Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Treatment
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of age-related macular degeneration in Chinese populations worldwide: A systematic review and meta-analysis. Clin. Exp. Ophthalmol. 2019, 47, 1019–1027. [Google Scholar]
- Blasiak, J.; Salminen, A.; Kaarniranta, K. Potential of epigenetic mechanisms in AMD pathology. Front. Biosci. (Schol. Ed). 2013, 5, 12–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, X.; Gharahkhani, P.; Mitchell, P.; Liew, G.; Hewitt, A.W.; MacGregor, S. Genome-wide meta-analysis identifies novel loci associated with age-related macular degeneration. J. Hum. Genet. 2020, 65, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef]
- Blasiak, J.; Watala, C.; Tuuminen, R.; Kivinen, N.; Koskela, A.; Uusitalo-Järvinen, H.; Tuulonen, A.; Winiarczyk, M.; Mackiewicz, J.; Zmorzyński, S.; et al. Expression of VEGFA-regulating miRNAs and mortality in wet AMD. J. Cell. Mol. Med. 2019, 23, 8464–8471. [Google Scholar] [CrossRef]
- Seddon, J.M.; Rosner, B. Validated Prediction Models for Macular Degeneration Progression and Predictors of Visual Acuity Loss Identify High-Risk Individuals. Am. J. Ophthalmol. 2019, 198, 223–261. [Google Scholar] [CrossRef]
- Karesvuo, P.; Hakkala, L.; Kaarniranta, K.; Uusitalo, H.; Ojamo, M.; Tuuminen, R. Correlation between the rate of intravitreal injections, use of aflibercept as a second-line treatment and visual impairment for wet AMD in Finland. Acta Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Taipale, C.; Lindholm, J.M.; Kaarniranta, K.; Tuuminen, R. Comparison of Two Different Treat-and-Extend Protocols with Aflibercept in Wet Age-Related Macular Degeneration: Two-Year Results. Adv. Ther. 2020, 37, 2256–2266. [Google Scholar] [CrossRef]
- Schroeder, M.; Westborg, I.; Lövestam, A.M. Twelve per cent of 6142 eyes treated for neovascular age-related macular degeneration (nAMD) presented with low visual outcome within 2 years. Analysis from the Swedish Macula Registry (SMR). Acta Ophthalmol. 2020, 98, 274–278. [Google Scholar] [CrossRef]
