffeine (2.5 mg/kg) treatment (at D1 and D2: 08:30, 14:30).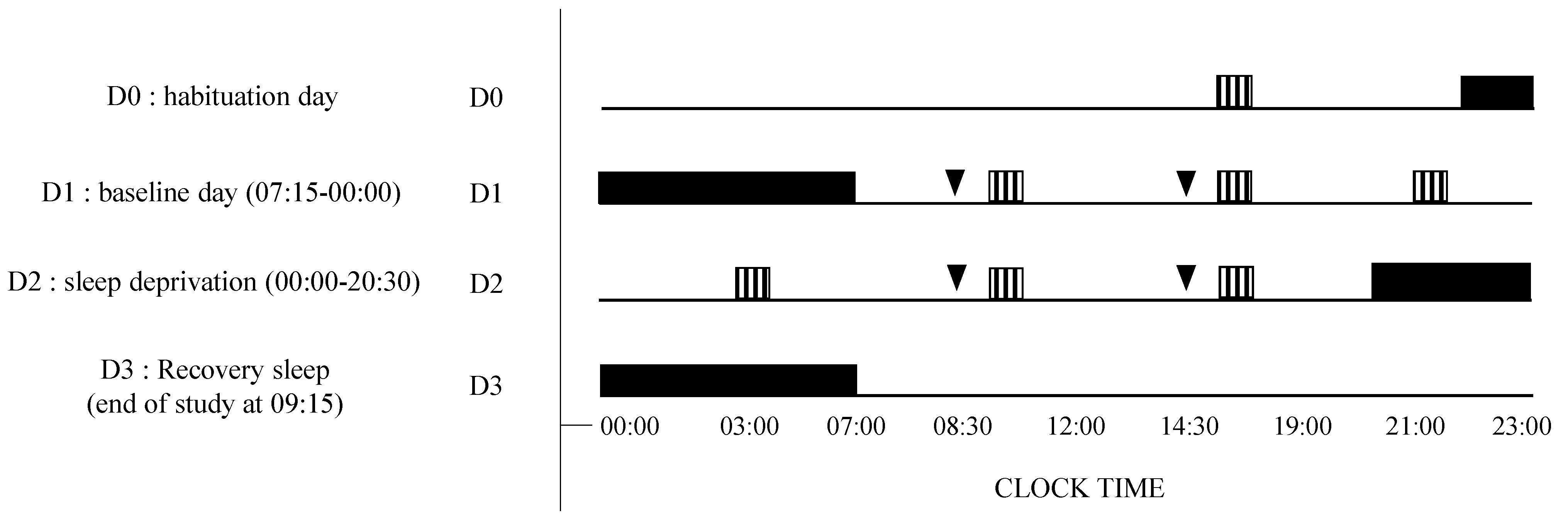

| Genetic Polymorphism (Chromosome, Location) | Genotypes | N (%) | 1000 Genomes (%) | Age (Years) | Gender (♀, %) | Habitual Caffeine Consumption (mg/day) | TST (Hours) |
|---|---|---|---|---|---|---|---|
| rs1800629_TNF-α | G/G (ancestral) | 24 (64.9%) | 74.4% | 34.8 ± 1.8 | 58.3% | 244 ± 42 | 7.37 ± 0.2 |
| (6:31.575.254) | G/A-A/A | 13 (35.1%) | 25.6% | 31.2 ± 1.8 | 53.8% | 261 ± 49 | 6.83 ± 0.3 |
| rs5751876_ADORA2A | C/C (ancestral) | 14 (37.8%) | 37.4% | 32.9 ± 1.8 | 57.1% | 168 ± 53 | 7.23 ± 0.3 |
| (22:24.441.33) | C/T—T/T | 23 (62.2%) | 62.6% | 33.9 ± 1.8 | 56.5% | 300 ± 36 * | 7.14 ± 0.2 |
| rs228697_PER3 | C/C (ancestral) | 31 (83.8%) | 81.7% | 34.4 ± 1.5 | 58.1% | 241 ± 35 | 7.10 ± 0.2 |
| (1:7.827.519) | C/G | 6 (16.2%) | 18.3% | 29.0 ± 2.5 | 50.0% | 296 ± 84 | 7.58 ± 0.3 |
| rs4680_COMT | G/G (ancestral) | 11 (29.7%) | 26.4% | 32.7 ± 1.7 | 72.7% | 249 ± 69 | 6.83 ± 0.3 |
| (22:19.963.748) | G/A | 14 (37.8%) | 47.1% | 37.4 ± 2.1 | 35.7% | 312 ± 47 | 7.62 ± 0.2 |
| A/A | 12 (32.4%) | 26.5% | 30.0 ± 2.5 | 66.6% | 178 ± 48 | 6.97 ± 0.3 |
| Parameters | SNPs | Awakening × Treatment | Polymorphism × Awakening | Polymorphism × Treatment | 3-Way Interaction |
|---|---|---|---|---|---|
| TSD × TRT F5, 175 (p) | SNP × TSD F5, 175 (p) * | SNP × TRT F1, 35 (p) ** | SNP × TSD × TRT F5, 175 (p) * | ||
| PVT Lapses | rs1800629_TNF-α | 19.17 (<0.01) | 1.87 (0.17) | 6.03 (0.02) | 4.04 (0.05) |
| rs5751876_ADORA2A | 19.64 (<0.01) | 5.67 (0.02) | 0.59 (0.45) | 0.53 (0.47) | |
| rs228697_PER3 | 18.71 (<0.01) | 3.02 (0.08) | 10.2 (<0.01) | 2.82 (0.09) | |
| rs4680_COMT | 14.70 (<0.01) | 0.15 (0.81) | 1.86 (0.15) | 5.06 (0.04) | |
| PVT Speed | rs1800629_TNF-α | 0.74 (0.39) | 6.59 (0.01) | 1.29 (0.26) | 0.27 (0.61) |
| rs5751876_ADORA2A | 0.64 (0.42) | 0.68 (0.41) | 0.46 (0.50) | 0.00 (0.97) | |
| rs228697_PER3 | 0.64 (0.42) | 0.05 (0.82) | 5.80 (0.02) | 0.18 (0.67) | |
