ICU ‘Magic Numbers’: The Role of Biomarkers in Supporting Clinical Decision-Making
Abstract
:1. Introduction
2. Neuron-Specific Enolase (NSE) as a Prognostic Marker of Central Nervous System Damage and Poor Neurological Outcomes Following Cardiac Arrest
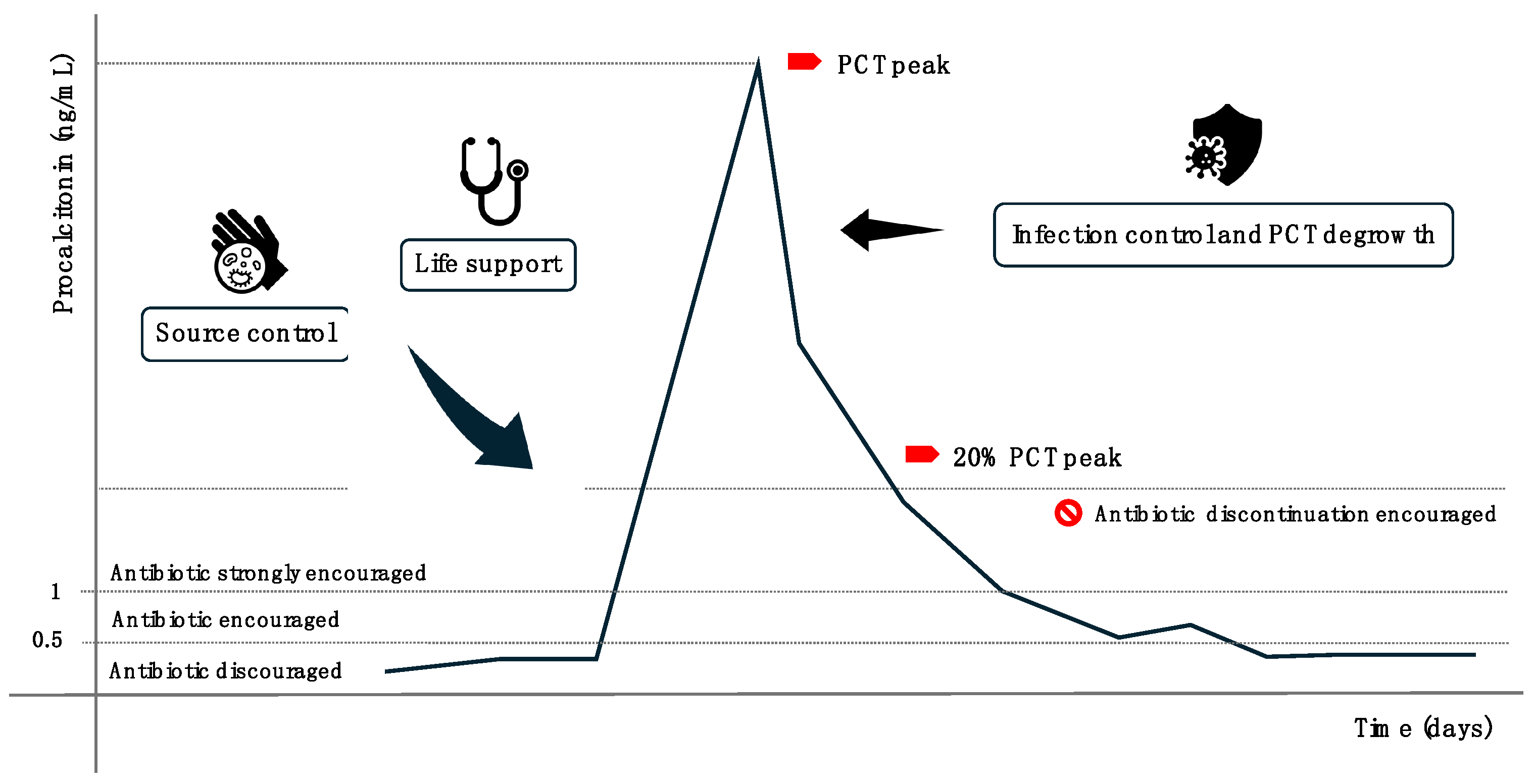
3. Procalcitonin (PCT): A Biomarker for Detecting Infections and Guiding Antibiotic Therapy
Emerging Biomarkers and Future Perspectives for Sepsis Detection: Presepsin
4. N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP): A Reliable Biomarker for Cardiac Failure Diagnosis and Management
Emerging Biomarkers and Future Perspectives for Heart Failure: Endotelin-1
5. Interleukin-6 (IL-6): A Key Biomarker of Systemic and Pulmonary Inflammation
6. Serum Creatinine (SCr) and Cystatin C (CysC): Essential Biomarkers for Renal Function Evaluation
Emerging Biomarkers and Future Perspectives for AKI: NGAL
7. Activated Clotting Time (ACT): A Rapid and Reliable Marker for Monitoring Anticoagulation Therapy
8. Prealbumin: A Marker for Differentiating Catabolic and Anabolic Phases in Critically Ill Patients
Emerging Biomarkers and Future Perspectives for Nutritional Assessment: Interleukin-6
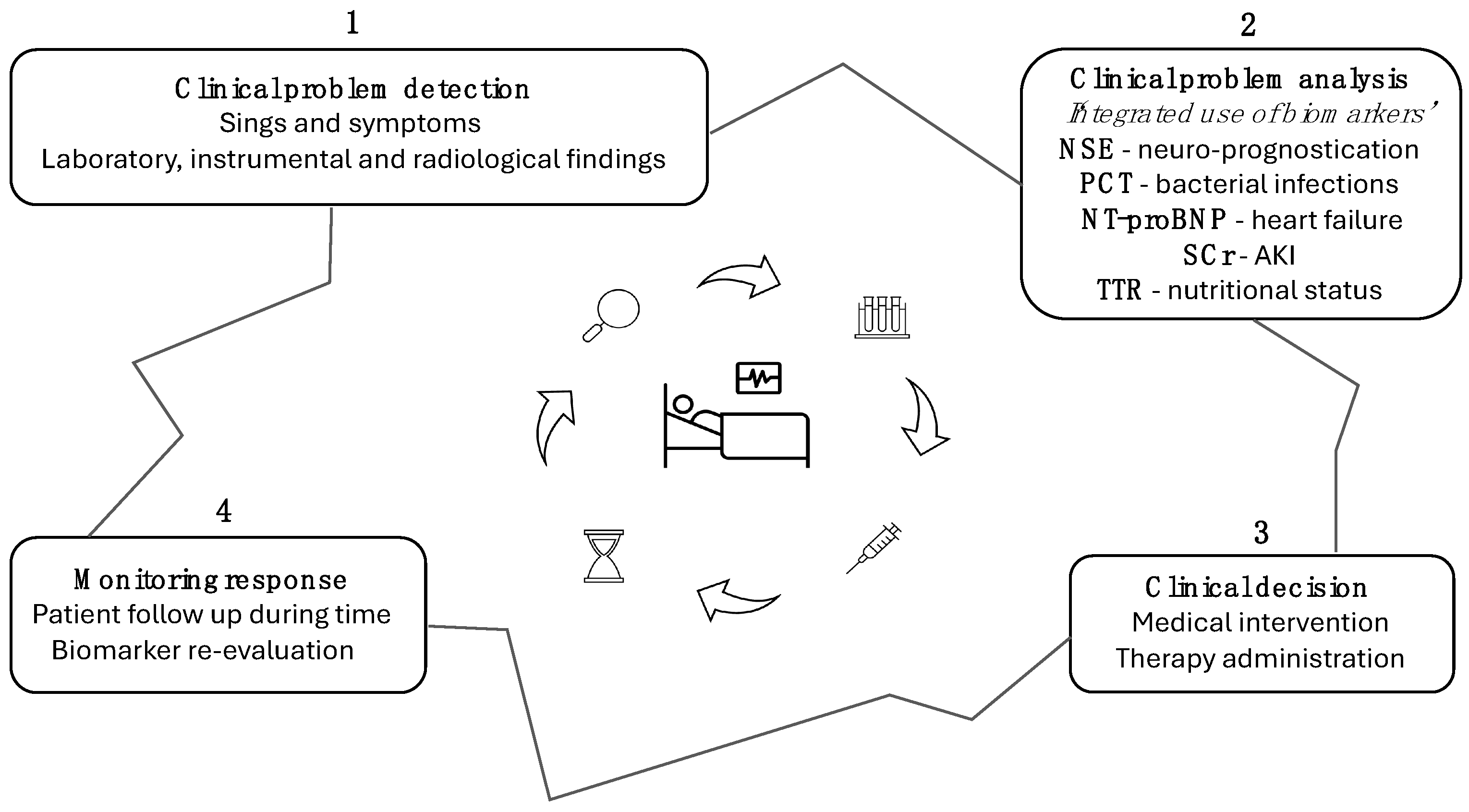
9. Clinical Tips: Practical Use of Biomarkers in the ICU
10. Future Advances in Biomarker Utilization in the ICU
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NSE | Neuron-specific enolase |
| PCT | Procalcitonin |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| SCr | Serum creatinine |
| CysC | Cystatin C |
| TTR | Prealbumin |
| ACT | Activated clotting time |
References
- Zhi, M.; Huang, J.; Jin, X. Clinical value of serum neuron-specific enolase in sepsis-associated encephalopathy: A systematic review and meta-analysis. Syst. Rev. 2024, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Hanin, A.; Denis, J.A.; Frazzini, V.; Cousyn, L.; Imbert-Bismut, F.; Rucheton, B.; Bonnefont-Rousselot, D.; Marois, C.; Lambrecq, V.; Demeret, S.; et al. Neuron Specific Enolase, S100-beta protein and progranulin as diagnostic biomarkers of status epilepticus. J. Neurol. 2022, 269, 3752–3760. [Google Scholar] [CrossRef] [PubMed]
- Vondrakova, D.; Kruger, A.; Janotka, M.; Malek, F.; Dudkova, V.; Neuzil, P.; Ostadal, P. Association of neuron-specific enolase values with outcomes in cardiac arrest survivors is dependent on the time of sample collection. Crit. Care 2017, 21, 172. [Google Scholar] [CrossRef]
- Sakimura, K.; Kushiya, E.; Takahashi, Y.; Suzuki, Y. The structure and expression of neuron-specific enolase gene. Gene 1987, 60, 103–113. [Google Scholar] [CrossRef]
- Marangos, P.J.; Schmechel, D.E. Neuron Specific Enolase, A Clinically Useful Marker for Neurons and Neuroendocrine Cells. Annu. Rev. Neurosci. 1987, 10, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Wu, L.; Ai, Y.H.; Deng, S.Y.; Ai, M.L.; Huang, L.; Liu, Z.Y.; Zhang, L.N. The diagnostic value of neuron-specific enolase, central nervous system specific protein and interleukin-6 in sepsis-associated encephalopathy. Zhonghua Nei Ke Za Zhi 2017, 56, 747–751. [Google Scholar]