- Kataja, M.; Hujanen, P.; Huhtala, H.; Kaarniranta, K.; Tuulonen, A.; Uusitalo-Jarvinen, H. Outcome of anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration in real-life setting. Br. J. Ophthalmol. 2018, 102, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Tuuminen, R.; Sipilä, R.; Komulainen, J.; Saarela, V.; Kaarniranta, K.; Tuulonen, A. The first ophthalmic Choosing Wisely recommendations in Finland for glaucoma and wet age-related macular degeneration. Acta Ophthalmol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Megías, A.; Veganzones-de-Castro, S.; Donate-López, J.; Maestro-de-las-Casas, M.L.; Megías-Fresno, A.; García-Feijoo, J. ARMS2 A69S polymorphism is associated with the number of ranibizumab injections needed for exudative age-related macular degeneration in a pro re nata regimen during 4 years of follow-up. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Lorés-Motta, L.; Riaz, M.; Grunin, M.; Corominas, J.; van Asten, F.; Pauper, M.; Leenders, M.; Richardson, A.J.; Muether, P.; Cree, A.J.; et al. Association of genetic variants with response to anti-vascular endothelial growth factor therapy in age-related macular degeneration. JAMA Ophthalmol. 2018, 136, 875–884. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cross, S.D.; Stanton, J.B.; Marmorstein, A.D.; Le, Y.Z.; Marmorstein, L.Y. Early AMD-like defects in the RPE and retinal degeneration in aged mice with RPE-specific deletion of Atg5 or Atg7. Mol. Vis. 2017, 23, 228. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef]
- Felszeghy, S.; Viiri, J.; Paterno, J.J.; Hyttinen, J.M.T.; Koskela, A.; Chen, M.; Leinonen, H.; Tanila, H.; Kivinen, N.; Koistinen, A.; et al. Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol. 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Gurubaran, I.S.; Viiri, J.; Koskela, A.; Hyttinen, J.M.T.; Paterno, J.J.; Kis, G.; Antal, M.; Urtti, A.; Kauppinen, A.; Felszeghy, S.; et al. Mitophagy in the Retinal Pigment Epithelium of Dry Age-Related Macular Degeneration Investigated in the NFE2L2/PGC-1α-/- Mouse Model. Int. J. Mol. Sci. 2020, 21, 1976. [Google Scholar] [CrossRef]
- Lahiri, V.; Hawkins, W.D.; Klionsky, D.J. Watch What You (Self-) Eat: Autophagig Mechanisms that Modulate Metabolism. Cell Metab. 2019, 29, 803–826. [Google Scholar] [CrossRef]
- Blasiak, J.; Pawlowska, E.; Szczepanska, J.; Kaarniranta, K. Interplay between Autophagy and the Ubiquitin-Proteasome System and Its Role in the Pathogenesis of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 210. [Google Scholar] [CrossRef]
