Efficacy and Safety of Bifidobacterium longum Supplementation in Infants: A Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment and Statistical Analysis
3. Results
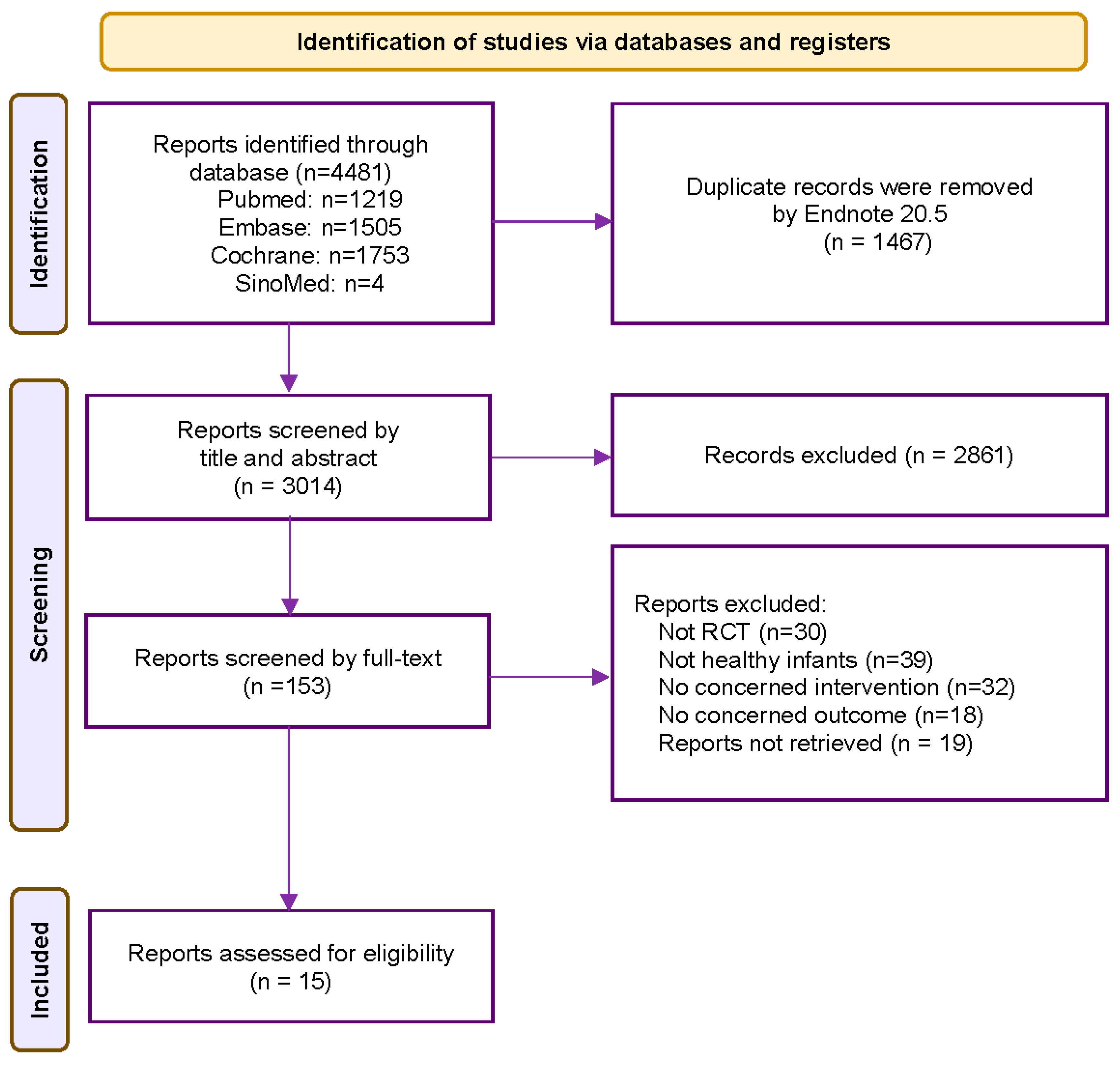
3.1. Study Selection and Characteristics
3.2. Study Quality
3.3. Efficacy Outcomes
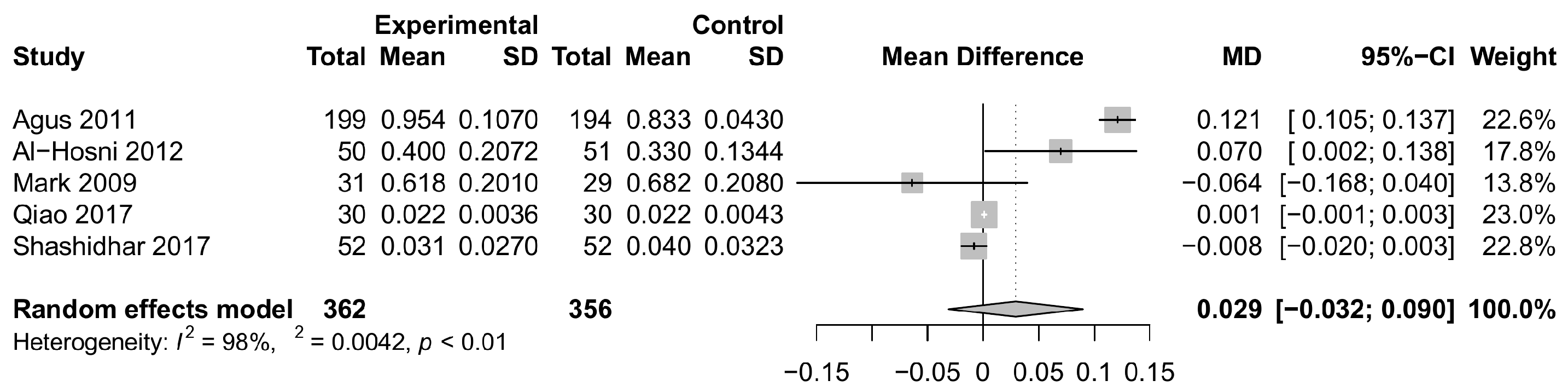
3.3.1. Weight Gain
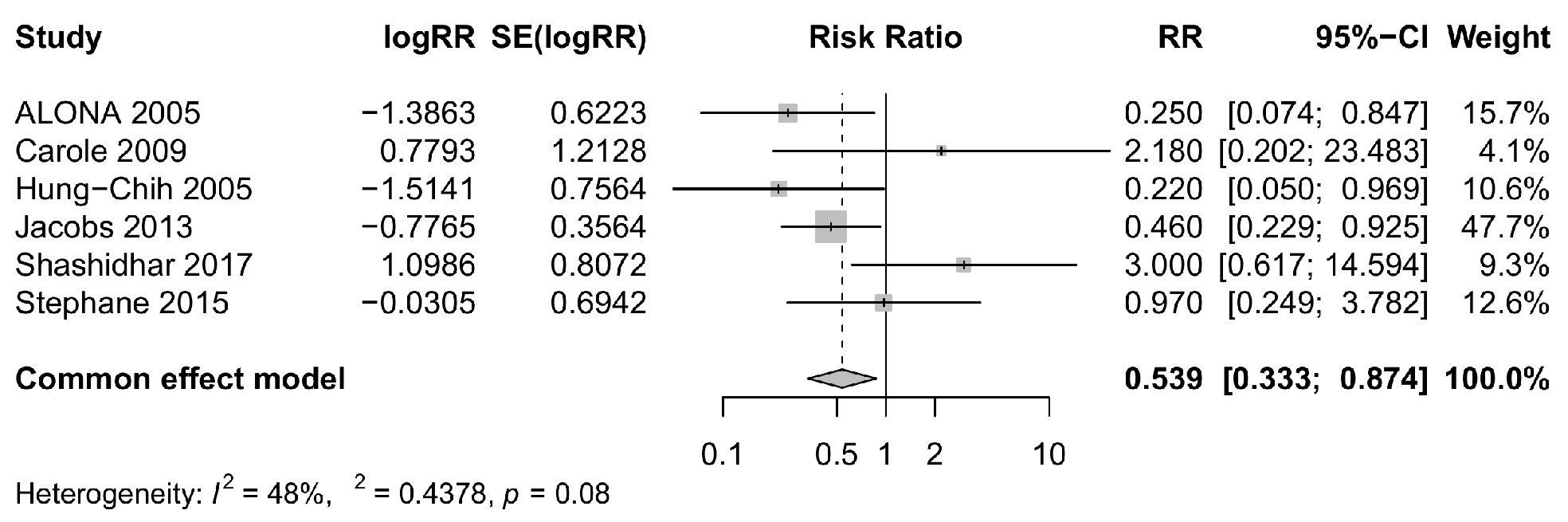
3.3.2. Risk of NEC
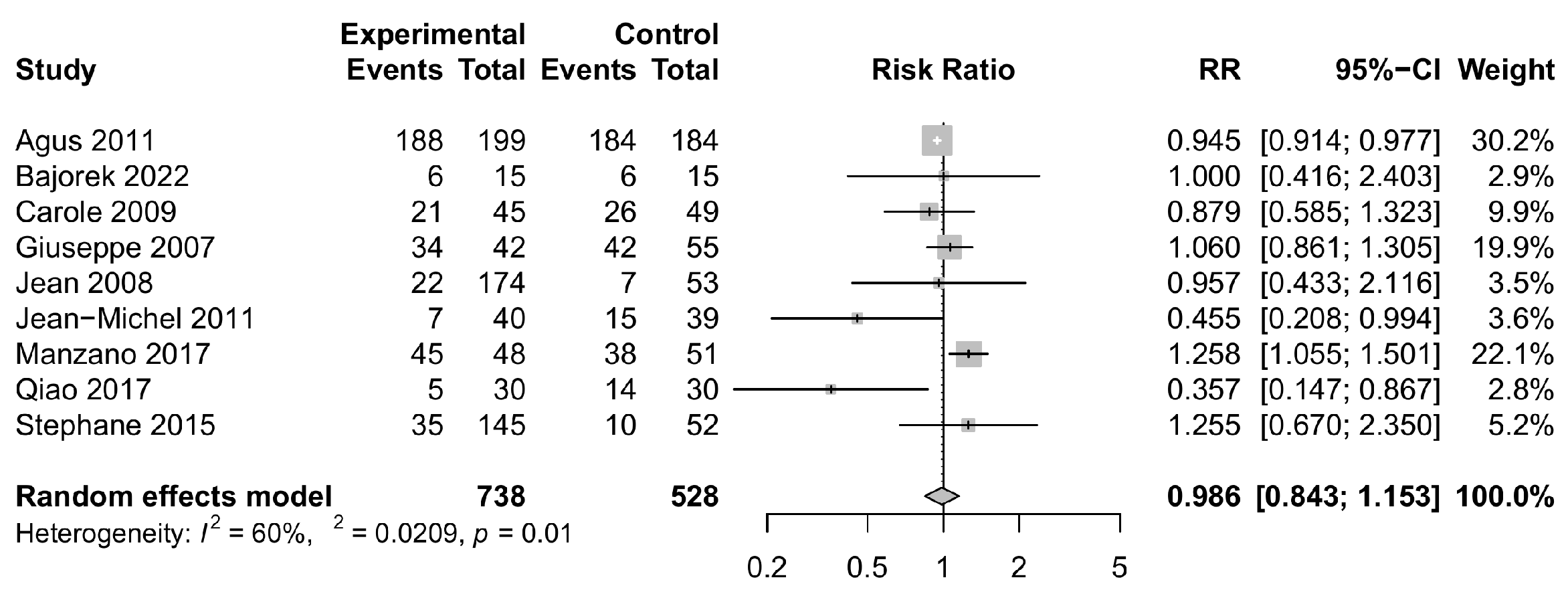
3.4. Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Brooks, B.; Firek, B.A.; Miller, C.S.; Sharon, I.; Thomas, B.C.; Baker, R.; Morowitz, M.J.; Banfield, J.F. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.W.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; Gilbert, J.A. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.C.; Stewart, C.J. The role of the preterm intestinal microbiome in sepsis and necrotising enterocolitis. Early Hum. Dev. 2019, 138, 104854. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef]
- Al-Hosni, M.; Duenas, M.; Hawk, M.; Stewart, L.A.; Borghese, R.A.; Cahoon, M.; Atwood, L.; Howard, D.; Ferrelli, K.; Soll, R. Probiotics-supplemented feeding in extremely low-birth-weight infants. J. Perinatol. 