- Kim, Y.-J.; Kim, Y.H.; Youn, C.S.; Cho, I.S.; Kim, S.J.; Wee, J.H.; Park, Y.S.; Oh, J.S.; Lee, B.K.; Kim, W.Y. Different neuroprognostication thresholds of neuron-specific enolase in shockable and non-shockable out-of-hospital cardiac arrest: A prospective multicenter observational study in Korea (the KORHN-PRO registry). Crit. Care 2023, 27, 313. [Google Scholar] [CrossRef]
- Chung-Esaki, H.M.; Mui, G.; Mlynash, M.; Eyngorn, I.; Catabay, K.; Hirsch, K.G. The neuron specific enolase (NSE) ratio offers benefits over absolute value thresholds in post-cardiac arrest coma prognosis. J. Clin. Neurosci. 2018, 57, 99–104. [Google Scholar] [CrossRef]
- Scarpino, M.; Lolli, F.; Lanzo, G.; Carrai, R.; Spalletti, M.; Valzania, F.; Lombardi, M.; Audenino, D.; Celani, M.G.; Marrelli, A.; et al. Neurophysiology and neuroimaging accurately predict poor neurological outcome within 24 hours after cardiac arrest: The ProNeCA prospective multicentre prognostication study. Resuscitation 2019, 143, 115–123. [Google Scholar] [CrossRef]
- Mastroianni, A.; Panella, R.; Morelli, D. Invisible hemolysis in serum samples interferes in NSE measurement. Tumori J. 2020, 106, 79–81. [Google Scholar] [CrossRef]
- Müller, J.; Bissmann, B.; Becker, C.; Beck, K.; Loretz, N.; Gross, S.; Amacher, S.A.; Bohren, C.; Pargger, H.; Tisljar, K.; et al. Neuron-Specific Enolase (NSE) Predicts Long-Term Mortality in Adult Patients after Cardiac Arrest: Results from a Prospective Trial. Medicines 2021, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- De Macedo, R.C.; Tomasi, C.D.; Giombelli, V.R.; Alves, S.C.; Bristot, M.D.L.U.; Locks, M.F.T.; Petronilho, F.; Grandi, C.; Quevedo, J.; Dal-Pizzol, F.; et al. Lack of association of S100β and neuron-specific enolase with mortality in critically ill patients. Rev. Bras. Psiquiatr. 2013, 35, 267–270. [Google Scholar] [CrossRef]
- Davies, J. Procalcitonin. J. Clin. Pathol. 2015, 68, 675–679. [Google Scholar] [CrossRef]
- Algeciras-Schimnich, A.; Preissner, C.M.; Theobald, J.P.; Finseth, M.S.; Grebe, S.K.G. Procalcitonin: A marker for the diagnosis and follow-up of patients with medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2009, 94, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M. Update on Procalcitonin Measurements. Ann. Lab. Med. 2014, 34, 263–273. [Google Scholar] [CrossRef]
- Gaïni, S.; Koldkjaer, O.G.; Pedersen, C.; Pedersen, S.S. Procalcitonin, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in community-acquired infections and sepsis: A prospective study. Crit. Care 2006, 10, R53. [Google Scholar] [CrossRef]
- Bouadma, L.; Luyt, C.-E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; Schortgen, F.; Lasocki, S.; Veber, B.; Dehoux, M.; et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 2010, 375, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Jona, V.; Bharadwaj, R.; Swindler, J.; Stokes, C. False Negative Procalcitonin Levels in Patients With Positive Blood Cultures. Chest 2012, 142, 230A. [Google Scholar] [CrossRef]
