Evaluating the Role of Circulating Dendritic Cells in Methimazole-Treated Pediatric Graves’ Disease Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Flow Cytometry
2.3. Statistical Analysis
3. Results
3.1. Graves’ Disease Is Associated with Higher Numbers of Circulating Dendritic Cells Compared to Healthy Control Groups
3.2. Treatment with Methimazole Influences Changes in Dendritic Cell Distribution in Graves’ Disease Pediatric Patients
3.3. Dendritic Cells Are Demonstrated to Correlate with Disease-Related Clinical Parameters in a Treatment-Dependent Manner
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, T.J.; Hegedüs, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef]
- Bossowski, A.T.; Reddy, V.; Perry, L.A.; Johnston, L.B.; Banerjee, K.; Blair, J.C.; Savage, M.O. Clinical and endocrine features and long-term outcome of Graves’ disease in early childhood. J. Endocrinol. Investig. 2007, 30, 388–392. [Google Scholar] [CrossRef]
- Kaguelidou, F.; Carel, J.C.; Léger, J. Graves’ disease in childhood: Advances in management with antithyroid drug therapy. Horm. Res. 2009, 71, 310–317. [Google Scholar] [CrossRef]
- Vita, R.; Lapa, D.; Trimarchi, F.; Vita, G.; Fallahi, P.; Antonelli, A.; Benvenga, S. Certain HLA alleles are associated with stress-triggered Graves’ disease and influence its course. Endocrine 2017, 55, 93–100. [Google Scholar] [CrossRef]
- Falgarone, G.; Heshmati, H.M.; Cohen, R.; Reach, G. Mechanisms in endocrinology. Role of emotional stress in the pathophysiology of Graves’ disease. Eur. J. Endocrinol. 2013, 168, R13–R18. [Google Scholar] [CrossRef] [PubMed]
- Stożek, K.; Bossowski, A.; Ziora, K.; Bossowska, A.; Mrugacz, M.; Noczyńska, A.; Walczak, M.; Petriczko, E.; Pyrżak, B.; Kucharska, A.; et al. Functional TSH receptor antibodies in children with autoimmune thyroid diseases. Autoimmunity 2018, 51, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Huang, Y.; Wang, N.; Zhang, S.; Zhong, S.; Li, Y.; Sun, J.; Liu, X.; Wang, Y.; Gu, P.; et al. Insights Into Local Orbital Immunity: Evidence for the Involvement of the Th17 Cell Pathway in Thyroid-Associated Ophthalmopathy. J. Clin. Endocrinol. Metab. 2019, 104, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Huang, Y.; Wang, S.; Zhang, Y.; Luo, X.; Liu, L.; Zhong, S.; Liu, X.; Li, D.; Liang, R.; et al. IL-17A Exacerbates Fibrosis by Promoting the Proinflammatory and Profibrotic Function of Orbital Fibroblasts in TAO. J. Clin. Endocrinol. Metab. 2016, 101, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Rydzewska, M.; Jaromin, M.; Pasierowska, I.E.; Stożek, K.; Bossowski, A. Role of the T and B lymphocytes in pathogenesis of autoimmune thyroid diseases. Thyroid Res. 2018, 11, 2. [Google Scholar] [CrossRef]
