Anti-Hair Loss Potential of Perilla Seed Extracts: In Vitro Molecular Insights from Supercritical Fluid Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Extract Preparation
2.3. Phytochemical Evaluation
2.3.1. Evaluation of Total Phenolic Content
2.3.2. Evaluation of Polyphenol Composition
2.3.3. Evaluation of Tocopherol Composition
2.3.4. Evaluation of Antioxidant Activities
ABTS Radical Scavenging Assay
DPPH Radical Scavenging Assay
Ferric Reducing Antioxidant Power (FRAP) Assay
Lipid Peroxidation Inhibition Using Ferric Thiocyanate (FTC) Assay
2.3.5. Evaluation of Fatty Acid Composition
2.4. Measurement of Cell Viability and Proliferation
2.5. Anti-Inflammatory Activity Assay
2.6. Thiobarbituric Acid-Reactive Substances (TBARS) Assay
2.7. Semi-Quantitative Reverse Transcription and Polymerase Chain Reaction Analysis
3. Results and Discussion
3.1. Extraction Yield, Bioactive Constituents, and Scavenging Potential of Perilla Seed Extracts
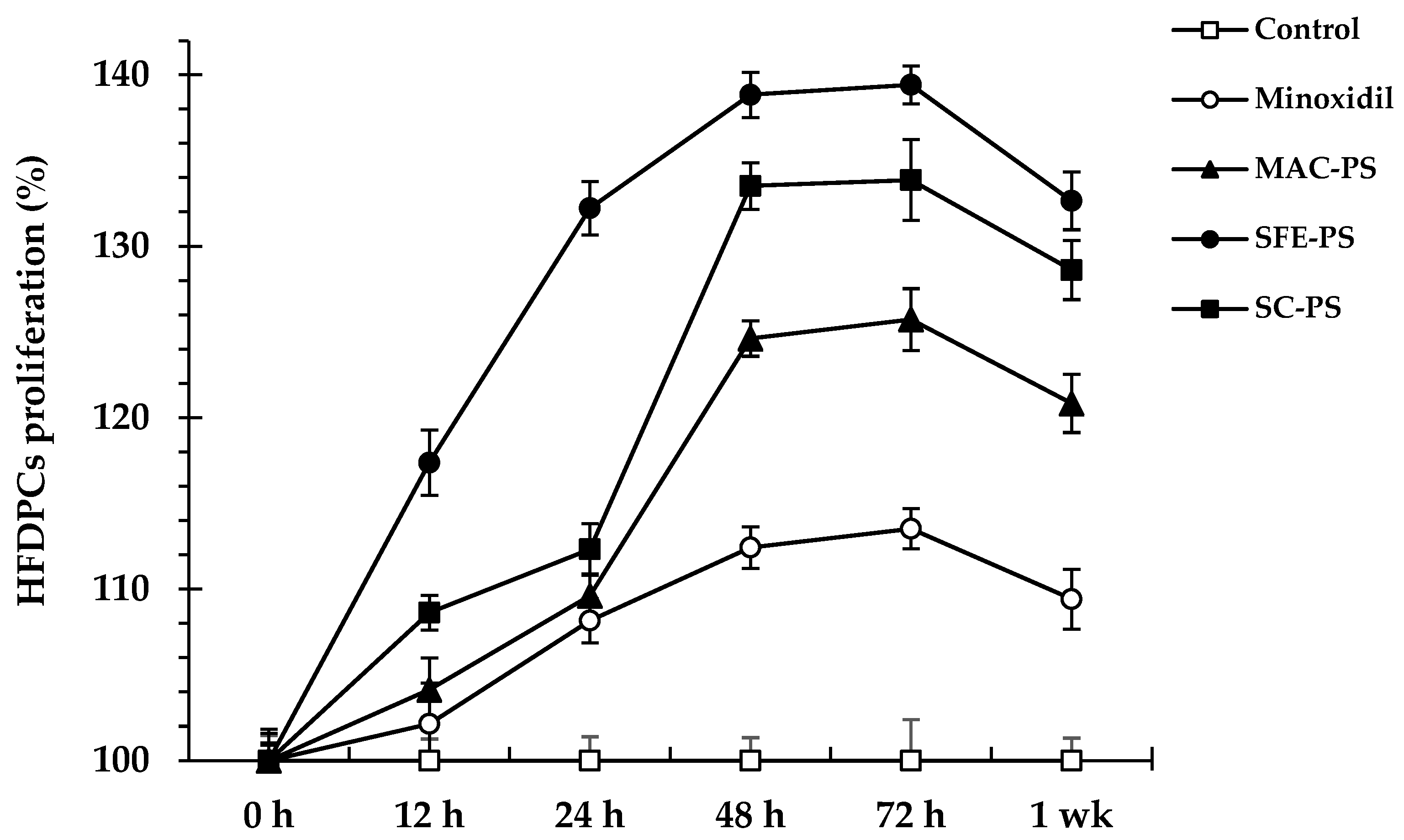
3.2. Effect of Perilla Seed Extracts on Cell Viability and Proliferation
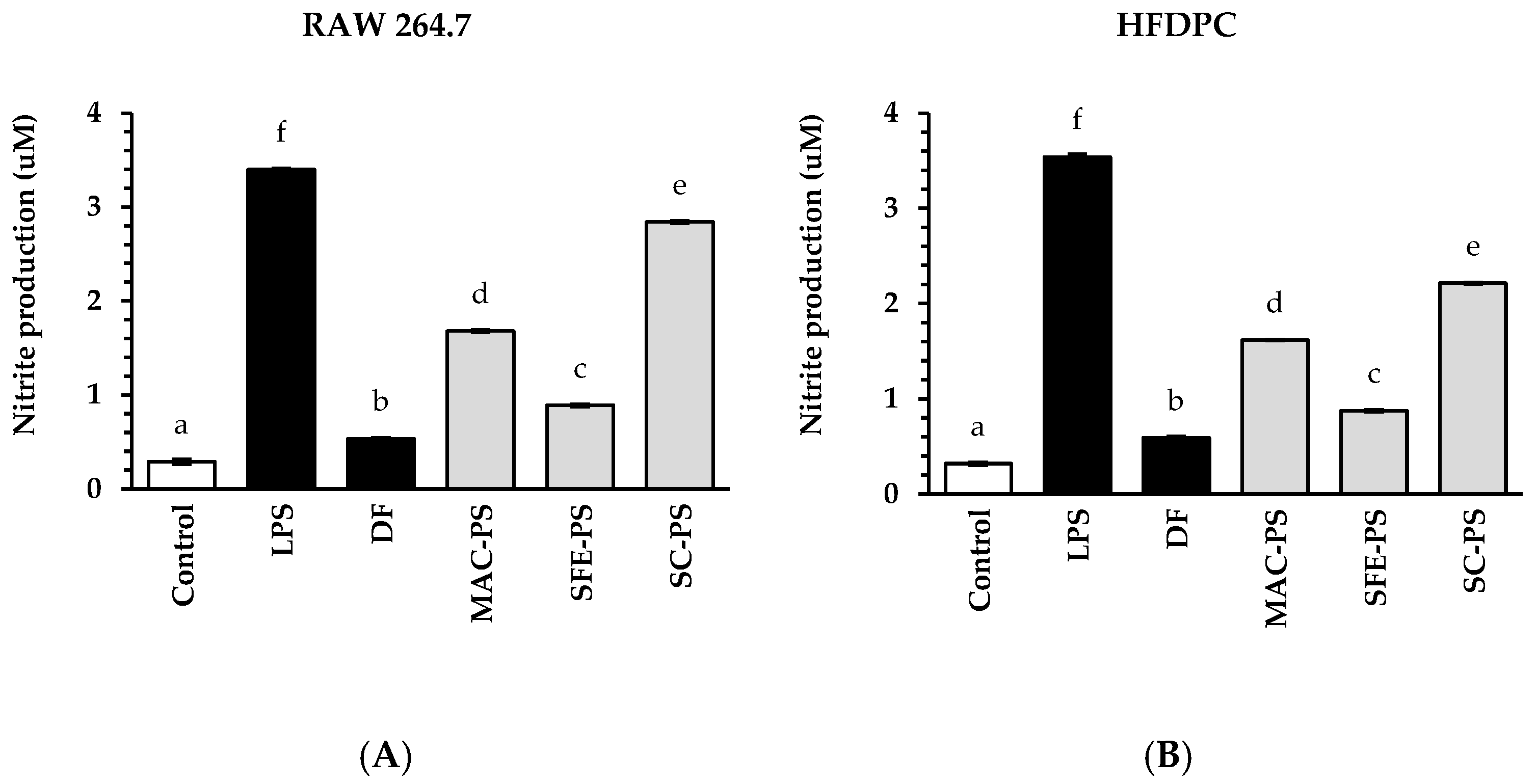
3.3. Effect of Perilla Seed Extracts on Anti-Inflammatory Activities
