Effect of Sugar-Free Jelly on Glycemic Metabolism and Its Potential Health Benefits in Non-Diabetic Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment and Population
2.2. Experimental Design
2.3. Products
2.4. Blood Analysis
2.5. Satiety
2.6. Gut Quotient
2.7. Statistical Analysis
3. Results
3.1. General Participant Characteristics
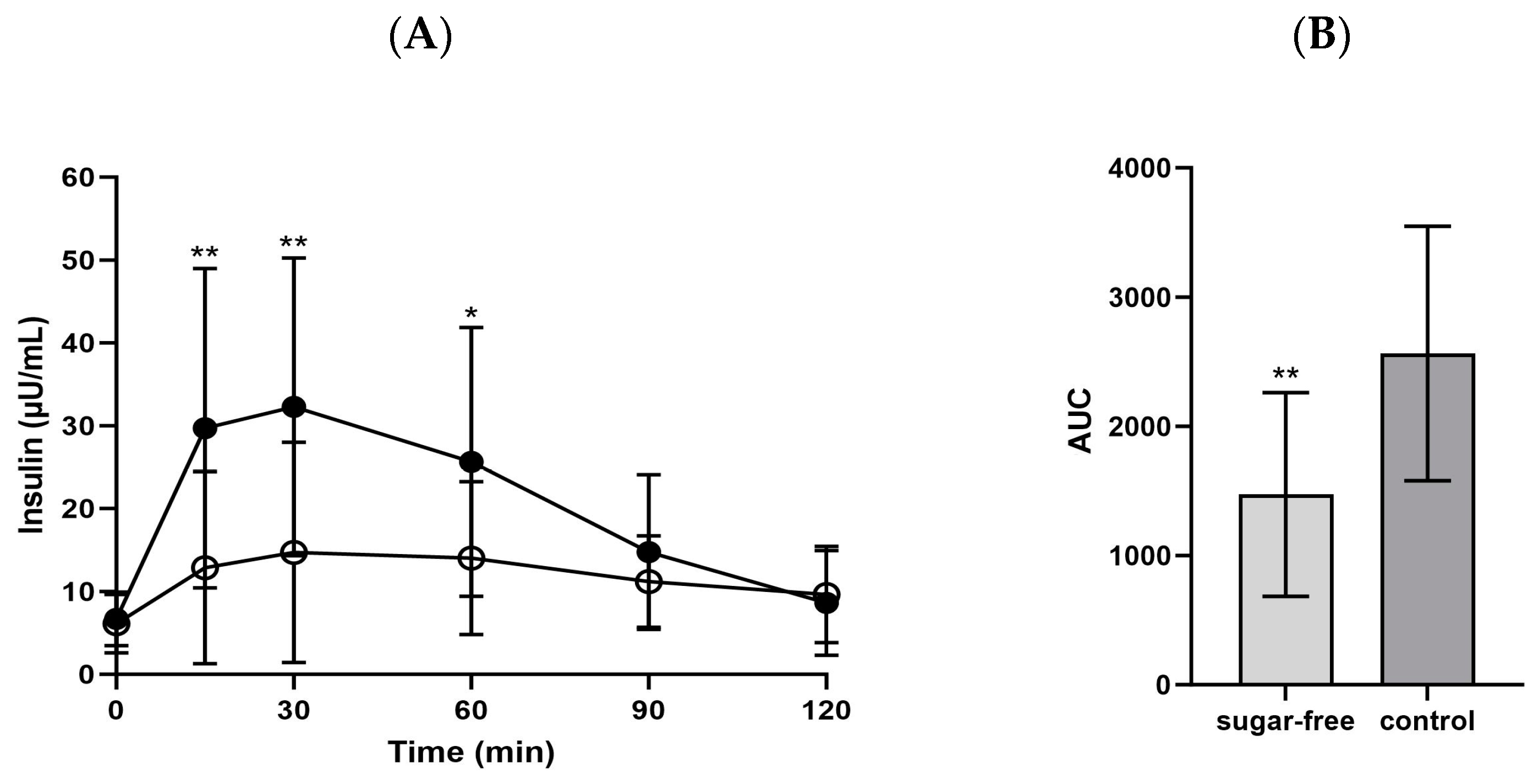
3.2. Glucose Metabolism Associated Biomarkers
3.3. Satiety and Ghrelin
3.4. GQ
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olivia Lawe Davies, C.L. WHO Calls on Countries to Reduce Sugars Intake among Adults and Children. Available online: https://www.who.int/news/item/04-03-2015-who-calls-on-countries-to-reduce-sugars-intake-among-adults-and-children (accessed on 7 November 2023).