- Chun, K.; Chung, W.; Kim, A.J.; Kim, H.; Ro, H.; Chang, J.H.; Lee, H.H.; Jung, J.Y. Association between acute kidney injury and serum procalcitonin levels and their diagnostic usefulness in critically ill patients. Sci. Rep. 2019, 9, 4777. [Google Scholar] [CrossRef]
- Piccioni, A.; Santoro, M.C.; de Cunzo, T.; Tullo, G.; Cicchinelli, S.; Saviano, A.; Valletta, F.; Pascale, M.M.; Candelli, M.; Covino, M.; et al. Presepsin as Early Marker of Sepsis in Emergency Department: A Narrative Review. Medicina 2021, 57, 770. [Google Scholar] [CrossRef]
- Bayés-Genís, A.; Santaló-Bel, M.; Zapico-Muñiz, E.; López, L.; Cotes, C.; Bellido, J.; Leta, R.; Casan, P.; Ordóñez-Llanos, J. N-terminal probrain natriuretic peptide (NT-proBNP) in the emergency diagnosis and in-hospital monitoring of patients with dyspnoea and ventricular dysfunction. Eur. J. Heart Fail. 2004, 6, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Forfia, P.R.; Watkins, S.P.; Rame, J.E.; Stewart, K.J.; Shapiro, E.P. Relationship Between B-Type Natriuretic Peptides and Pulmonary Capillary Wedge Pressure in the Intensive Care Unit. J. Am. Coll. Cardiol. 2005, 45, 1667–1671. [Google Scholar] [CrossRef]
- Christenson, R.H. What is the value of B-type natriuretic peptide testing for diagnosis, prognosis or monitoring of critically ill adult patients in intensive care? Clin. Chem. Lab. Med. 2008, 46, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Baggish, A.L.; van Kimmenade, R.R.J.; Januzzi, J.L. The differential diagnosis of an elevated amino-terminal pro-B-type natriuretic peptide level. Am. J. Cardiol. 2008, 101, S43–S48. [Google Scholar] [CrossRef] [PubMed]
- Christ, M.; Mueller, C. Use of natriuretic peptide assay in dyspnea. Dtsch. Arztebl. Int. 2008, 105, 95–100. [Google Scholar] [CrossRef]
- Smit, B.; Spoelstra-de Man, A.M.; Girbes, A.R.; De Waard, M.C. NT-proBNP in cardiopulmonary resuscitated patients treated with mild therapeutic hypothermia is not independently associated with mortality: A retrospective observational study. BMC Anesthesiol. 2015, 15, 48. [Google Scholar] [CrossRef]
- Hou, J.-L.; Gao, K.; Li, M.; Ma, J.-Y.; Shi, Y.-K.; Wang, Y.; Zhao, Y.-F. Increased N-terminal pro-brain natriuretic peptide level predicts atrial fibrillation after surgery for esophageal carcinoma. World J. Gastroenterol. 2008, 14, 2582–2585. [Google Scholar]
- Yagnik, P.; Modem, V.; Dhar, A. 1301: N-Terminal Pro-B-Type Natriuretic Peptide as a Predictor of Volume Status in Critically Ill Children. Crit. Care Med. 2016, 44, 401. [Google Scholar] [CrossRef]
- Medina de Chazal, H.; Del Buono, M.G.; Keyser-Marcus, L.; Ma, L.; Moeller, F.G.; Berrocal, D.; Abbate, A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 1955–1971. [Google Scholar] [CrossRef]
- Jankowich, M.D.; Wu, W.-C.; Choudhary, G. Association of Elevated Plasma Endothelin-1 Levels With Pulmonary Hypertension, Mortality, and Heart Failure in African American Individuals: The Jackson Heart Study. JAMA Cardiol. 2016, 1, 461. [Google Scholar] [CrossRef]