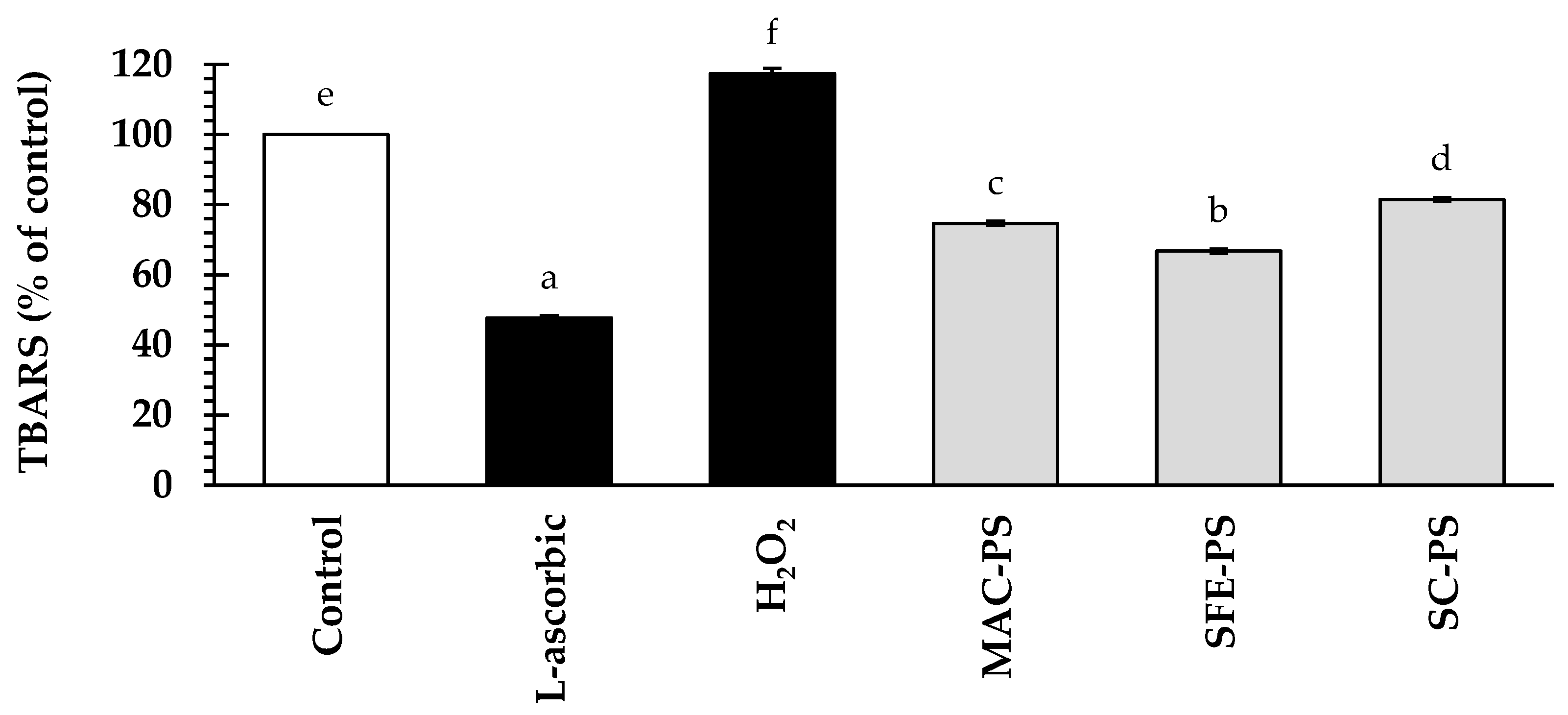
3.4. Effect of Perilla Seed Extracts on Antioxidant Activities
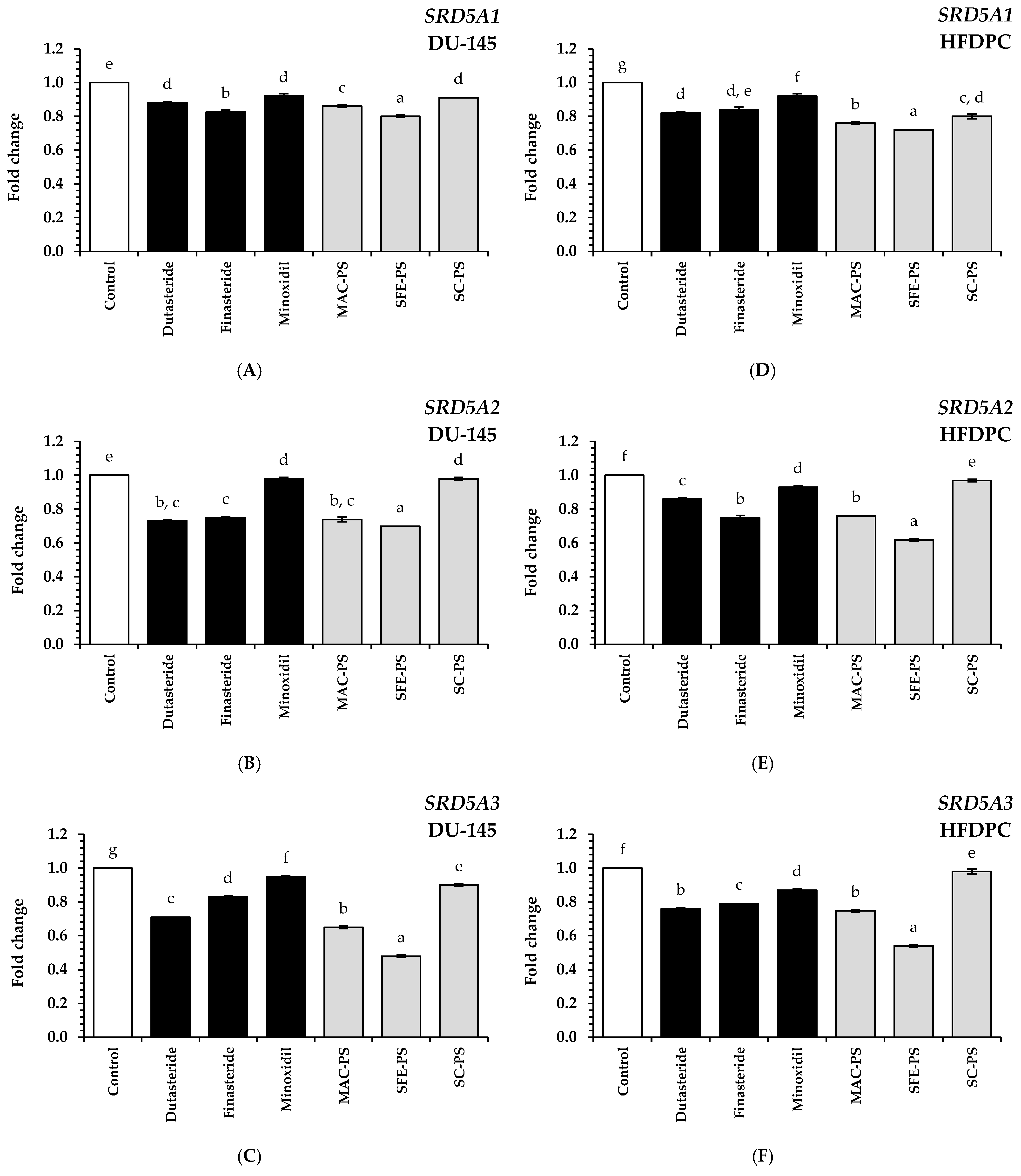
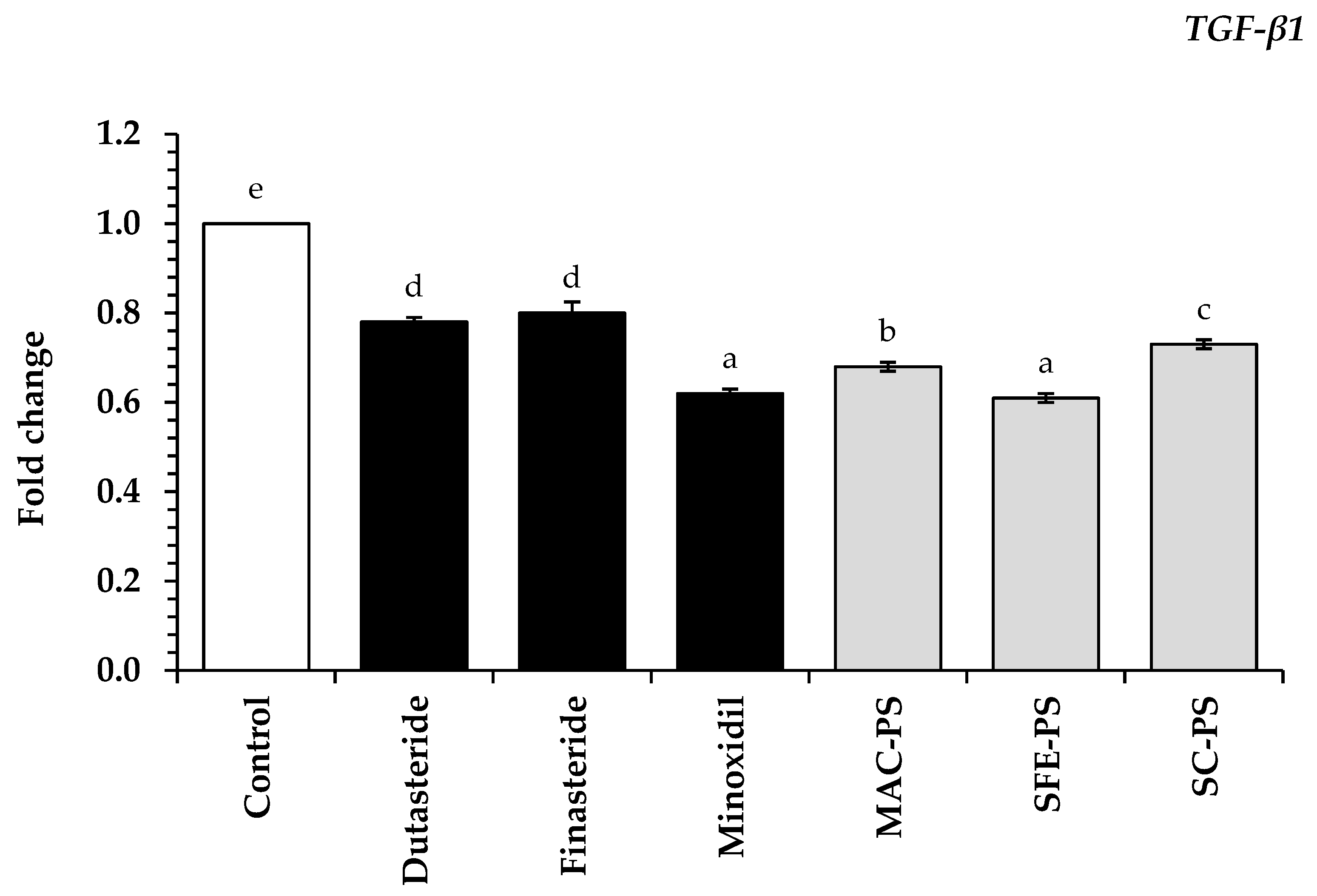
3.5. Effect of Perilla Seed Extracts on Genes Associated with Hair Loss and Hair Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ntshingila, S.; Oputu, O.; Arowolo, A.T.; Khumalo, N.P. Androgenetic alopecia: An update. JAAD Int. 2023, 13, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Muangsanguan, A.; Ruksiriwanich, W.; Linsaenkart, P.; Jantrawut, P.; Rachtanapun, P.; Jantanasakulwong, K.; Sommano, S.R.; Sringarm, K.; Arjin, C.; Sainakham, M. Synergistic phytochemical and pharmacological actions of Hair RiseTM microemulsion: A novel herbal formulation for androgenetic alopecia and hair growth stimulation. Plants 2024, 13, 2802. [Google Scholar] [CrossRef] [PubMed]
- Hibino, T.; Nishiyama, T. Role of TGF-β2 in the human hair cycle. J. Dermatol. Sci. 2004, 35, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Insights, F.B. Herbal Medicine Market Size, Share & Trends, 2024–2032. Available online: https://www.fortunebusinessinsights.com/herbal-medicine-market-106320 (accessed on 20 July 2025).