2011, 32, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Tobin, J.M.; Opie, G.F.; Donath, S.; Tabrizi, S.N.; Pirotta, M.; Morley, C.J.; Garland, S.M. Probiotic Effects on Late-onset Sepsis in Very Preterm Infants: A Randomized Controlled Trial. Pediatrics 2013, 132, 1055–1062. [Google Scholar] [CrossRef]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef]
- Duar, R.M.; Kyle, D.; Casaburi, G. Colonization Resistance in the Infant Gut: The Role of B. infantis in Reducing pH and Preventing Pathogen Growth. High Throughput. 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Chouraqui, J.P.; Grathwohl, D.; Labaune, J.M.; Hascoet, J.M.; de Montgolfier, I.; Leclaire, M.; Giarre, M.; Steenhout, P. Assessment of the safety, tolerance, and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial. Am. J. Clin. Nutr. 2008, 87, 1365–1373. [Google Scholar] [CrossRef]
- Hascoët, J.-M.; Hubert, C.; Rochat, F.; Legagneur, H.; Gaga, S.; Emady-Azar, S.; Steenhout, P.G. Effect of Formula Composition on the Development of Infant Gut Microbiota. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 756–762. [Google Scholar] [CrossRef]
- Manzano, S.; De Andrés, J.; Castro, I.; Rodríguez, J.M.; Jiménez, E.; Espinosa-Martos, I. Safety and tolerance of three probiotic strains in healthy infants: A multi-centre randomized, double-blind, placebo-controlled trial. Benef Microbes. 