- Ganguly, D.; Haak, S.; Sisirak, V.; Reizis, B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 2013, 13, 566–577. [Google Scholar] [CrossRef]
- Steinman, R.M. Decisions about dendritic cells: Past, present, and future. Annu. Rev. Immunol. 2012, 30, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.; Miller, J.; Merad, M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 2011, 35, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, A.T.; Wu, X.; Albring, J.C.; Murphy, K.M. Re(de)fining the dendritic cell lineage. Nat. Immunol. 2012, 13, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Stożek, K.; Grubczak, K.; Marolda, V.; Eljaszewicz, A.; Moniuszko, M.; Bossowski, A. Lower proportion of CD19. Autoimmunity 2020, 53, 46–55. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Mok, W.H.; Radford, K.J. Human dendritic cell subsets and function in health and disease. Cell. Mol. Life Sci. 2015, 72, 4309–4325. [Google Scholar] [CrossRef]
- Mbongue, J.C.; Nieves, H.A.; Torrez, T.W.; Langridge, W.H. The Role of Dendritic Cell Maturation in the Induction of Insulin-Dependent Diabetes Mellitus. Front. Immunol. 2017, 8, 327. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Jin, J.O.; Zhang, W.; Du, J.Y.; Yu, Q. BDCA1-positive dendritic cells (DCs) represent a unique human myeloid DC subset that induces innate and adaptive immune responses to Staphylococcus aureus Infection. Infect. Immun. 2014, 82, 4466–4476. [Google Scholar] [CrossRef]
- Jongbloed, S.L.; Kassianos, A.J.; McDonald, K.J.; Clark, G.J.; Ju, X.; Angel, C.E.; Chen, C.J.; Dunbar, P.R.; Wadley, R.B.; Jeet, V.; et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010, 207, 1247–1260. [Google Scholar] [CrossRef]
- Lewis, K.L.; Reizis, B. Dendritic cells: Arbiters of immunity and immunological tolerance. Cold. Spring Harb. Perspect. Biol. 2012, 4, a007401. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V.; Haniffa, M.; Hambleton, S. Human dendritic cell deficiency: The missing ID? Nat. Rev. Immunol. 2011, 11, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Hwang, J.S. The treatment of Graves’ disease in children and adolescents. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Audiger, C.; Rahman, M.J.; Yun, T.J.; Tarbell, K.V.; Lesage, S. The Importance of Dendritic Cells in Maintaining Immune Tolerance. J. Immunol. 2017, 198, 2223–2231. [Google Scholar] [CrossRef]