- Adam, G.; Robu, S.; Flutur, M.-M.; Cioanca, O.; Vasilache, I.-A.; Adam, A.-M.; Mircea, C.; Nechita, A.; Harabor, V.; Harabor, A. Applications of Perilla frutescens extracts in clinical practice. Antioxidants 2023, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Netala, V.R.; Zhang, H.; Xing, Y.; Li, H.; Zhang, Z. Perilla frutescens: A rich source of pharmacological active compounds. Molecules 2022, 27, 3578. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Seem, K.; Ali, A.; Jaiswal, S.; Gumachanamardi, P.; Kaur, G.; Singh, N.; Touthang, L.; Kumar, S.; Bhardwaj, R.A. comprehensive review on nutritional, nutraceutical, and industrial perspectives of perilla (Perilla frutscens L.) seeds–An orphan oilseed crop. Heliyon 2024, 10, e33281. [Google Scholar] [CrossRef] [PubMed]
- Muangsanguan, A.; Ruksiriwanich, W.; Arjin, C.; Jamjod, S.; Prom-u-Thai, C.; Jantrawut, P.; Rachtanapun, P.; Hnorkaew, P.; Satsook, A.; Sainakham, M. Comparison of in vitro hair growth promotion and anti-hair loss potential of Thai rice by-product from Oryza sativa L. cv. buebang 3 CMU and sanpatong. Plants 2024, 13, 3079. [Google Scholar] [CrossRef] [PubMed]
- Soukupova, J.; Malfettone, A.; Bertran, E.; Hernández-Alvarez, M.I.; Peñuelas-Haro, I.; Dituri, F.; Giannelli, G.; Zorzano, A.; Fabregat, I. Epithelial–mesenchymal transition (Emt) induced by TGF-β in hepatocellular carcinoma cells reprograms lipid metabolism. Int. J. Mol. Sci. 2021, 22, 5543. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, D.G.D.S. A comprehensive review of conventional and non-conventional solvent extraction techniques. J. Pharmacogn. Phytochem. 2023, 12, 202–211. [Google Scholar] [CrossRef]
- Tangjaidee, P.; Xiang, J.; Yin, H.; Wen, X.; Quek, S.Y. Selenium, fibre, and protein enrichment of rice product: Extrusion variables and product properties. Food Qual. Saf. 2019, 3, 40–51. [Google Scholar] [CrossRef]
- Muangrat, R.; Chalermchat, Y.; Siriwoharn, T.; Jirarattanarangsri, W.; Tangjaidee, P.; Pongsirikul, I.; Pannasai, S. Ultrasound and low-pressure supercritical CO2 extraction: A synergistic approach to hemp seed oil extraction. J. Appl. Res. Med. Aromat. Plants 2024, 43, 100595. [Google Scholar] [CrossRef]
- Muangsanguan, A.; Linsaenkart, P.; Chaitep, T.; Sangta, J.; Sommano, S.R.; Sringarm, K.; Arjin, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y. Hair growth promotion and anti-hair loss effects of by-products arabica coffee pulp extracts using supercritical fluid extraction. Foods 2023, 12, 4116. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Khantham, C.; Linsaenkart, P.; Chaitep, T.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Režek Jambrak, A.; Nazir, Y.; Yooin, W. Anti-inflammation of bioactive compounds from ethanolic extracts of edible bamboo mushroom (Dictyophora indusiata) as functional health promoting food ingredients. Int. J. Food Sci. Technol. 2022, 57, 110–122. [Google Scholar] [CrossRef]
- Preedalikit, W.; Chittasupho, C.; Leelapornpisid, P.; Potprommanee, S.; Kiattisin, K. Comparison of biological activities and protective effects on PAH-induced oxidative damage of different coffee cherry pulp extracts. Foods 2023, 12, 4292. [Google Scholar] [CrossRef] [PubMed]
- Sringarm, K.; Chaiwang, N.; Wattanakul, W.; Mahinchai, P.; Satsook, A.; Norkeaw, R.; Seel-Audom, M.; Moonmanee, T.; Mekchay, S.; Sommano, S.R. Improvement of intramuscular fat in longissimus muscle of finishing Thai crossbred black pigs by perilla cake supplementation in a low-lysine diet. Foods 2022, 11, 907. [Google Scholar] [CrossRef] [PubMed]
- Vieitez, I.; Maceiras, L.; Jachmanián, I.; Alborés, S. Antioxidant and antibacterial activity of different extracts from herbs obtained by maceration or supercritical technology. J. Supercrit. Fluids 2018, 133, 58–64. [Google Scholar] [CrossRef]
- De Melo, M.; Silvestre, A.; Silva, C. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Zhao, S.; Baik, O.-D.; Choi, Y.J.; Kim, S.-M. Pretreatments for the efficient extraction of bioactive compounds from plant-based biomaterials. Crit. Rev. Food Sci. Nutr. 2014, 54, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Palaiogiannis, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Successive solvent extraction of polyphenols and flavonoids from Cistus creticus L. leaves. Oxygen 2023, 3, 274–286. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The application of supercritical fluid extraction in phenolic compounds isolation from natural plant materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, K.H.; Lee, M.-H.; Kim, H.-T.; Seo, W.D.; Kim, J.Y.; Baek, I.-Y.; Jang, D.S.; Ha, T.J. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013, 136, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Radzali, S.A.; Markom, M.; Saleh, N.M. Co-solvent selection for supercritical fluid extraction (SFE) of phenolic compounds from Labisia pumila. Molecules 2020, 25, 5859. [Google Scholar] [CrossRef] [PubMed]