- Kobayashi, A.; Hashimoto, S.; Kooguchi, K.; Kitamura, Y.; Onodera, H.; Urata, Y.; Ashihara, T. Expression of Inducible Nitric Oxide Synthase and Inflammatory Cytokines in Alveolar Macrophages of ARDS Following Sepsis. Chest 1998, 113, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Singanayagam, A.; Hill, A.T. C-Reactive Protein Is an Independent Predictor of Severity in Community-acquired Pneumonia. Am. J. Med. 2008, 121, 219–225. [Google Scholar] [CrossRef]
- Ware, L.B.; Koyama, T.; Zhao, Z.; Janz, D.R.; Wickersham, N.; Bernard, G.R.; May, A.K.; Calfee, C.S.; Matthay, M.A. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit. Care 2013, 17, R253. [Google Scholar] [CrossRef]
- Gamarra-Morales, Y.; Molina-López, J.; Santiago-Ruiz, F.-C.; Herrera-Quintana, L.; Vázquez-Lorente, H.; Gascón-Luna, F.; Planells, E. Efficiency of IL-6 in Early Prognosis and Follow-Up in Critically Ill Patients with Septic Shock. Diseases 2024, 12, 298. [Google Scholar] [CrossRef]
- Li, L.; Yao, Y.; Feng, X.; Chen, L.; Wu, R.; Chang, Y.; Lou, Q.; Pan, J.; Wang, Z. Analysis of Clinical Manifestations and Imaging of COVID-19 Patients in Intensive Care. Contrast Media Mol. Imaging 2022, 2022, 9697285. [Google Scholar] [CrossRef]
- Boretti, A.; Banik, B. Modulation of COVID-19 cytokine storm by tocilizumab. J. Med. Virol. 2022, 94, 823–828. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Mei, S.; Xu, Q.; Qin, S.; Feng, J.; Wang, J.; Xing, S.; Wang, W.; Li, F.; et al. Identifying risk factors for acute respiratory distress syndrome in critically ill patients: A retrospective study. Front. Med. 2024, 11, 1469291. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, H.; Kang, Y. Comparison of diagnostic accuracy among procalcitonin, C-reactive protein, and interleukin 6 for blood culture positivity in general ICU patients. Crit. Care 2018, 22, 339. [Google Scholar] [CrossRef]
- De Rosa, S.; Samoni, S.; Ronco, C. Creatinine-based definitions: From baseline creatinine to serum creatinine adjustment in intensive care. Crit. Care 2016, 20, 69. [Google Scholar] [CrossRef]
- De Rosa, S.; Greco, M.; Rauseo, M.; Annetta, M.G. The Good, the Bad, and the Serum Creatinine: Exploring the Effect of Muscle Mass and Nutrition. Blood Purif. 2023, 52, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Baxmann, A.C.; Ahmed, M.S.; Marques, N.C.; Menon, V.B.; Pereira, A.B.; Kirsztajn, G.M.; Heilberg, I.P. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin. J. Am. Soc. Nephrol. 2008, 3, 348–354. [Google Scholar] [CrossRef]
- Lorenz, G.; Hettwer, S.; McCallum, W.; Angermann, S.; Wen, M.; Schmaderer, C.; Heemann, U.; Roos, M.; Renders, L.; Steubl, D. Plasma C-terminal agrin fragment and rapid kidney function decline in chronic kidney disease patients. Medicine 2019, 98, e15597. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Mattes, M.D.; Peralta, C.A. Update on cystatin C: Incorporation into clinical practice. Am. J. Kidney Dis. 2013, 62, 595–603. [Google Scholar] [CrossRef]
- Peng, P.; Fu, X.C.; Wang, Y.; Zheng, X.; Bian, L.; Zhati, N.; Zhang, S.; Wei, W. The value of serum cystatin c in predicting acute kidney injury after cardiac surgery: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0310049. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Legrand, M.; Meersch, M.; Srisawat, N.; Zarbock, A.; Kellum, J.A. Biomarkers in acute kidney injury. Ann. Intensive Care 2024, 14, 145. [Google Scholar] [CrossRef]