- Baek, A.; Yoon, S.; Kim, J.; Baek, Y.M.; Park, H.; Lim, D.; Chung, H.; Kim, D.E. Autophagy and KRT8/keratin 8 protect degeneration of retinal pigment epithelium under oxidative stress. Autophagy 2017, 13, 248–263. [Google Scholar] [CrossRef]
- Song, C.; Mitter, S.K.; Qi, X.; Beli, E.; Rao, H.V.; Ding, J.; Ip, C.S.; Gu, H.; Akin, D.; Dunn, W.A., Jr.; et al. Oxidative stress-mediated NFkappaB phosphorylation upregulates p62/SQSTM1 and promotes retinal pigmented epithelial cell survival through increased autophagy. PLoS ONE 2017. [Google Scholar] [CrossRef]
- Yao, J.; Jia, L.; Khan, N.; Lin, C.; Mitter, S.K.; Boulton, M.E.; Dunaief, J.L.; Klionsky, D.J.; Guan, J.L.; Thompson, D.A.; et al. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy 2015, 11, 939–953. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Paananen, J.; Nevalainen, T.; Sorri, I.; Seitsonen, S.; Immonen, I.; Salminen, A.; Pulkkinen, L.; Uusitupa, M. Adiponectin receptor 1 gene (ADIPOR1) variant is associated with advanced age-related macular degeneration in Finnish population. Neurosci. Lett. 2012, 513, 233–237. [Google Scholar] [CrossRef]
- Tuuminen, R.; Uusitalo-Järvinen, H.; Aaltonen, V.; Hautala, N.; Kaipiainen, S.; Laitamäki, N.; Ollila, M.; Rantanen, J.; Välimäki, S.; Sipilä, R.; et al. The Finnish national guideline for diagnosis, treatment and follow-up of patients with wet age-related macular degeneration. Acta Ophthalmol. 2017, 95 (Suppl. A105), 1–9. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 40–70. [Google Scholar] [CrossRef]
- Han, S.; Chen, J.; Hua, J.; Hu, X.; Jian, S.; Zheng, G.; Wang, J.; Li, H.; Yang, J.; Hejtmancik, J.F.; et al. MITF protects against oxidative damage-induced retinal degeneration by regulating the NRF2 pathway in the retinal pigment epithelium. Redox Biol. 2020, 34. [Google Scholar] [CrossRef]
- Cobos, E.; Recalde, S.; Anter, J.; Hernandez-Sanchez, M.; Barreales, C.; Olavarrieta, L.; Valverde, A.; Suarez-Figueroa, M.; Cruz, F.; Abraldes, M.; et al. Association between CFH, CFB, ARMS2, SERPINF1, VEGFR1 and VEGF polymorphisms and anatomical and functional response to ranibizumab treatment in neovascular age-related macular degeneration. Acta Ophthalmol. 2018, 96, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Smailhodzic, D.; Muether, P.S.; Chen, J.; Kwestro, A.; Zhang, A.Y.; Omar, A.; Van de Ven, J.P.; Keunen, J.E.; Kirchhof, B.; Hoyng, C.B.; et al. Cumulative effect of risk alleles in CFH, ARMS2, and VEGFA on the response to ranibizumab treatment in age-related macular degeneration. Ophthalmology 2012, 119, 2304–2311. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010. [Google Scholar] [CrossRef]