- Jayalath, V.; de Souza, R.; Ha, V.; Mirrahimi, A.; Blanco-Mejia, S.; Di Buono, M.; Jenkins, A.; Leiter, L.; Wolever, T.; Beyene, J. Sugar-sweetened beverage consumption and incident hypertension: A systematic review and meta-analysis of prospective cohorts. Am. J. Clin. Nutr. 2015, 102, 914–921. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Zelek, L.; Agaesse, C.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; et al. Total and added sugar intakes, sugar types, and cancer risk: Results from the prospective NutriNet-Sante cohort. Am. J. Clin. Nutr. 2020, 112, 1267–1279. [Google Scholar] [CrossRef]
- Yeung, C.H.C.; Gohil, P.; Rangan, A.M.; Flood, V.M.; Arcot, J.; Gill, T.P.; Louie, J.C.Y. Modelling of the impact of universal added sugar reduction through food reformulation. Sci. Rep. 2017, 7, 17392. [Google Scholar] [CrossRef]
- Kim, E.; Ahn, J.A.; Jang, J.K.; Lee, M.A.; Seo, S.H.; Lee, E.-J. Consumer Perceptions and Attitudes towards Reducing Sugar Intake. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1865–1872. [Google Scholar] [CrossRef]
- Bang, S.K.; Son, E.-J.; Kim, H.-J.; Park, S. Quality Characteristics and Glycemic Index of Oatmeal Cookies Made with Artificial Sweeteners. J. Korean Soc. Food Sci. Nutr. 2013, 42, 877–884. [Google Scholar] [CrossRef]
- Shin, I.-Y.; Kim, H.-I.; Kim, C.-S.; Whang, K. Characteristics of sugar cookies with replacement of sucrose with sugar alcohols (I) Organoleptic characteristics of sugar alcohol cookies. J. Korean Soc. Food Sci. Nutr. 1999, 28, 850–857. [Google Scholar]
- Park, S.-M.; Kim, Y.-S.; Yoon, I.-C.; Seo, E.-H.; Ko, B.-S.; Choi, S.-B. Development and hypoglycemic effect of low-fat and sugar-free cookie. Korean J. Food Sci. Technol. 2002, 34, 487–492. [Google Scholar]
- Anton, S.D.; Martin, C.K.; Han, H.; Coulon, S.; Cefalu, W.T.; Geiselman, P.; Williamson, D.A. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 2010, 55, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ni, J. WHO Advises Not to Use Non-Sugar Sweeteners for Weight Control in Newly Released Guideline. Available online: https://www.who.int/news/item/15-05-2023-who-advises-not-to-use-non-sugar-sweeteners-for-weight-control-in-newly-released-guideline (accessed on 7 November 2023).