- Khantham, C.; Linsaenkart, P.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Prom-U-Thai, C. Antioxidation, anti-inflammation, and regulation of SRD5A gene expression of Oryza sativa cv. Bue Bang 3 CMU husk and bran extracts as androgenetic alopecia molecular treatment substances. Plants 2022, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, C.Y. The Role of Vitamin E Isoforms and Metabolites in Cancer Prevention: Mechanistic Insights into Sphingolipid Metabolism Modulation. Nutrients 2024, 16, 4115. [Google Scholar] [CrossRef] [PubMed]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural deep eutectic solvents (NADES): Phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Jirarattanarangsri, W.; Muangrat, R. Comparison of supercritical CO2 and screw press extraction methods for producing oil from Camellia sinensis var. assamica seeds: Physicochemical properties and antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100413. [Google Scholar] [CrossRef]
- Barouh, N.; Bourlieu-Lacanal, C.; Figueroa-Espinoza, M.C.; Durand, E.; Villeneuve, P. Tocopherols as antioxidants in lipid-based systems: The combination of chemical and physicochemical interactions determines their efficiency. Compr. Rev. Food Sci. Food Saf. 2022, 21, 642–688. [Google Scholar] [CrossRef] [PubMed]
- Pamunuwa, G.K.; Prasadani, M.; Nanayakkara, T.U.; Atapattu, S.N. Effect of liposomal encapsulation of curcumin and α-tocopherol on sensory and physicochemical properties, and retention of antioxidant capacity of fortified cookies during baking. Food Chem. Adv. 2023, 3, 100504. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Amorati, R.; Cariola, A.; Valgimigli, L.; Napolitano, A. Role of sulphur and heavier chalcogens on the antioxidant power and bioactivity of natural phenolic compounds. Biomolecules 2022, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Antônio, M.; Araújo, D.; Galberto, M.; da Costa, J. Chemical properties of caffeic and ferulic acids in biological system: Implications in cancer therapy. A review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Hussain, S.; Hussain, N.; Kakar, K.U.; Shah, J.M.; Zaidi, S.H.R.; Jan, M.; Zhang, K.; Khan, M.A.; Imtiaz, M. Tocopherol as plant protector: An overview of Tocopherol biosynthesis enzymes and their role as antioxidant and signaling molecules. Acta Physiol. Plant. 2022, 44, 20. [Google Scholar] [CrossRef]
- Peterle, L.; Sanfilippo, S.; Borgia, F.; Cicero, N.; Gangemi, S. Alopecia areata: A review of the role of oxidative stress, possible biomarkers, and potential novel therapeutic approaches. Antioxidants 2023, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Li, J.; Zhang, S.; Zeng, X.; Nie, J.; Li, Z. Oxidative stress in hair follicle development and hair growth: Signalling pathways, intervening mechanisms and potential of natural antioxidants. J. Cell. Mol. Med. 2024, 28, e18486. [Google Scholar] [CrossRef] [PubMed]
- Prayitno, T.; Widyorini, R.; Lukmandaru, G. Chemical variation of five natural extracts by non-polar solvent. Maderas. Cienc. Y Tecnol. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; He, H. The role of linoleic acid in skin and hair health: A review. Int. J. Mol. Sci. 2024, 26, 246. [Google Scholar] [CrossRef] [PubMed]
- Khantham, C.; Ruksiriwanich, W.; Sringarm, K.; Prom-U-Thai, C.; Jamjod, S.; Arjin, C.; Muangsanguan, A.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y. Effects of bioactive composition in Oryza sativa L. cv. KDML105 bran extract on gene expression related to hair cycle in human hair follicle dermal papilla cells. Agronomy 2023, 13, 295. [Google Scholar] [CrossRef]
- Thangaraju, P.; Varthya, S.B. ISO 10993: Biological evaluation of medical devices. In Medical Device Guidelines and Regulations Handbook; Timiri Shanmugam, P.S., Thangaraju, P., Palani, N., Sampath, T., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 163–187. [Google Scholar]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional hair follicle regeneration: An updated review. Signal Transduct. Target. Ther. 2021, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates β-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Park, S. Hair follicle morphogenesis during embryogenesis, neogenesis, and organogenesis. Front. Cell Dev. Biol. 2022, 10, 933370. [Google Scholar] [CrossRef] [PubMed]