- Poventud-Fuentes, I.; Garnett, E.; Akcan-Arikan, A.; Devaraj, S. Comparison of Cystatin C and Creatinine-Based Equations with Measured Glomerular Filtration Rate in a Diverse Pediatric Population. J. Appl. Lab. Med. 2022, 7, 1016–1024. [Google Scholar] [CrossRef]
- Miyazaki, S.; Iino, N.; Koda, R.; Narita, I.; Kaneko, Y. Brain-derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatr. Gerontol. Int. 2021, 21, 27–33. [Google Scholar] [CrossRef]
- Nourie, N.; Ghaleb, R.; Lefaucheur, C.; Louis, K. Toward Precision Medicine: Exploring the Landscape of Biomarkers in Acute Kidney Injury. Biomolecules 2024, 14, 82. [Google Scholar] [CrossRef]
- Chew, J.S.C.; Saleem, M.; Florkowski, C.M.; George, P.M. Cystatin C—A paradigm of evidence based laboratory medicine. Clin. Biochem. Rev. 2008, 29, 47–62. [Google Scholar] [PubMed]
- Yong, Z.; Pei, X.; Zhu, B.; Yuan, H.; Zhao, W. Predictive value of serum cystatin C for acute kidney injury in adults: A meta-analysis of prospective cohort trials. Sci. Rep. 2017, 7, 41012. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Cheungpasitporn, W.; Ronco, C. Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin. Chem. Lab. Med. 2017, 55, 1074–1089. [Google Scholar] [CrossRef]
- Bufkin, K.B.; Karim, Z.A.; Silva, J. Review of the limitations of current biomarkers in acute kidney injury clinical practices. SAGE Open Med. 2024, 12, 20503121241228446. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil Gelatinase-Associated Lipocalin: A Promising Biomarker for Human Acute Kidney Injury. Biomark. Med. 2010, 4, 265–280. [Google Scholar] [CrossRef]
- Erkinaro, T.; Moilanen, J.; Lahtinen, J.; Mosorin, M.; Savolainen, E.-R. The Standard Point-of-Care Hemochron Jr. ACT+ Test in Monitoring Heparin Administration for Cardiopulmonary Bypass in Severe Factor XII Deficiency. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2031–2034. [Google Scholar] [CrossRef]
- Chen, Y.; Phoon, P.H.Y.; Hwang, N.C. Heparin Resistance During Cardiopulmonary Bypass in Adult Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2022, 36, 4150–4160. [Google Scholar] [CrossRef] [PubMed]
- Roosendaal, L.C.; Hoebink, M.; Wiersema, A.M.; Blankensteijn, J.D.; Jongkind, V. Activated clotting time-guided heparinization during open AAA surgery: A pilot study. Pilot Feasibility Stud. 2024, 10, 73. [Google Scholar] [CrossRef]
- Wehner, J.E.; Boehne, M.; David, S.; Brand, K.; Tiede, A.; Bikker, R. Activated Clotting Time (ACT) for Monitoring of Low-Dose Heparin: Performance Characteristics in Healthy Adults and Critically Ill Patients. Clin. Appl. Thromb./Hemost. 2020, 26, 1076029620975494. [Google Scholar] [CrossRef]