| rs4680_COMT | 0.74 (0.39) | 0.68 (0.41) | 1.60 (0.21) | 0.30 (0.61) | |
| KSS | rs1800629_TNF-α | 0.94 (0.33) | 1.89 (0.35) | 0.75 (0.39) | 0.48 (0.49) |
| rs5751876_ADORA2A | 0.87 (0.35) | 2.83 (0.09) | 0.76 (0.39) | 0.24 (0.62) | |
| rs228697_PER3 | 0.94 (0.33) | 0.02 (0.90) | 1.70 (0.20) | 3.67 (0.06) | |
| rs4680_COMT | 0.89 (0.32) | 2.81 (0.52) | 0.73 (0.34) | 1.55 (0.23) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erblang, M.; Sauvet, F.; Drogou, C.; Quiquempoix, M.; Van Beers, P.; Guillard, M.; Rabat, A.; Trignol, A.; Bourrilhon, C.; Erkel, M.-C.; et al. Genetic Determinants of Neurobehavioral Responses to Caffeine Administration during Sleep Deprivation: A Randomized, Cross Over Study (NCT03859882). Genes 2021, 12, 555. https://doi.org/10.3390/genes12040555
Erblang M, Sauvet F, Drogou C, Quiquempoix M, Van Beers P, Guillard M, Rabat A, Trignol A, Bourrilhon C, Erkel M-C, et al. Genetic Determinants of Neurobehavioral Responses to Caffeine Administration during Sleep Deprivation: A Randomized, Cross Over Study (NCT03859882). Genes. 2021; 12(4):555. https://doi.org/10.3390/genes12040555
Chicago/Turabian StyleErblang, Mégane, Fabien Sauvet, Catherine Drogou, Michaël Quiquempoix, Pascal Van Beers, Mathias Guillard, Arnaud Rabat, Aurélie Trignol, Cyprien Bourrilhon, Marie-Claire Erkel, and et al. 2021. "Genetic Determinants of Neurobehavioral Responses to Caffeine Administration during Sleep Deprivation: A Randomized, Cross Over Study (NCT03859882)" Genes 12, no. 4: 555. https://doi.org/10.3390/genes12040555
APA StyleErblang, M., Sauvet, F., Drogou, C., Quiquempoix, M., Van Beers, P., Guillard, M., Rabat, A., Trignol, A., Bourrilhon, C., Erkel, M.-C., Léger, D., Thomas, C., Gomez-Merino, D., & Chennaoui, M. (2021). Genetic Determinants of Neurobehavioral Responses to Caffeine Administration during Sleep Deprivation: A Randomized, Cross Over Study (NCT03859882). Genes, 12(4), 555. https://doi.org/10.3390/genes12040555