- Coutant, F.; Miossec, P. Altered dendritic cell functions in autoimmune diseases: Distinct and overlapping profiles. Nat. Rev. Rheumatol. 2016, 12, 703–715. [Google Scholar] [CrossRef]
- Rönnblom, L.; Alm, G.V. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 2001, 194, F59–F63. [Google Scholar] [CrossRef]
- Breton, G.; Lee, J.; Liu, K.; Nussenzweig, M.C. Defining human dendritic cell progenitors by multiparametric flow cytometry. Nat. Protoc. 2015, 10, 1407–1422. [Google Scholar] [CrossRef]
- Hughes, C.E.; Benson, R.A.; Bedaj, M.; Maffia, P. Antigen-Presenting Cells and Antigen Presentation in Tertiary Lymphoid Organs. Front. Immunol. 2016, 7, 481. [Google Scholar] [CrossRef]
- Kim, B.; Kim, T.H. Fundamental role of dendritic cells in inducing Th2 responses. Korean J. Intern. Med. 2018, 33, 483–489. [Google Scholar] [CrossRef]
- Mailliard, R.B.; Egawa, S.; Cai, Q.; Kalinska, A.; Bykovskaya, S.N.; Lotze, M.T.; Kapsenberg, M.L.; Storkus, W.J.; Kalinski, P. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: Helper role of CD8+ T cells in the development of T helper type 1 responses. J. Exp. Med. 2002, 195, 473–483. [Google Scholar] [CrossRef]
- Boldison, J.; Da Rosa, L.C.; Davies, J.; Wen, L.; Wong, F.S. Dendritic cells license regulatory B cells to produce IL-10 and mediate suppression of antigen-specific CD8 T cells. Cell. Mol. Immunol. 2020, 17, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R.; Johnson, C.N.; Corbett, K.S.; Edwards, G.C.; Graham, B.S. Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus. PLoS ONE 2011, 6, e16458. [Google Scholar] [CrossRef] [PubMed]
- Lechpammer, M.; Lukac, J.; Lechpammer, S.; Kusić, Z. Antithyroid drug-induced immunomodulation in Graves’ disease patients. Acta Med Croatica 2002, 56, 21–26. [Google Scholar] [PubMed]
- Pedro, A.B.; Romaldini, J.H.; Takei, K. Changes of serum cytokines in hyperthyroid Graves’ disease patients at diagnosis and during methimazole treatment. Neuroimmunomodulation 2011, 18, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Giusti, C. The Th1 chemokine MIG in Graves’ Disease: A narrative review of the literature. Clin. Ther. 2019, 170, e285–e290. [Google Scholar] [CrossRef]