- Saunier, V.; Merle, B.M.J.; Delyfer, M.N.; Cougnard-Grégoire, A.; Rougier, M.B.; Amouyel, P.; Lambert, J.C.; Dartigues, J.F.; Korobelnik, J.F.; Delcourt, C. Incidence of and Risk Factors Associated with Age-Related Macular Degeneration: Four-Year Follow-up from the ALIENOR Study. JAMA Ophthalmol. 2018, 136, 473–481. [Google Scholar] [CrossRef]
- Al-Holou, S.N.; Tucker, W.R.; Agrón, E.; Clemons, T.E.; Cukras, C.; Ferris, F.L., III; Chew, E.Y. Age-Related Eye Disease Study 2 Research Group. The Association of Statin Use with Age-Related Macular Degeneration Progression: The Age-Related Eye Disease Study 2 Report Number 9. Ophthalmology 2015, 122, 2490–2496. [Google Scholar] [CrossRef]
- Ouimet, M. Autophagy in obesity and atherosclerosis: Interrelationships between cholesterol homeostasis, lipoprotein metabolism and autophagy in macrophages and other systems. Biochim. Biophys. Acta 2013, 1831, 1124–1133. [Google Scholar] [CrossRef]
- Barbero-Camps, E.; Roca-Agujetas, V.; Bartolessis, I.; de Dios, C.; Fernández-Checa, J.C.; Marí, M.; Morales, A.; Hartmann, T.; Colell, A. Cholesterol impairs autophagy-mediated clearance of amyloid β while promoting its secretion. Autophagy 2018, 14, 1129–1154. [Google Scholar] [CrossRef] [PubMed]
- Small, K.W.; Garabetian, C.A.; Shaya, F.S. Macular degeneration and aspirin use. Retina 2017, 37, 1630–1635. [Google Scholar] [CrossRef]
- Ying, G.S.; Maguire, M.G.; Daniel, E.; Grunwald, J.E.; Ahmed, O.; Martin, D.F.; Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Association between Antiplatelet or Anticoagulant Drugs and Retinal or Subretinal Hemorrhage in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 352–360. [Google Scholar] [CrossRef]
- Modjtahedi, B.S.; Fong, D.S.; Jorgenson, E.; Van Den Eeden, S.K.; Quinn, V.; Slezak, J.M. The Relationship Between Nonsteroidal Anti-inflammatory Drug Use and Age-related Macular Degeneration. Am. J. Ophthalmol. 2018, 188, 111–122. [Google Scholar] [CrossRef]