- Hansen, R.; Koster, A.; Kukucka, M.; Mertzlufft, F.; Kuppe, H. A quick anti-Xa-activity-based whole blood coagulation assay for monitoring unfractionated heparin during cardiopulmonary bypass: A pilot investigation. Anesth. Analg. 2000, 91, 533–538. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Otsuka, Y.; Furukawa, T.; Amitani, S.; Kimura, N.; Sanui, M. Correlation between activated clotting time monitoring and heparin concentration measurement in a patient with antiphospholipid syndrome during cardiac valve surgery: A case report. JA Clin. Rep. 2021, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Yousef, D.; Palmen, M.; Klautz, R.; Eikenboom, J.; Wink, J. Accuracy of point-of-care coagulation testing during cardiopulmonary bypass in a patient post COVID-19 infection. J. Cardiothorac. Surg. 2022, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Helin, T.; Tirri, T.; Korkala, H.; Lappalainen, K.; Joutsi-Korhonen, L. Laboratory Assessment of Unfractionated Heparin (UFH) with Activated Clotting Time (ACT) and Anti-Xa Activity during Peripheral Arterial Angiographic Procedure. Diagnostics 2023, 13, 1489. [Google Scholar] [CrossRef]
- McLaughlin, K.; Rimsans, J.; Sylvester, K.W.; Fanikos, J.; Dorfman, D.M.; Senna, P.; Connors, J.M.; Goldhaber, S.Z. Evaluation of Antifactor-Xa Heparin Assay and Activated Partial Thromboplastin Time Values in Patients on Therapeutic Continuous Infusion Unfractionated Heparin Therapy. Clin. Appl. Thromb./Hemost. 2019, 25, 1076029619876030. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Ingenbleek, Y.; Young, V. Transthyretin (prealbumin) in health and disease: Nutritional implications. Annu. Rev. Nutr. 1994, 14, 495–533. [Google Scholar] [CrossRef] [PubMed]
- Dellière, S.; Cynober, L. Is transthyretin a good marker of nutritional status? Clin. Nutr. 2017, 36, 364–370. [Google Scholar] [CrossRef]
- Wajner, S.M.; Goemann, I.M.; Bueno, A.L.; Larsen, P.R.; Maia, A.L. IL-6 promotes nonthyroidal illness syndrome by blocking thyroxine activation while promoting thyroid hormone inactivation in human cells. J. Clin. Investig. 2011, 121, 1834–1845. [Google Scholar] [CrossRef]
- Linden, M.A.; Freitas, R.G.B.D.O.N.; Teles, L.O.D.S.; Morcillo, A.M.; Ferreira, M.T.; Nogueira, R.J.N. Transthyretin and Nutritional Status in Critically Ill Adults on Parenteral Nutrition: A Prospective Cohort Study. Nutrients 2024, 16, 2448. [Google Scholar] [CrossRef]
- Devakonda, A.; George, L.; Raoof, S.; Esan, A.; Saleh, A.; Bernstein, L.H. Transthyretin as a marker to predict outcome in critically ill patients. Clin. Biochem. 2008, 41, 1126–1130. [Google Scholar] [CrossRef]
- Sreedhara, R.; Avram, M.M.; Blanco, M.; Batish, R.; Avram, M.M.; Mittman, N. Prealbumin is the best nutritional predictor of survival in hemodialysis and peritoneal dialysis. Am. J. Kidney Dis. 1996, 28, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Minami, Y.; Haruki, S.; Jujo, K.; Hagiwara, N. Prognostic implications of prealbumin level on admission in patients with acute heart failure referred to a cardiac intensive care unit. J. Cardiol. 2019, 73, 114–119. [Google Scholar] [CrossRef]
- Lourenço, P.; Silva, S.; Friões, F.; Alvelos, M.; Amorim, M.; Torres-Ramalho, P.; Teles, M.J.; Guimarães, J.T.; Bettencourt, P. Does pre-albumin predict in-hospital mortality in heart failure? Int. J. Cardiol. 2013, 166, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ren, D.; Wang, C.-S.; Li, T.; Yao, H.-C. High sensitivity C-reactive protein to prealbumin ratio measurement as a marker of the prognosis in acute coronary syndrome. Sci. Rep. 2019, 9, 11583. [Google Scholar] [CrossRef] [PubMed]