2017, 8, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.X.; Zhu, W.Y.; Zhang, H.Y.; Wang, H. Effect of early administration of probiotics on gut microflora and feeding in pre-term infants: A randomized controlled trial. J. Matern. Fetal. Neonatal. Med. 2017, 30, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, A.; Dwipoerwantoro, P.G.; Kadim, M.; Alatas, S.; Conus, N.; Lestarina, L.; Bouisset, F.; Steenhout, P. Improved growth of toddlers fed a milk containing synbiotics. Asia Pac. J. Clin. Nutr. 2011, 20, 69–76. [Google Scholar] [PubMed]
- Puccio, G.; Cajozzo, C.; Meli, F.; Rochat, F.; Grathwohl, D.; Steenhout, P. Clinical evaluation of a new starter formula for infants containing live Bifidobacterium longum BL999 and prebiotics. Nutrition 2007, 23, 1–8. [Google Scholar] [CrossRef]
- Hays, S.; Jacquot, A.; Gauthier, H.; Kempf, C.; Beissel, A.; Pidoux, O.; Jumas-Bilak, E.; Decullier, E.; Lachambre, E.; Beck, L.; et al. Probiotics and growth in preterm infants: A randomized controlled trial, PREMAPRO study. Clin. Nutr. 2015, 35, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Shashidhar, A.; Rao, P.N.S.; Nesargi, S.; Bhat, S.; Chandrakala, B.S. Probiotics for promoting feed tolerance in very low birth weight neonates—A randomized controlled trial. Indian Pediatr. 2017, 54, 363–367. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpstonm, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Jiang, H.; Liu, C.; Sun, B.; Tang, W. Cyclin D1 rs9344 G> A polymorphism and gastric cancer risk: A meta-analysis. Int. J. Clin. Exp. Med. 2016, 9, 1594–1602. [Google Scholar]
- Bajorek, S.; Duar, R.M.; Corrigan, M.; Matrone, C.; Winn, K.A.; Norman, S.; Mitchell, R.D.; Cagney, O.; Aksenov, A.A.; Melnik, A.V.; et al. B. infantis EVC001 Is Well-Tolerated and Improves Human Milk Oligosaccharide Utilization in Preterm Infants in the Neonatal Intensive Care Unit. Front. Pediatr. 2022, 9, 795970. [Google Scholar] [CrossRef]
- Bin-Nun, A.; Bromiker, R.; Wilschanski, M.; Kaplan, M.; Rudensky, B.; Caplan, M.; Hammerman, C. Oral Probiotics Prevent Necrotizing Enterocolitis in Very Low Birth Weight Neonates. J. Pediatr. 2005, 147, 192–196. [Google Scholar] [CrossRef]
- Lin, H.-C.; Su, B.-H.; Chen, A.-C.; Lin, T.-W.; Tsai, C.-H.; Yeh, T.-F.; Oh, W. Oral Probiotics Reduce the Incidence and Severity of Necrotizing Enterocolitis in Very Low Birth Weight Infants. Pediatrics 2005, 115, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Salzman, N.H.; Bennett, S.H.; Barman, M.; Mills, D.; Marcobal, A. Effects of Probiotic and Prebiotic Combinations on Premature Infants. 2006. Available online: https://clinicaltrials.gov/show/NCT00282113 (accessed on 15 October 2023).