- Rim, T.H.; Yoo, T.K.; Kwak, J.; Lee, J.S.; Kim, S.H.; Kim, D.W.; Kim, S.S. Long-Term Regular Use of Low-Dose Aspirin and Neovascular Age-Related Macular Degeneration: National Sample Cohort 2010–2015. Ophthalmology 2019, 126, 274–282. [Google Scholar] [CrossRef]
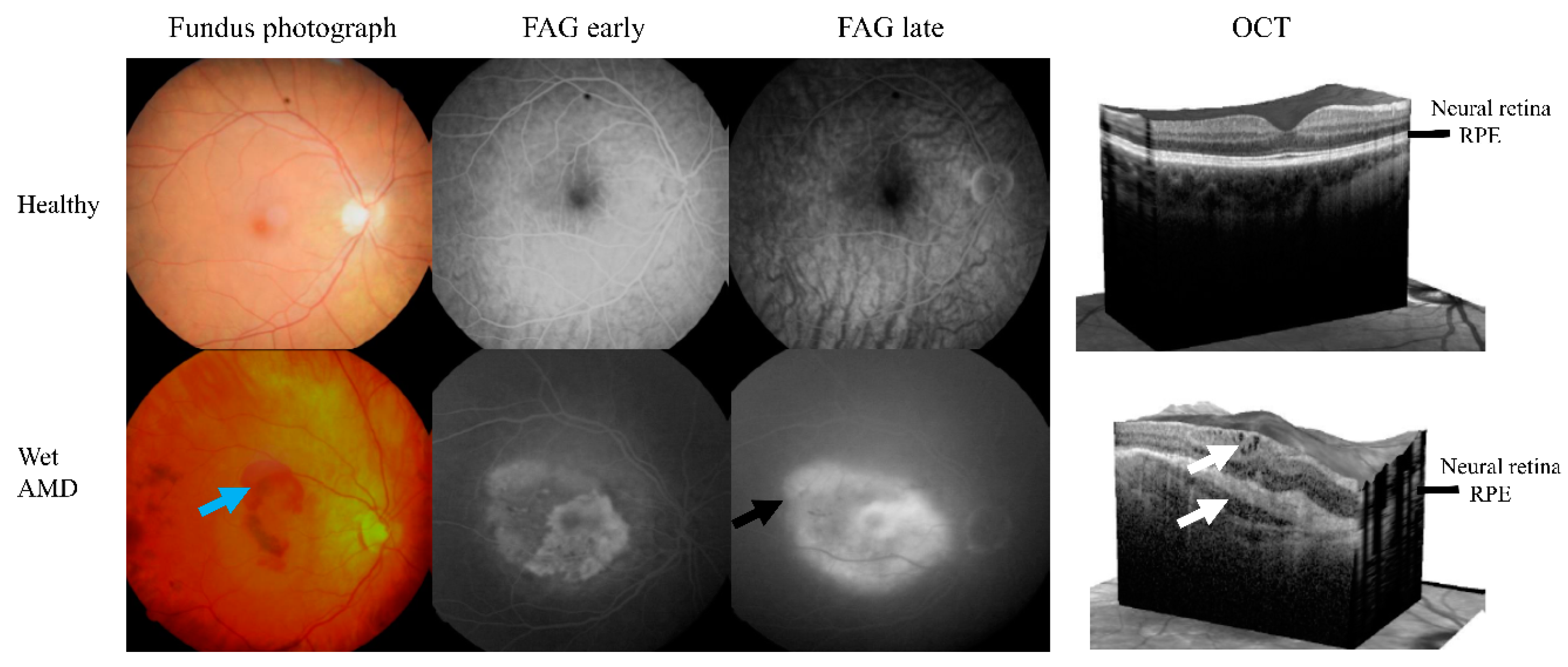
| Feature | Control (n = 161) | Wet AMD (n = 225) | p3 |
|---|---|---|---|
| Age (years) | 74.1 ± 6.3 | 78.4 ± 6.9 | 0.000 |
| Sex (male/female) | 52/109 | 70/155 | 0.368 |
| BMI (mean) | 25.7 ± 4.5 1 | 26.2 ± 4.1 2 | 0.161 |
| Smoking | 0.001 | ||
| Non-smoker | 93 (57.8%) | 140 (62.2%) | |
| Occasionally | 16 (9.9%) | 26 (11.6%) | |
| Smoker | 6 (3.7%) | 27 (12.0%) | |
| No information | 46 (28.6%) | 32 (14.2%) | |
| Medication | |||
| Blood pressure | 115 (79.3%) 1 | 164 (76.3%) 2 | 0.340 |
| Anti-cholesterol | 77 (53.1%) 1 | 93 (43.5%) 2 | 0.011 |
| Anticoagulant | 43 (29.5%) 1 | 64 (30.2%) 2 | 0.832 |
| Antiplatelet | 66 (44.6%) 1 | 109 (50.9%) 2 | 0.094 |
| Polymorphism | Gene | Change | Location within Gene |
|---|---|---|---|
| rs2295080 | mTOR—Mechanistic Target of Rapamycin Kinase | g.11262571G > T, C | 5′UTR (promoter) |
| rs11121704 | g.11233902C > A, T | Intron | |
| rs1057079 | c.1437C > T | Exon | |
| rs1064261 | c.2997C > T | Exon | |
| rs573775 | ATG5—Autophagy Related 5 | g. 106316991G > A | Intron |
| rs11246867 | ULK1—Unc-51 Like Autophagy Activating Kinase 1 | g. 131893472G > A | 2 KB Upstream Variant |
| rs3088051 | ULK1—Unc-51 Like Autophagy Activating Kinase 1 | g. 131922463T > C | 3′UTR |
| rs10902469 | g. 131893588G > A, C | 2 KB Upstream Variant | |
| rs73105013 | MAP1LC3A—Microtubule Associated Protein 1 Light Chain 3 α | g. 179837731T > A, C | Intron |
| rs10277 | SQSTM1—Sequestosome 1 | g. 179837731T > A, C | 3′UTR |
| Polymorphism | Control | AMD | ||
|---|---|---|---|---|
| Chi-square | p1 | Chi-square | p1 | |
| rs2295080 | 6.720 | 0.035 | 1.201 | 0.548 |
| rs11121704 | 6.090 | 0.048 | 2.192 | 0.334 |
| rs1057079 | 1.464 | 0.481 | 0.673 | 0.714 |
| rs1064261 | 5.081 | 0.079 | 0.898 | 0.638 |
| rs573775 | 0.456 | 0.796 | 6.555 | 0.038 |
| rs11246867 | 1.044 | 0.593 | 0.109 | 0.947 |
| rs3088051 | 0.220 | 0.896 | 0.401 | 0.818 |
| rs73105013 | 1.884 | 0.390 | 1.987 | 0.370 |
| rs10277 | 0.668 | 0.716 | 0.033 | 0.984 |
| rs10902469 | 1.044 | 0.593 | 0.109 | 0.947 |