- Haltmeier, T.; Inaba, K.; Durso, J.; Khan, M.; Siboni, S.; Cheng, V.; Schnüriger, B.; Benjamin, E.; Demetriades, D. Transthyretin at Admission and Over Time as a Marker for Clinical Outcomes in Critically Ill Trauma Patients: A Prospective Single-Center Study. World J. Surg. 2020, 44, 115–123. [Google Scholar] [CrossRef]
- Yildirim, M.; Yildirim, Z.S.; Deniz, M. Effect of the modified NUTRIC score in predicting the prognosis of patients admitted to intensive care units. BMC Anesthesiol. 2024, 24, 473. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.J.; Park, K.N.; Choi, S.P.; Lee, B.K.; Oh, S.H.; Jeung, K.W.; Cho, I.S.; Youn, C.S. Neuron-specific enolase and neuroimaging for prognostication after cardiac arrest treated with targeted temperature management. PLoS ONE 2020, 15, e0239979. [Google Scholar] [CrossRef]
- Westwood, M.; Ramaekers, B.; Whiting, P.; Tomini, F.; Joore, M.; Armstrong, N.; Ryder, S.; Stirk, L.; Severens, J.; Kleijnen, J. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2015, 19, v–xxv, 1–236. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef]
- Lagos-Arevalo, P.; Palijan, A.; Vertullo, L.; Devarajan, P.; Bennett, M.R.; Sabbisetti, V.; Bonventre, J.V.; Ma, Q.; Gottesman, R.D.; Zappitelli, M. Cystatin C in acute kidney injury diagnosis: Early biomarker or alternative to serum creatinine? Pediatr. Nephrol. 2015, 30, 665–676. [Google Scholar] [CrossRef]
- Tolan, N.V.; Vidal-Folch, N.; Algeciras-Schimnich, A.; Singh, R.J.; Grebe, S.K.G. Individualized correction of neuron-specific enolase (NSE) measurement in hemolyzed serum samples. Clin. Chim. Acta 2013, 424, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Mat-Nor, M.B.; Md Ralib, A.; Abdulah, N.Z.; Pickering, J.W. The diagnostic ability of procalcitonin and interleukin-6 to differentiate infectious from noninfectious systemic inflammatory response syndrome and to predict mortality. J. Crit. Care 2016, 33, 245–251. [Google Scholar] [CrossRef]
- Michelhaugh, S.A.; Januzzi, J.L. Using Artificial Intelligence to Better Predict and Develop Biomarkers. Heart Fail. Clin. 2022, 18, 275–285. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.-Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, R. Transforming Clinical Research: The Power of High-Throughput Omics Integration. Proteomes 2024, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Nodari, S.; Parrinello, G.; Specchia, C.; Brentana, L.; Rocca, P.; Fracassi, F.; Bordonali, T.; Milani, P.; Danesi, R.; et al. The role of plasma biomarkers in acute heart failure. Serial changes and independent prognostic value of NT-proBNP and cardiac troponin-T. Eur. J. Heart Fail. 2007, 9, 776–786. [Google Scholar] [CrossRef]
- Méndez Hernández, R.; Ramasco Rueda, F. Biomarkers as Prognostic Predictors and Therapeutic Guide in Critically Ill Patients: Clinical Evidence. J. Pers. Med. 2023, 13, 333. [Google Scholar] [CrossRef]

| Condition | Biological Mechanism | Cut-Off NSE | Sensitivity (%) | Specificity (%) | Main Findings |