- Linsaenkart, P.; Ruksiriwanich, W.; Muangsanguan, A.; Sommano, S.R.; Sringarm, K.; Arjin, C.; Rachtanapun, P.; Jantanasakulwong, K.; Castagnini, J.M.; Chutoprapat, R. Antioxidant, anti-inflammation, and melanogenesis inhibition of Sang 5 CMU rice (Oryza sativa) byproduct for cosmetic applications. Plants 2024, 13, 1795. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Schönfelder, G.; Paul, M.; Blume-Peytavi, U. Nitric oxide in the human hair follicle: Constitutive and dihydrotestosterone-induced nitric oxide synthase expression and NO production in dermal papilla cells. J. Mol. Med. 2003, 81, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Yanez, D.A.; Lacher, R.K.; Vidyarthi, A.; Colegio, O.R. The role of macrophages in skin homeostasis. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Sowden, H.; Naseem, K.; Tobin, D. Differential expression of nitric oxide synthases in human scalp epidermal and hair follicle pigmentary units: Implications for regulation of melanogenesis. Br. J. Dermatol. 2005, 153, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Role of dietary polyphenols in the activity and expression of nitric oxide synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J.B. Caffeic acid derivatives: In vitro and in vivo anti-inflammatory properties. Free Radic. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Faralli, A.; Shekarforoush, E.; Mendes, A.C.; Chronakis, I.S. Enhanced transepithelial permeation of gallic acid and (−)-epigallocatechin gallate across human intestinal caco-2 cells using electrospun xanthan nanofibers. Pharmaceutics 2019, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, J.B.; Walle, T. Transport and metabolism of the tea flavonoid (–)-epicatechin by the human intestinal cell line Caco-2. Pharm. Res. 2001, 18, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, H.; Zheng, S.; Zhang, B.; Deng, Z.-y. Implication of the significance of dietary compatibility: Based on the antioxidant and anti-inflammatory interactions with different ratios of hydrophilic and lipophilic antioxidants among four daily agricultural crops. J. Agric. Food Chem. 2018, 66, 7461–7474. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant activity, metabolism, and bioavailability of polyphenols in the diet of animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Cwynar, A.; Olszewska-Słonina, D.; Czajkowski, R.; Zegarska, B.; Białecka, A.; Męcińska-Jundziłł, K.; Piskorska, E.; Lampka, M. Investigation of oxidative stress in patients with alopecia areata by measuring the levels of malondialdehyde and ceruloplasmin in the blood. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2018, 35, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Koonyosying, P.; Kusirisin, W.; Kusirisin, P.; Kasempitakpong, B.; Sermpanich, N.; Tinpovong, B.; Salee, N.; Pattanapanyasat, K.; Srichairatanakool, S.; Paradee, N. Perilla Fruit Oil-Fortified Soybean Milk Intake Alters Levels of Serum Triglycerides and Antioxidant Status, and Influences Phagocytotic Activity among Healthy Subjects: A Randomized Placebo-Controlled Trial. Nutrients 2022, 14, 1721. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Phytochemical study of polyphenols in Perilla Frutescens as an antioxidant. Avicenna J. Phytomedi. 2012, 2, 169. [Google Scholar]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [PubMed]
- York, K.; Meah, N.; Bhoyrul, B.; Sinclair, R. A review of the treatment of male pattern hair loss. Expert Opin. Pharmaco. 2020, 21, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Tang, X.; Zhang, X.; Cao, C.; Yu, M.; Wan, M. Adipose mesenchymal stromal cell-derived exosomes carrying MiR-122-5p antagonize the inhibitory effect of dihydrotestosterone on hair follicles by targeting the TGF-β1/SMAD3 signaling pathway. Int. J. Mol. Sci. 2023, 24, 5703. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Pan, Z.; Yang, C.; Chen, L.; Wang, Y.; Xu, D.; Xia, H.; Wang, S.; Chen, S. Potential “therapeutic” effects of tocotrienol-rich fraction (TRF) and carotene “against” bleomycin-induced pulmonary fibrosis in rats via TGF-β/Smad, PI3K/Akt/mTOR and NF-κB signaling pathways. Nutrients 2022, 14, 1094. [Google Scholar] [CrossRef] [PubMed]
- Rabbaa, S.; Bouchab, H.; Laaziouez, Y.; Limami, Y.; Nasser, B.; Andreoletti, P.; Cherkaoui-Malki, M.; El Kebbaj, R. Argan Oil: A natural bioactive lipid modulating oxidative stress and inflammation. Antioxidants 2025, 14, 515. [Google Scholar] [CrossRef] [PubMed]