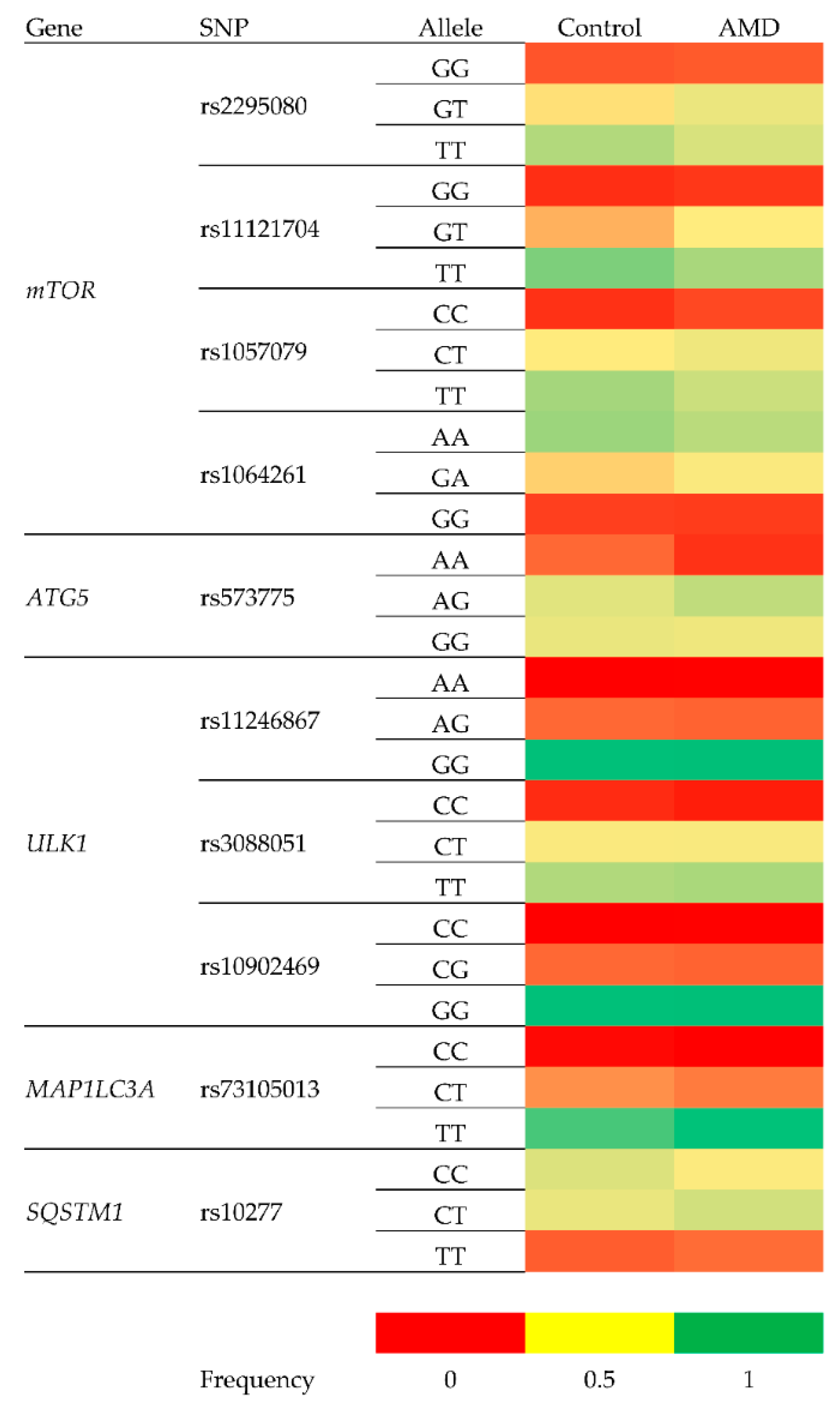
| Genotype/Allele | Frequency | Crude OR (95% CI) | p | Adjusted OR 1 (95% CI) | p | |
|---|---|---|---|---|---|---|
| Control | AMD | |||||
| rs2295080 148/216 (Control/AMD cases) | ||||||
| GG | 0.12 | 0.12 | 1.07 (0.57–2.00) | 0.836 | 1.28 (0.65–2.53) | 0.474 |
| GT | 0.32 | 0.42 | 1.51 (0.98–2.34) | 0.062 | 1.49 (0.94–2.38) | |
| TT | 0.56 | 0.46 | 0.661 (0.435–1.004) 0.664 (0.454–0.971) 2 | 0.052 0.035 | 0.621 (0.398–0.968) 2 0.617 (0.393–0.968)0.651 | 0.035 0.036 |
| G | 0.22 | 0.27 | 1.296 (0.941–1.785) | 0.113 | ||
| T | 0.28 | 0.23 | 0.772 (0.560–1.063) | 0.113 | 0.709 (0.503–0.998) 2 0.703 (0.493–0.995)0.725 | 0.048 0.049 |
| rs11121704 152/209 (Control/AMD cases) | ||||||
| CC | 0.07 | 0.09 | 1.15 (0.53–2.52) | 0.720 | 1.23 (0.54–2.82) | 0.616 |
| CT | 0.26 | 0.33 | 1.489 (0.940–2.357) 1.481 (0.936–2.345) 2 | 0.089 0.093 2 | 1.609 (0.985–2.628) 2 1.634 (0.982–2.717)0.459 | 0.056 0.059 |
| TT | 0.67 | 0.58 | 0.671 (0.436–1.034) 0.663 (0.433–1.016) 2 | 0.071 0.059 2 | 0.613 (0.386–0.972) 2 0.605 (0.375–0.975)0.717 | 0.037 0.039 |
| C | 0.16 | 0.21 | 1.34 (0.94–1.91) | 0.102 | 1.447 (0.993–2.109) 2 1.467 (1.016–2.119)0.479 | 0.054 0.041 |
| T | 0.33 | 0.29 | 0.74 (0.52–1.06) | 0.102 | 0.691 (0.474–1.007) 2 0.681 (0.474–0.985)0.809 | 0.054 0.041 |
| rs1057079 161/217 (Control/AMD cases) | ||||||
| CC | 0.07 | 0.10 | 1.47 (0.71–3.04) | 0.293 | 1.69 (0.77–3.71) | 0.191 |
| CT | 0.33 | 0.41 | 1.37 (0.90–2.10) | 0.141 | 1.39 (0.88–2.18) | 0.153 |
| TT | 0.60 | 0.49 | 0.653 (0.434–0.984) 0.654 (0.435–0.982) 2 | 0.041 0.041 2 | 0.621 (0.402–0.961) 2 0.620 (0.396–0.972)0.675 | 0.032 0.037 |
| C | 0.20 | 0.25 | 1.413 (1.021–1.955) 1.403 (1.017–1.936) 2 | 0.037 0.039 2 | 1.500 (1.059–2.123) 2 1.507 (1.059–2.144)0.612 | 0.022 0.023 |
| T | 0.30 | 0.25 | 0.708 (0.511–0.979) 0.707 (0.513–976) 2 | 0.037 0.035 2 | 0.667 (0.471–0.944) 2 0.661 (0.470–0.929)0.869 | 0.022 0.017 |
| rs1064261 138/215 (Control/AMD cases) | ||||||
| GG | 0.09 | 0.09 | 0.94 (0.45–1.96) | 0.874 | 0.98 (0.45–2.13) | 0.961 |
| GA | 0.30 | 0.37 | 1.42 (0.90–2.24) | 0.131 | 1.50 (0.92–2.44) | 0.101 |
| AA | 0.61 | 0.53 | 0.74 (0.48–1.14) | 0.174 | 0.70 (0.44–1.10) | 0.123 |
| G | 0.19 | 0.23 | 1.19 (0.85–1.68) | 0.313 | 1.25 (0.87–1.80) | 0.229 |
| A | 0.30 | 0.27 | 0.84 (0.59–1.18) | 0.313 | 0.80 (0.55–1.15) | 0.230 |