- Crescioli, C.; Cosmi, L.; Borgogni, E.; Santarlasci, V.; Gelmini, S.; Sottili, M.; Sarchielli, E.; Mazzinghi, B.; Francalanci, M.; Pezzatini, A.; et al. Methimazole inhibits CXC chemokine ligand 10 secretion in human thyrocytes. J. Endocrinol. 2007, 195, 145–155. [Google Scholar] [CrossRef]
- Bossowski, A.; Moniuszko, M.; Idźkowska, E.; Grubczak, K.; Singh, P.; Bossowska, A.; Diana, T.; Kahaly, G.J. Decreased proportions of CD4 + IL17+/CD4 + CD25 + CD127- and CD4 + IL17+/CD4 + CD25 + CD127 - FoxP3+ T cells in children with autoimmune thyroid diseases (.). Autoimmunity 2016, 49, 320–328. [Google Scholar] [CrossRef]
- Heufelder, A.E.; Bahn, R.S. Soluble intercellular adhesion molecule-1 (sICAM-1) in sera of patients with Graves’ ophthalmopathy and thyroid diseases. Clin. Exp. Immunol. 1993, 92, 296–302. [Google Scholar] [CrossRef]
- Jublanc, C.; Beaudeux, J.L.; Aubart, F.; Raphael, M.; Chadarevian, R.; Chapman, M.J.; Bonnefont-Rousselot, D.; Bruckert, E. Serum levels of adhesion molecules ICAM-1 and VCAM-1 and tissue inhibitor of metalloproteinases, TIMP-1, are elevated in patients with autoimmune thyroid disorders: Relevance to vascular inflammation. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 817–822. [Google Scholar] [CrossRef]
- Allen, J.S.; Pang, K.; Skowera, A.; Ellis, R.; Rackham, C.; Lozanoska-Ochser, B.; Tree, T.; Leslie, R.D.; Tremble, J.M.; Dayan, C.M.; et al. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes 2009, 58, 138–145. [Google Scholar] [CrossRef]
- Glitzner, E.; Korosec, A.; Brunner, P.M.; Drobits, B.; Amberg, N.; Schonthaler, H.B.; Kopp, T.; Wagner, E.F.; Stingl, G.; Holcmann, M.; et al. Specific roles for dendritic cell subsets during initiation and progression of psoriasis. EMBO Mol. Med. 2014, 6, 1312–1327. [Google Scholar] [CrossRef] [PubMed]
- Mascanfroni, I.; Montesinos, M.e.M.; Susperreguy, S.; Cervi, L.; Ilarregui, J.M.; Ramseyer, V.D.; Masini-Repiso, A.M.; Targovnik, H.M.; Rabinovich, G.A.; Pellizas, C.G. Control of dendritic cell maturation and function by triiodothyronine. FASEB J. 2008, 22, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.A.; Ferris, S.T.; Unanue, E.R. Macrophages and dendritic cells in islets of Langerhans in diabetic autoimmunity: A lesson on cell interactions in a mini-organ. Curr. Opin. Immunol. 2016, 43, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Quadbeck, B.; Eckstein, A.K.; Tews, S.; Walz, M.; Hoermann, R.; Mann, K.; Gieseler, R. Maturation of thyroidal dendritic cells in Graves’ disease. Scand. J. Immunol. 2002, 55, 612–620. [Google Scholar] [CrossRef]