| Target Pathway | Primers | Gene Bank No. | Sequences | Primer Sequences (5’ to 3’) | Annealing Temperature (°C) |
|---|---|---|---|---|---|
| Internal control | GAPDH | NM_001289745.3 | Sense Antisense | GGAAGGTGAAGGTCGGAGTC CTCAGCCTTGACGGTGCCATG | 55 |
| 5α-reductase | SRD5A1 | NM_001047.4 | Sense Antisense | AGCCATTGTGCAGTGTATGC AGCCTCCCCTTGGTATTTTG | 52 |
| SRD5A2 | NM_000348.4 | Sense Antisense | TGAATACCCTGATGGGTGG CAAGCCACCTTGTGGAATC | 52 | |
| SRD5A3 | NM_024592.5 | Sense Antisense | TCCTTCTTTGCCCAAACATC TCCTTCTTTGCCCAAACATC | 50 | |
| Transforming growth factor beta 1 | TGF-β1 | NM_00600.7 | Sense Antisense | AACCCACAACGAAATCTATG CTTTTAACTTGAGCCTCAGC | 58 |
| Results | Extracts | |||
|---|---|---|---|---|
| MAC-PS | SFE-PS | SC-PS | ||
| Extraction yield (%) | 42.34 ± 0.11 | 33.93 ± 0.28 | 22.46 ± 0.20 | |
| Total phenolic content (mg GAE/g extract) | 23.07 ± 0.32 | 8.22 ± 0.73 | 1.17 ± 0.39 | |
| Polyphenols (μg/g extract) | Gallic acid | 8.27 ± 0.01 | ND | ND |
| Epicatehchin | 29.14 ± 4.36 | ND | ND | |
| Caffeic acid | 17.54 ± 0.28 b | 12.80 ± 0.00 a | ND | |
| Epicathechin gallate | 8.69 ± 0.78 | ND | ND | |
| Naringin | 7.32 ± 0.11 | ND | ND | |
| Rosmarinic acid | 16.35 ± 0.05 | ND | ND | |
| o-Coumaric acid | 6.24 ± 0.33 | ND | ND | |
| p-Coumaric acid | 13.23 ± 0.01 | ND | ND | |
| Quercetin | 7.58 ± 0.12 | ND | ND | |
| Tocopherol (μg/g extract) | α-Tocopherol | ND | 20.58 ± 1.87 a | 25.68 ± 0.36 b |
| β- and γ-Tocopherol | ND | 485.84 ± 0.84 a | 513.16 ± 3.77 b | |
| δ-Tocopherol | ND | 13.40 ± 0.50 a | 14.08 ± 0.02 b | |
| Antioxidant activity | ABTS radical scavenging (%) | 85.72 ± 0.96 c | 38.30 ± 0.57 b | 28.89 ± 1.82 a |
| DPPH radical scavenging (%) | 75.70 ± 2.71 c | 45.29 ± 0.72 b | 32.19 ± 0.90 a | |
| FRAP (mM Fe2+/g extract) | 68.32 ± 2.11 a | 121.21 ± 2.72 c | 92.38 ± 1.14 b | |
| FTC (mg TEAC/g extract) | 9.85 ± 1.36 a | 17.38 ± 1.02 b | 19.53 ± 1.04 c | |
| Classification | Name | MAC-PS | SFE-PS | SC-PS |
|---|---|---|---|---|
| Saturated Fatty Acids | Pentadecanoic acid | ND | 0.01 a | 0.01 a |
| Palmitic acid | ND | 6.83 a | 6.88 b | |
| Heptadecanoic acid | ND | 0.14 a | 0.14 a | |
| Stearic acid | ND | 2.88 a | 2.90 b | |
| Arachidic acid | ND | 0.18 a | 0.18 a | |
| Behenic acid | ND | 0.02 a | 0.03 b | |
| Heneicosanoic acid | ND | 0.05 a | 0.05 a | |
| Butyric acid | ND | 0.76 a | 0.86 b | |
| Myristic acid | ND | 0.02 a | 0.02 a | |
| Monounsaturated Fatty Acids | Palmitoleic acid | ND | 0.01 a | 0.01 a |
| Heptadecenoic acid | ND | 0.01 a | 0.01 a | |
| Oleic acid | ND | 11.75 a | 11.74 a | |
| Gondoic acid | ND | 0.04 a | 0.07 b | |
| Erucic acid | ND | 0.03 b | 0.01 a | |
| Nervonic acid | ND | 0.01 a | 0.01 a | |
| Polyunsaturated Fatty Acids | Trans-Linoleic acid | ND | 0.07 b | 0.06 a |
| Linoleic acid | ND | 17.45 b | 17.36 a | |
| γ-Linolenic acid | ND | 0.21 a | 0.21 a | |
| α-Linolenic acid | ND | 59.43 b | 59.23 a | |
| Eicosadienoic acid | ND | 0.01 a | 0.02 b | |
| Dihomo-γ-linolenic acid | ND | 0.02 a | 0.02 a | |
| Eicosatrienoic acid | ND | 0.01 a | 0.01 a | |
| Eicosapentaenoic acid | ND | 0.02 a | 0.02 a | |
| Docosahexaenoic acid | ND | 0.01 a | 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muangsanguan, A.; Ruksiriwanich, W.; Tangjaidee, P.; Sringarm, K.; Arjin, C.; Rachtanapun, P.; Sommano, S.R.; Chaisu, K.; Satsook, A.; Castagnini, J.M. Anti-Hair Loss Potential of Perilla Seed Extracts: In Vitro Molecular Insights from Supercritical Fluid Extraction. Foods 2025, 14, 2583. https://doi.org/10.3390/foods14152583
Muangsanguan A, Ruksiriwanich W, Tangjaidee P, Sringarm K, Arjin C, Rachtanapun P, Sommano SR, Chaisu K, Satsook A, Castagnini JM. Anti-Hair Loss Potential of Perilla Seed Extracts: In Vitro Molecular Insights from Supercritical Fluid Extraction. Foods. 2025; 14(15):2583. https://doi.org/10.3390/foods14152583
Chicago/Turabian StyleMuangsanguan, Anurak, Warintorn Ruksiriwanich, Pipat Tangjaidee, Korawan Sringarm, Chaiwat Arjin, Pornchai Rachtanapun, Sarana Rose Sommano, Korawit Chaisu, Apinya Satsook, and Juan Manuel Castagnini. 2025. "Anti-Hair Loss Potential of Perilla Seed Extracts: In Vitro Molecular Insights from Supercritical Fluid Extraction" Foods 14, no. 15: 2583. https://doi.org/10.3390/foods14152583
APA StyleMuangsanguan, A., Ruksiriwanich, W., Tangjaidee, P., Sringarm, K., Arjin, C., Rachtanapun, P., Sommano, S. R., Chaisu, K., Satsook, A., & Castagnini, J. M. (2025). Anti-Hair Loss Potential of Perilla Seed Extracts: In Vitro Molecular Insights from Supercritical Fluid Extraction. Foods, 14(15), 2583. https://doi.org/10.3390/foods14152583