- Rougé, C.; Piloquet, H.; Butel, M.-J.; Berger, B.; Rochat, F.; Ferraris, L.; Robert, C.D.; Legrand, A.; de la Cochetière, M.-F.; N’guyen, J.-M.; et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2009, 89, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Duar, R.M.; Casaburi, G.; Mitchell, R.D.; Scofield, L.N.C.; Ortega Ramirez, C.A.; Barile, D. Comparative Genome Analysis of Bifidobacterium longum subsp. infantis Strains Reveals Variation in Human Milk Oligosaccharide Utilization Genes among Commercial Probiotics. Nutrients 2020, 12, 3247. [Google Scholar] [CrossRef]
- Nguyen, M.; Holdbrooks, H.; Mishra, P.; Abrantes, M.A.; Eskew, S.; Garma, M.; Oca, C.-G.; McGuckin, C.; Hein, C.B.; Mitchell, R.D.; et al. Impact of Probiotic B. infantis EVC001 Feeding in Premature Infants on the Gut Microbiome, Nosocomially Acquired Antibiotic Resistance, and Enteric Inflammation. Front. Pediatr. 2021, 9, 618009. [Google Scholar] [CrossRef]
- Beghetti, I.; Panizza, D.; Lenzi, J.; Gori, D.; Martini, S.; Corvaglia, L.; Aceti, A. Probiotics for Preventing Necrotizing Enterocolitis in Preterm Infants: A Network Meta-Analysis. Nutrients 2021, 13, 192. [Google Scholar] [CrossRef]
- Cooke, R.J.; Ainsworth, S.B.; Fenton, A.C. Postnatal growth retardation: A universal problem in preterm infants. Arch. Dis. Child.—Fetal Neonatal Ed. 2004, 89, F428–F430. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.; Lam, K.; Funk, A.; Lodha, N.; Lorenzetti, D.L.; Freedman, S.B. Comparison of Publication of Pediatric Probiotic vs Antibiotic Trials Registered on ClinicalTrials.gov. JAMA Netw. Open. 2021, 4, e2125236. [Google Scholar] [CrossRef] [PubMed]


| Study | Year | Country | Patients 1 | Female | Birth Weight (g) | Gestational Age (Week) | Cesarean 1 | Postnatal Age at Starting (Month) | Probiotic Strains | Total Dose (CFU/d) 2 | Duration of Supplementation (Week) | Antibiotic Use | Type of Milk Feeding | Outcomes 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Hosni et al. [7] | 2012 | America | 50/51 | 49.50% | 778 | 26 | 22/30 | 4 | Bifidobacterium longum infantis, Lactobacillus rhamnosus GG | 5 × 108 | 34 | no | breast milk | a |
| Mark et al. [26] | 2006 | America | 31/29 | 36.70% | 1428 | 30 | 23/23 | 7 | Bifidobacterium longum, Bifidobacterium longum infantis Lactobacillus acidophilus, Bifidobacterium bifidum | 5 × 108 | 5 | no | - | a |
| Shashidhar et al. [18] | 2017 | India | 52/52 | 54.80% | 1223 | 31 | 27/38 | 0 | Bifidobacterium longum Lactobacillus acidophilus, Lactobacillus rhamnosus, Saccharomyces boulardii | 1.25 × 109 | 4 | no | breast milk | a, b, d |
| Qiao et al. [14] | 2017 | China | 30/30 | 55.00% | 1593 | 32 | -/- | 0 | Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis | 1 × 107 | 2 | yes | breast milk | a, c |
| Agus et al. [15] | 2011 | Indonesia | 199/194 | 48.30% | - | - | -/- | 12 | Bifidobacterium longum BL999, Lactobacillus rhamonosus LPR | 3 × 107 | 64 | no | breast milk | a, c, d |