- Han, J.-S.; Kim, G.-E.; Park, J.-W.; Park, C.-J. Polydextrose and sugar alcohols: Their properties and uses in foods. Food Sci. Ind. 2019, 52, 375–386. [Google Scholar]
- Kim, Y.H.; Kim, S.-B.; Kim, S.J.; Park, S.-W. Market and trend of alternative sweeteners. Food Sci. Ind. 2016, 49, 17–28. [Google Scholar]
- Grembecka, M. Sugar alcohols—Their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef]
- Bayindir GÜMÜŞ, A.; Keser, A.; TunÇEr, E.; AltuntaŞ Yildiz, T.; Kepenekcİ Bayram, İ. Does Saccharin Have Effect on Appetite, Energy Intake, and Serum Ghrelin? A Randomized, Controlled, Cross-Over Study in Healthy Males. Konuralp Tıp Derg. 2022, 14, 415–423. [Google Scholar] [CrossRef]
- Higgins, K.A.; Mattes, R.D. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am. J. Clin. Nutr. 2019, 109, 1288–1301. [Google Scholar] [CrossRef]
- Higgins, K.A.; Considine, R.V.; Mattes, R.D. Aspartame Consumption for 12 Weeks Does Not Affect Glycemia, Appetite, or Body Weight of Healthy, Lean Adults in a Randomized Controlled Trial. J. Nutr. 2018, 148, 650–657. [Google Scholar] [CrossRef] [PubMed]
- ISO 26642:2010; Food Products—Determination of the Glycaemic Index (GI) and Recommendation for Food Classification. ISO: Geneva, Switzerland, 2010.
- Quilez, J.; Bullo, M.; Salas-Salvado, J. Improved postprandial response and feeling of satiety after consumption of low-calorie muffins with maltitol and high-amylose corn starch. J. Food Sci. 2007, 72, S407–S411. [Google Scholar] [CrossRef]
- Jones, A.G.; Hattersley, A.T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 2013, 30, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Kohzuma, T.; Tao, X.; Koga, M. Glycated albumin as biomarker: Evidence and its outcomes. J. Diabetes Complicat. 2021, 35, 108040. [Google Scholar] [CrossRef] [PubMed]
- Little, R.R.; Sacks, D.B. HbA1c: How do we measure it and what does it mean? Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Lee, E.S.; Oh, S.W.; Kim, Y.H.; Lee, D.E.; Hwang, C.K.; Lee, E.Y.; Yoon, Y.S.; Yang, Y.J. Validity, Reproducibility of Visual Analogue Scales in Assessment of Appetite Sensations. J. Korean Acad. Fam. Med. 2008, 29, 736–745. [Google Scholar]
- Choi, Y.J.; Cho, J.H.; Lee, D.H.; Song, D.J.; Kwon, Y.J.; Baek, S.M.; Kim, Y.J.; Jang, M.H.; Lee, D.H.; Park, H.Y.; et al. Development of Koreans Gut Quotient Measurement Scales. Korean J. Gastroenterol. 2019, 73, 341–349. [Google Scholar] [CrossRef]
- Argyri, K.; Sotiropoulos, A.; Psarou, E.; Papazafiropoulou, A.; Zampelas, A.; Kapsokefalou, M. Dessert formulation using sucralose and dextrin affects favorably postprandial response to glucose, insulin, and C-peptide in type 2 diabetic patients. Rev. Diabet. Stud. 2013, 10, 39–48. [Google Scholar] [CrossRef]