- Pawlowski, P.; Grubczak, K.; Kostecki, J.; Ilendo-Poskrobko, E.; Moniuszko, M.; Pawlowska, M.; Rejdak, R.; Reszec, J.; Mysliwiec, J. Decreased Frequencies of Peripheral Blood CD4+CD25+CD127-Foxp3+ in Patients with Graves’ Disease and Graves’ Orbitopathy: Enhancing Effect of Insulin Growth Factor-1 on Treg Cells. Horm. Metab. Res. 2017, 49, 185–191. [Google Scholar] [CrossRef]
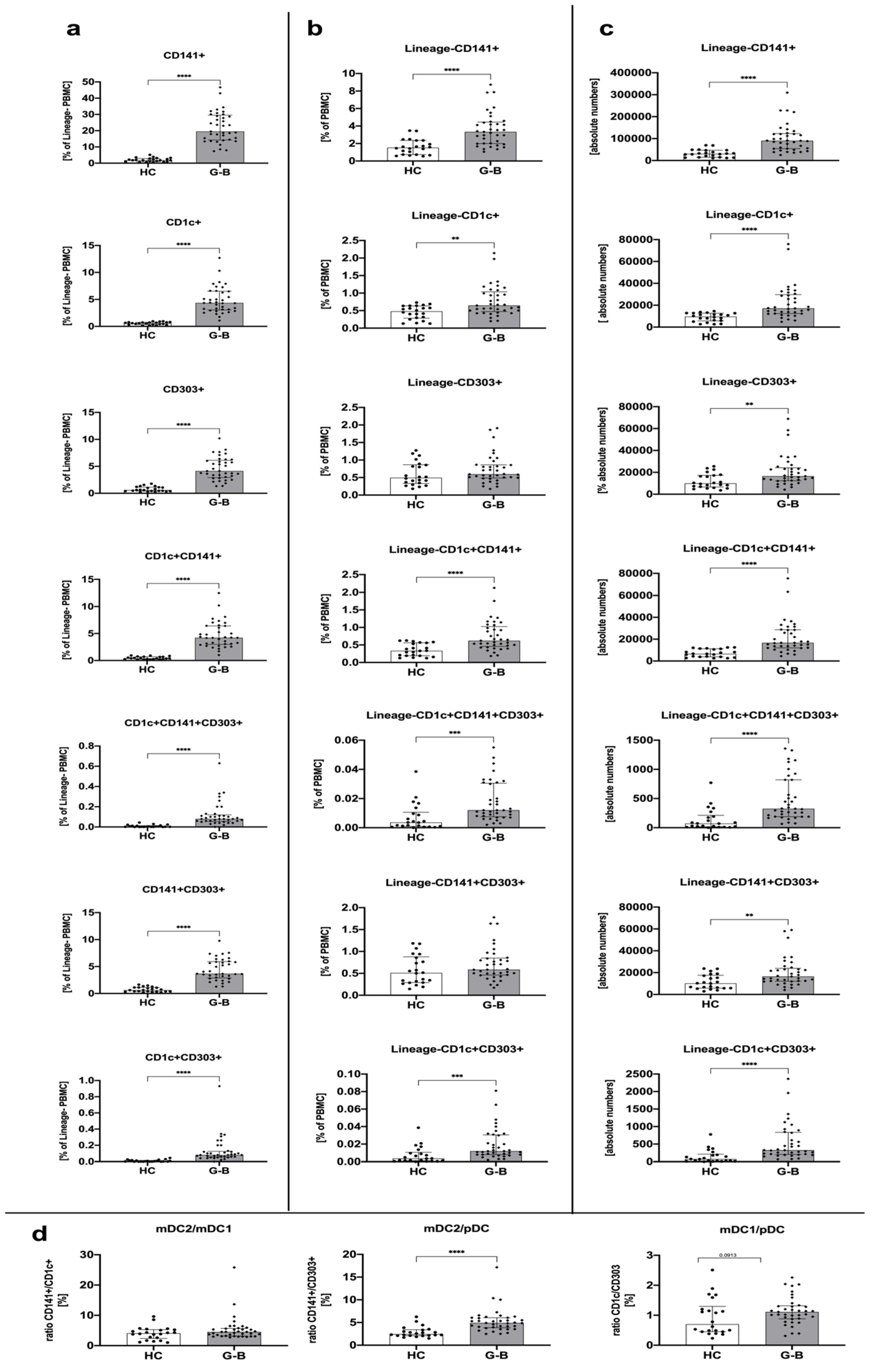
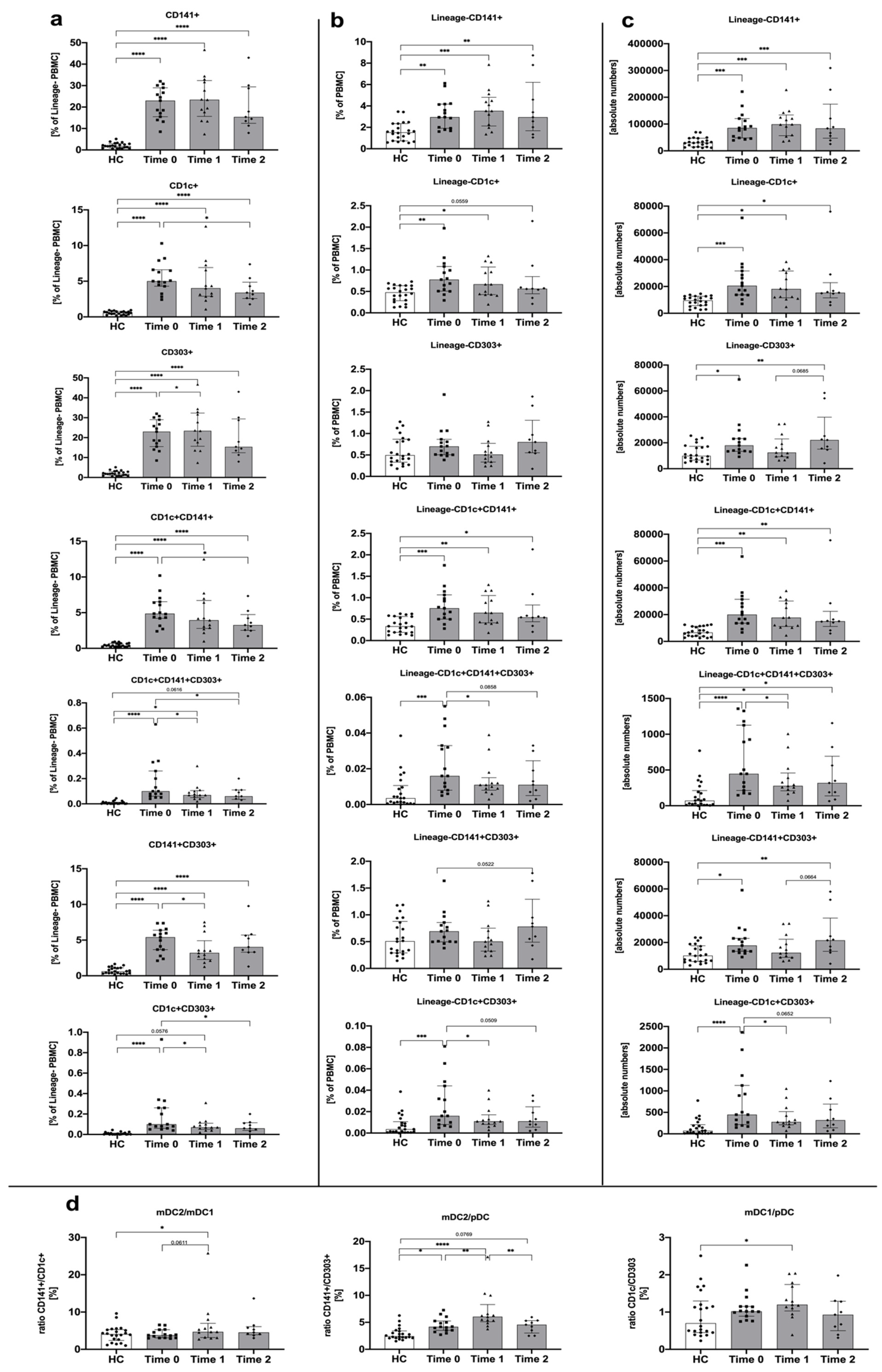

| Parameter | Graves’ Disease (GD) Time 0 | Graves’ Disease (GD) Time 1 | Graves’ Disease (GD) Time 2 | Control Group (HC) |
|---|---|---|---|---|
| Age (years) | 14 (10.75; 16.00) | 13.5 (9.50; 14.50) | ||
| Sex distribution (male to female) | 18% to 82% 4 to 18 | 39% to 61% 12 to 19 | ||
| TSH (mIU/L) Ref. range: 0.32–5.0 | 0.02 *** (0.01; 0.48) | 0.23 ** (0.04; 1.89) | 1.56 (0.78; 15.21) | 2.39 (1.40; 2.80) |
| fT4 (ng/dL) Ref. range: 0.71–1.55 | 7.77 (2.28; 7.77) | 1.08 (0.42; 1.81) | 1.21 (0.41; 1.50) | |
| fT3 (ng/L) Ref. range: 2.6–5.4 | 24.59 (5.31; 32.55) | 3.92 (2.15; 5.58) | 3.52 (1.86; 6.26) | |
| TRAb (IU/L) Ref. range: 0–1.7 | 27.90 (15.20; 36.77) | 11.39 (3.60; 24.28) | 6.46 (2.42; 11.28) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starosz, A.; Stożek, K.; Moniuszko, M.; Grubczak, K.; Bossowski, A. Evaluating the Role of Circulating Dendritic Cells in Methimazole-Treated Pediatric Graves’ Disease Patients. Genes 2021, 12, 164. https://doi.org/10.3390/genes12020164
Starosz A, Stożek K, Moniuszko M, Grubczak K, Bossowski A. Evaluating the Role of Circulating Dendritic Cells in Methimazole-Treated Pediatric Graves’ Disease Patients. Genes. 2021; 12(2):164. https://doi.org/10.3390/genes12020164
Chicago/Turabian StyleStarosz, Aleksandra, Karolina Stożek, Marcin Moniuszko, Kamil Grubczak, and Artur Bossowski. 2021. "Evaluating the Role of Circulating Dendritic Cells in Methimazole-Treated Pediatric Graves’ Disease Patients" Genes 12, no. 2: 164. https://doi.org/10.3390/genes12020164
APA StyleStarosz, A., Stożek, K., Moniuszko, M., Grubczak, K., & Bossowski, A. (2021). Evaluating the Role of Circulating Dendritic Cells in Methimazole-Treated Pediatric Graves’ Disease Patients. Genes, 12(2), 164. https://doi.org/10.3390/genes12020164