| rs573775 160/214 (Control/AMD cases) | ||||||
| AA | 0.14 | 0.07 | 0.492 (0.253–0.954) 0.487 (0.246–0.964) 2 | 0.036 0.039 2 | 0.611 (0.304–1.227) | 0.165 |
| AG | 0.44 | 0.53 | 1.42 (0.94–1.14) | 0.092 2 | 1.31 (0.85–2.03) | 0.221 |
| GG | 0.42 | 0.40 | 0.92 (0.61–1.39) | 0.699 | 0.92 (0.59–1.43) | 0.717 |
| A | 0.29 | 0.30 | 0.90 (0.67–1.21) | 0.493 | 0.94 (0.68–1.29) | 0.707 |
| G | 0.21 | 0.20 | 1.11 (0.82–1.50) | 0.493 | 1.06 (0.77–1.46) | 0.707 |
| rs11246867 161/217 (Control/AMD cases) | ||||||
| AA | 0.00 | 0.00 | 2.1 × 107 (0–0) | 0.997 | 1.6077 × 1010 (0–0) | 1.000 |
| AG | 0.15 | 0.15 | 0.98 (0.55–1.72) | 0.933 | 0.95 (0.52–1.73) | 0.860 |
| GG | 0.85 | 0.85 | 0.99 (0.56–1.40) | 0.970 | 1.00 (0.55–1.83) | 0.994 |
| A | 0.07 | 0.08 | 1.04 (0.61–1.79) | 0.881 | 1.05 (0.59–1.85) | 0.878 |
| G | 0.42 | 0.42 | 0.96 (0.56–1.65) | 0.881 | 0.96 (0.54–1.69) | 0.878 |
| rs3088051 149/216 (Control/AMD cases) | ||||||
| CC | 0.07 | 0.05 | 0.71 (0.30–1.73) | 0.455 | 0.69 (0.26–1.84) | 0.462 |
| CT | 0.36 | 0.38 | 1.10 (0.72–0.69) | 0.665 | 1.13 (0.71–1.80) | 0.599 |
| TT | 0.58 | 0.57 | 0.98 (0.65–1.50) | 0.941 | 0.96 (0.61–1.50) | 0.855 |
| C | 0.21 | 0.22 | 0.96 (0.68–1.35) | 0.822 | 0.98 (0.68–1.41) | 0.903 |
| T | 0.29 | 0.29 | 1.04 (0.74–1.46) | 0.822 | 1.02 (0.71–1.48) | 0.903 |
| rs73105013 156/210 (Control/AMD cases) | ||||||
| CT | 0.21 | 0.18 | 0.79 (0.47–1.32) | 0.366 | 0.73 (0.42–1.23) | 0.252 |
| TT | 0.76 | 0.82 | 1.53 (0.92–2.53) | 0.102 | 1.65 (0.96–2.83) | 0.069 |
| C | 0.12 | 0.09 | 0.597 (0.376–0.949) 0.601 (0.376–0.960) 2 | 0.029 0.033 2 | 0.561 (0.344–0.919) 2 0.565 (0.337–0.947)0.695 | 0.021 0.030 |
| T | 0.38 | 0.41 | 1.674 (1.054–2.660) 1.686 (1.052–2.703) 2 | 0.029 0.030 2 | 1.779 (1.089–2.910) 2 1.776 (1.078–2.927)0.993 | 0.021 0.024 |
| rs10277 136/212 (Control/AMD cases) | ||||||
| CC | 0.46 | 0.35 | 0.657 (0.426–1.015) 0.656 (0.429–1.004) 2 | 0.059 0.052 2 | 0.658 (0.414–1.047) | 0.077 |
| CT | 0.41 | 0.49 | 1.34 (0.87–2.06) | 0.182 | 1.28 (0.80–2.03) | 0.297 |
| TT | 0.13 | 0.16 | 1.27 (0.68–2.38) | 0.450 | 1.41 (0.72–2.76) | 0.316 |
| C | 0.44 | 0.42 | 0.75 (0.54–1.03) | 0.071 | 0.74 (0.53–1.03) | 0.074 |
| T | 0.06 | 0.08 | 1.34 (0.97–1.36) | 0.078 | 1.36 (0.97–1.91) | 0.074 |
| rs10902469 161/217 (Control/AMD cases) | ||||||