| ALONA et al. [24] | 2005 | America | 72/73 | 44.10% | 1131 | 30 | 56/57 | 0 | Bifidobacteria longum infantis, Streptococcus thermophilus, Bifidobacteria bifidus | 1.05 × 109 | 6 | yes | breast milk | b |
| Hung-Chih et al. [25] | 2005 | China | 180/187 | 49.90% | 1087 | 28 | 104/100 | 0 | Bifidobacterium longum infantis, Lactobacillus acidophilus | - | no | breast milk | b | |
| Jacobs et al. [8] | 2013 | Australia | 548/551 | 52.00% | 1055 | 28 | 359/377 | 0 | Bifidobacterium longum infantis BB02, Streptococcus thermophilus (TH–4), Bifidobacterium lactis (BB-12) | 1 × 109 | no | breast milk | b | |
| Carole et al. [27] | 2009 | France | 45/49 | 42.60% | 1085 | 28 | 28/35 | 0 | Bifidobacterium longum BB536, Lactobacillus rhamnosus GG | 1 × 108 | 2 | yes | breast milk, preterm formula | b, c |
| Bajorek et al. [23] | 2021 | America | 15/15 | 60.00% | 1468 | 31 | 13/10 | 0 | Bifidobacterium longum infantis EVC001 | 8 × 109 | 4 | yes | breast milk | c, d |
| Jean et al. [11] | 2008 | France | 174/53 | 51.10% | 3400 | 40 | 49/19 | 0 | Bifidobacterium longum BL999, Lactobacillus paracasei ST11, Lactobacillus rhamnosus LPR | 1.29 × 108 | 16 | no | - | c |
| Jean-Michel et al. [12] | 2011 | France | 40/39 | 48.10% | 3300 | 39 | 3/3 | 0 | Bifidobacterium longum BL999 | 2 × 107 | 16 | no | preterm formula | c |
| Manzano et al. [13] | 2017 | Spain | 48/51 | 51.50% | - | full-term (≥37 weeks) | 8/9 | 6 | Bifidobacterium longum infantis R0033 | 3 × 109 | 8 | no | breast milk, preterm formula | c |
| Giuseppe et al. [16] | 2007 | Italy | 42/55 | 53.60% | - | 39 | 15/24 | 0 | Bifidobacterium longum BL999 | 2 × 107 | 16 | no | preterm formula | c, d |
| Stephane et al. [17] | 2015 | France | 145/52 | 48.70% | 1170 | 29 | 115/39 | 0 | Bifidobacterium longum, Bifidobacterium lactis | 1 × 109 | 3 | yes | breast milk, preterm formula | b, c, d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Fan, M.; Hou, T.; Li, Y.; Wang, S.; Wang, X.; Peng, H.; Wang, M.; Wu, T.; Zhang, Y. Efficacy and Safety of Bifidobacterium longum Supplementation in Infants: A Meta-Analysis of Randomized Controlled Trials. Foods 2023, 12, 4451. https://doi.org/10.3390/foods12244451
Guo H, Fan M, Hou T, Li Y, Wang S, Wang X, Peng H, Wang M, Wu T, Zhang Y. Efficacy and Safety of Bifidobacterium longum Supplementation in Infants: A Meta-Analysis of Randomized Controlled Trials. Foods. 2023; 12(24):4451. https://doi.org/10.3390/foods12244451
Chicago/Turabian StyleGuo, Huangda, Meng Fan, Tianjiao Hou, Yixin Li, Siyue Wang, Xueheng Wang, Hexiang Peng, Mengying Wang, Tao Wu, and Yumei Zhang. 2023. "Efficacy and Safety of Bifidobacterium longum Supplementation in Infants: A Meta-Analysis of Randomized Controlled Trials" Foods 12, no. 24: 4451. https://doi.org/10.3390/foods12244451