|---|---|---|---|---|---|
| Out-of-Hospital Cardiac Arrest (OHCA) | Cerebral ischemia and reperfusion injury. | >20 μg/L (Days 3–4) | 85 | 82 |
|
| Sepsis-Associated Encephalopathy (SAE) | Neuronal damage due to inflammation and BBB disruption. | 14.36 μg/L (Day 3) | 61.1 | 73.9 |
|
| Status Epilepticus (SE) | Neuronal damage from prolonged seizures. | 17.8 μg/L | 77.3 | 45.2 |
|
| Delirium in the ICU | Acute brain dysfunction, unclear NSE role. | Not defined | NA | NA |
|
| Ischemic/Traumatic Brain Injury | Neuronal apoptosis and necrosis. | >25 μg/L | 76 | 80 |
|
| Characteristic | Serum Creatinine (SCr) | Cystatin C (CysC) |
|---|---|---|
| Production | Derived from muscle metabolism | Produced constantly by all nucleated cells |
| Elimination | Filtered by glomeruli; partially secreted by tubules | Filtered and fully metabolized in the proximal tubules |
| Influencing Factors | Muscle mass, diet, age, and hydration | Minimal; thyroid and inflammation influence |
| Cost | Low (<€5) | High (~10 × SCr) |
| Half-Life | ~4 h | ~1.5–2 h; faster response to GFR changes |
| Sensitivity for AKI | Low; delayed detection | High; AUROC 0.89 for AKI |
| Specificity for CKD | Moderate | High; better predictor of CKD progression |
| Response to Therapy | Slow; lag in reflecting renal recovery | Fast; better indicator of therapy response |
| Utility in Pediatric Patients | Limited due to growth-related variability | Effective; reliable, even in pediatric populations |
| Utility in Post-Transplant Monitoring | Limited; less accurate for dynamic GFR changes | Superior marker for post-transplant renal function monitoring |
| Utility in Critical Care | Limited in ICU; confounded by muscle wasting | High; preferred in ICU settings for early AKI detection |
| Correlation with Inflammation or Other Conditions | Minimal inflammation impact | May be influenced by systemic inflammation |
| Primary Applications | CKD monitoring, basic renal evaluation | Early AKI detection, ICU, cardiovascular risk prediction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipulli, F.; Balzani, E.; Marini, G.; Lassola, S.; De Rosa, S.; Bellani, G. ICU ‘Magic Numbers’: The Role of Biomarkers in Supporting Clinical Decision-Making. Diagnostics 2025, 15, 975. https://doi.org/10.3390/diagnostics15080975
Cipulli F, Balzani E, Marini G, Lassola S, De Rosa S, Bellani G. ICU ‘Magic Numbers’: The Role of Biomarkers in Supporting Clinical Decision-Making. Diagnostics. 2025; 15(8):975. https://doi.org/10.3390/diagnostics15080975
Chicago/Turabian StyleCipulli, Francesco, Eleonora Balzani, Giuseppe Marini, Sergio Lassola, Silvia De Rosa, and Giacomo Bellani. 2025. "ICU ‘Magic Numbers’: The Role of Biomarkers in Supporting Clinical Decision-Making" Diagnostics 15, no. 8: 975. https://doi.org/10.3390/diagnostics15080975
APA StyleCipulli, F., Balzani, E., Marini, G., Lassola, S., De Rosa, S., & Bellani, G. (2025). ICU ‘Magic Numbers’: The Role of Biomarkers in Supporting Clinical Decision-Making. Diagnostics, 15(8), 975. https://doi.org/10.3390/diagnostics15080975