| CG | 0.15 | 0.15 | 1.34 (0.97–1.86) | 0.934 | 0.95 (0.52–1.73) | 0.086 |
| GG | 0.85 | 0.85 | 0.99 (0.56–1.74) | 0.970 | 1.00 (0.55–1.83) | 0.994 |
| C | 0.08 | 0.08 | 1.04 (0.61–1.79) | 0.881 | 1.05 (0.59–1.85) | 0.888 |
| G | 0.42 | 0.42 | 0.96 (0.56–1.65) | 0.881 | 0.95 (0.54–1.69) | 0.880 |
| Polymorphism | Genotype | R | p |
|---|---|---|---|
| rs2295080 | GG | 0.137 | 0.040 1 |
| GT | 0.136 | 0.041 1 | |
| TT | 0.088 | 0.377 | |
| rs11121704 | CC | 0.089 | 0.362 |
| CT | 0.099 | 0.313 | |
| TT | 0.089 | 0.367 | |
| rs1057079 | CC | 0.100 | 0.309 |
| CT | 0.106 | 0.281 | |
| TT | 0.131 | 0.049 1 | |
| rs1064261 | GG | 0.093 | 0.344 |
| GA | 0.129 | 0.053 | |
| AA | 0.111 | 0.257 | |
| rs573775 | AA | 0.083 | 0.398 |
| AG | 0.105 | 0.287 | |
| GG | 0.107 | 0.274 | |
| rs11246867 | AA | 0.106 | 0.114 |
| AG | 0.133 | 0.045 1 | |
| GG | 0.106 | 0.278 | |
| rs3088051 | CC | 0.148 | 0.026 1 |
| CT | 0.104 | 0.290 | |
| TT | 0.083 | 0.398 | |
| rs73105013 | CC | ||
| CT | 0.105 | 0.281 | |
| TT | 0.105 | 0.281 | |
| rs10277 | CC | 0.131 | 0.049 1 |
| CT | 0.086 | 0.380 | |
| TT | 0.087 | 0.194 | |
| rs10902469 | CC | ||
| CG | 0.084 | 0.391 | |
| GG | 0.084 | 0.391 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterno, J.J.; Koskela, A.; Hyttinen, J.M.T.; Vattulainen, E.; Synowiec, E.; Tuuminen, R.; Watala, C.; Blasiak, J.; Kaarniranta, K. Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study. Genes 2020, 11, 1318. https://doi.org/10.3390/genes11111318
Paterno JJ, Koskela A, Hyttinen JMT, Vattulainen E, Synowiec E, Tuuminen R, Watala C, Blasiak J, Kaarniranta K. Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study. Genes. 2020; 11(11):1318. https://doi.org/10.3390/genes11111318
Chicago/Turabian StylePaterno, Jussi J., Ali Koskela, Juha M.T. Hyttinen, Elina Vattulainen, Ewelina Synowiec, Raimo Tuuminen, Cezary Watala, Janusz Blasiak, and Kai Kaarniranta. 2020. "Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study" Genes 11, no. 11: 1318. https://doi.org/10.3390/genes11111318
APA StylePaterno, J. J., Koskela, A., Hyttinen, J. M. T., Vattulainen, E., Synowiec, E., Tuuminen, R., Watala, C., Blasiak, J., & Kaarniranta, K. (2020). Autophagy Genes for Wet Age-Related Macular Degeneration in a Finnish Case-Control Study. Genes, 11(11), 1318. https://doi.org/10.3390/genes11111318