- Thabuis, C.; Rodriguez, B.; Gala, T.; Salvi, A.; Parashuraman, M.; Wils, D.; Guerin-Deremaux, L. Evaluation of glycemic and insulinemic responses of maltitol in Indian healthy volunteers. Int. J. Diabetes Dev. Ctries. 2015, 35, 482–487. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Geiselman, P.J.; Novin, D. The role of carbohydrates in appetite, hunger and obesity. Appetite 1982, 3, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Khouryieh, H.A.; Aramouni, F.M.; Herald, T.J. Physical, chemical and sensory properties of sugar-free jelly. J. Food Qual. 2005, 28, 179–190. [Google Scholar] [CrossRef]





| Sugar-Free | Control | |
|---|---|---|
| Calorie (kcal) | 126.5 | 187.0 |
| Carbohydrate (g) | 40.2 | 42.3 |
| Sugar (g) | 0.0 | 31.5 |
| Sugar alcohols (g) | 30.7 | 0.0 |
| Protein (g) | 3.4 | 3.8 |
| Fat (g) | 0.1 | 0.3 |
| n = 16 | Reference Values | |
|---|---|---|
| Age (years) [Range] | 35.8 ± 7.5 [26–52] 1 | - |
| Male/female (n) | 3/13 | - |
| Height (cm) | 165.3 ± 7.1 | - |
| Weight (kg) | 63.4 ± 12.9 | - |
| Fasting glucose (mg/dL) | 107.9 ± 9.2 | 70–99 |
| BMI (kg/m2) | 23.1 ± 3.9 | 18.5–22.9 |
| Systolic blood pressure (mmHg) | 116.8 ± 14.1 | <120 |
| Diastolic blood pressure (mmHg) | 74.3 ± 13.1 | <80 |
| Pulse rate (bpm) | 77.6 ± 8.9 | 60–100 |
| C-peptide (ng/mL) | 2.1 ± 0.5 | 1.10–4.40 |
| HbA1c (%) | 5.3 ± 0.3 | <5.7 |
| Glycated Albumin (%) | 12.3 ± 1.2 | 11–16 |
| Baseline | Sugar-Free | Control | p-Value 2 | |
|---|---|---|---|---|
| C-peptide (ng/mL) | 2.06 ± 0.53 1 | 1.88 ± 0.50 | 1.79 ± 0.49 | 0.418 |
| HbA1c (%) | 5.31 ± 0.29 | 5.26 ± 0.27 | 5.23 ± 0.29 | 0.819 |
| GA (%) | 12.26 ± 1.19 | 12.52 ± 0.95 | 12.58 ± 0.90 | 0.792 |
| Sugar-Free | Control | p-Value 2 | |
|---|---|---|---|
| Ghrelin (pg/mL) | |||
| 0 min | 1368.7 ± 489.6 1 | 1303.1 ± 488.3 | 0.462 |
| 90 min | 1197.3 ± 329.7 | 1078.6 ± 389.9 | 0.142 |
| 120 min | 1162.6 ± 258.6 | 1185.8 ± 439.9 | 0.763 |
| % change | |||
| 90 min/0 min | −9.3 ± 12.8 | −16.5 ± 9.8 | 0.042 * |
| 120 min/90 min | −1.5 ± 10.2 | 9.7 ± 8.0 | 0.002 ** |
| Baseline | Sugar-Free | Control | p-Value 2 | |
|---|---|---|---|---|
| GQ | 85.75 ± 15.15 1 | 72.31 ± 25.62 | 87.81 ± 10.50 | 0.186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Kim, Y.; Gim, M.; Shin, H.; Jang, H.; Yoon, W.J.; Lee, G.-H.; Park, Y.K. Effect of Sugar-Free Jelly on Glycemic Metabolism and Its Potential Health Benefits in Non-Diabetic Adults. Foods 2024, 13, 920. https://doi.org/10.3390/foods13060920
Han H, Kim Y, Gim M, Shin H, Jang H, Yoon WJ, Lee G-H, Park YK. Effect of Sugar-Free Jelly on Glycemic Metabolism and Its Potential Health Benefits in Non-Diabetic Adults. Foods. 2024; 13(6):920. https://doi.org/10.3390/foods13060920
Chicago/Turabian StyleHan, Heejin, Yuri Kim, Minchul Gim, Hoyeon Shin, Hyunsook Jang, Won Joo Yoon, Gyeong-Hweon Lee, and Yoo Kyoung Park. 2024. "Effect of Sugar-Free Jelly on Glycemic Metabolism and Its Potential Health Benefits in Non-Diabetic Adults" Foods 13, no. 6: 920. https://doi.org/10.3390/foods13060920
APA StyleHan, H., Kim, Y., Gim, M., Shin, H., Jang, H., Yoon, W. J., Lee, G.-H., & Park, Y. K. (2024). Effect of Sugar-Free Jelly on Glycemic Metabolism and Its Potential Health Benefits in Non-Diabetic Adults. Foods, 13(6), 920. https://doi.org/10.3390/foods13060920







