Pancreatic Volume in Thalassemia: Determinants and Association with Alterations of Glucose Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
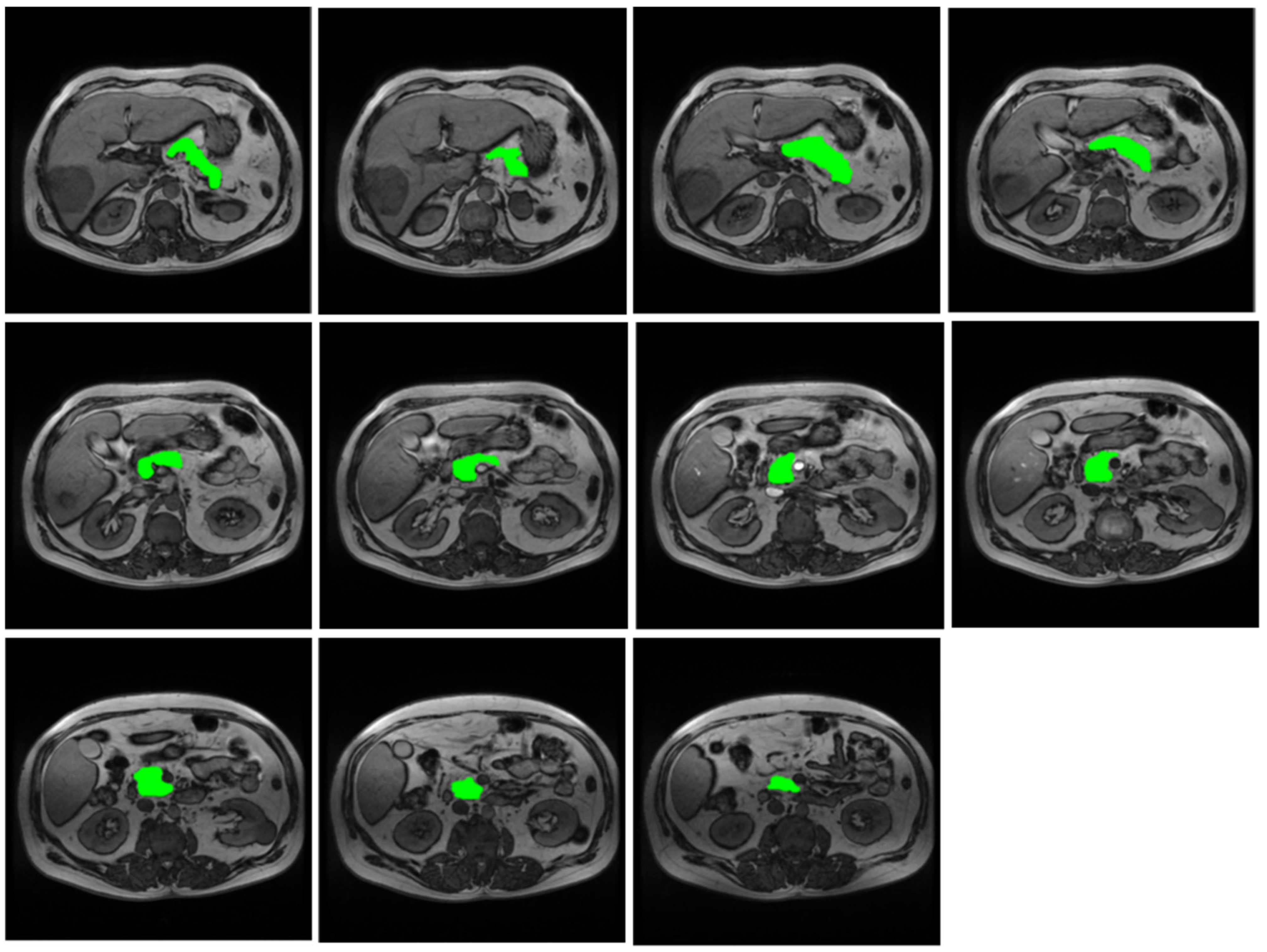
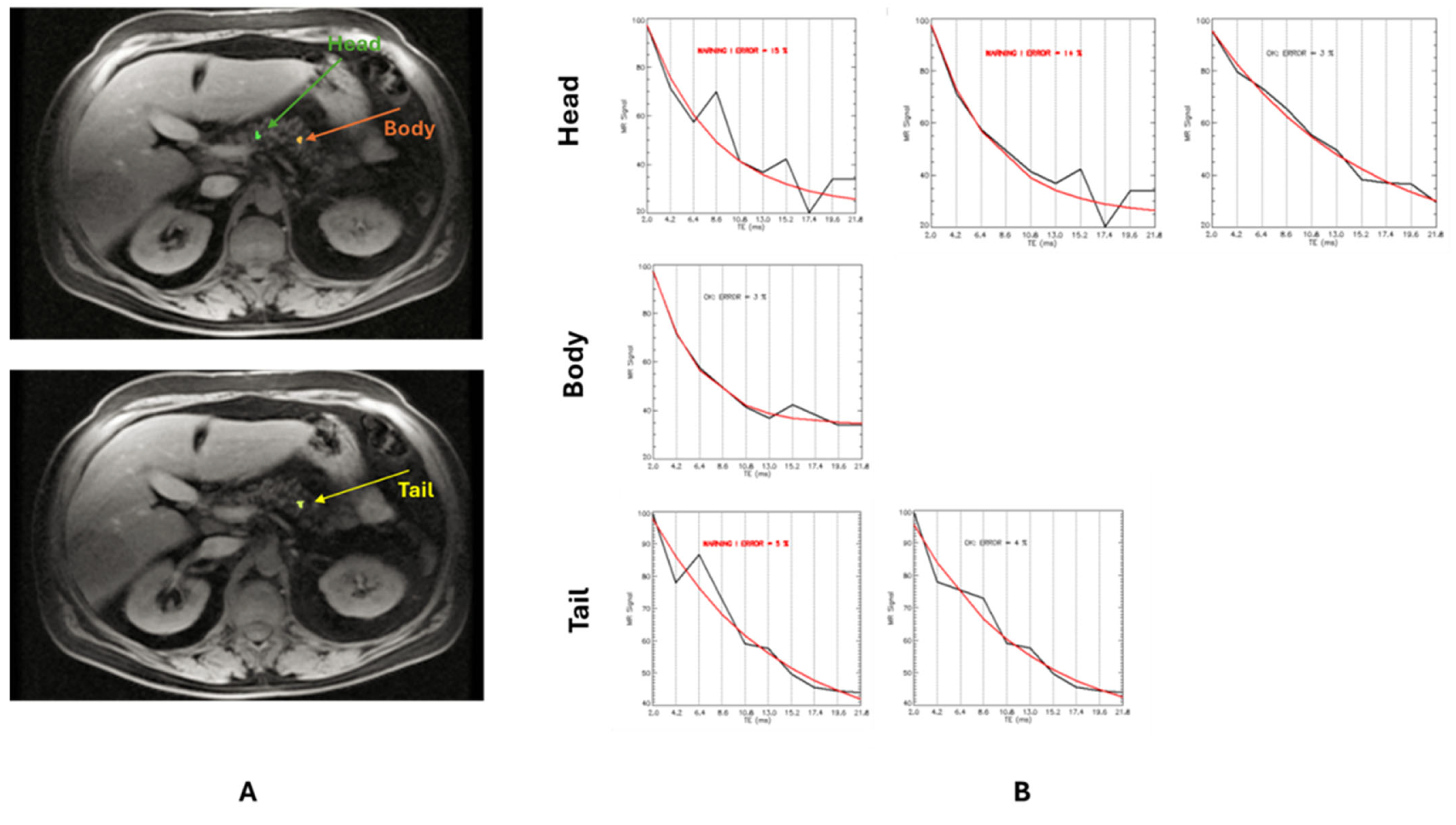
2.2. MRI
2.3. Biochemical Investigations
2.4. Diagnostic Criteria
2.5. Statistical Analysis
3. Results
3.1. Derivation of the Patient Population
3.2. Association Between Pancreatic Volume and Gender in Thalassemia
3.3. Comparison Between Thalassemia Patients and Healthy Individuals
3.4. Comparison Between β-TM and β-TI Patients
3.5. Correlates and Determinants of Pancreatic Volume in β-TM
3.6. Correlates and Determinants of Pancreatic Volume in β-TI
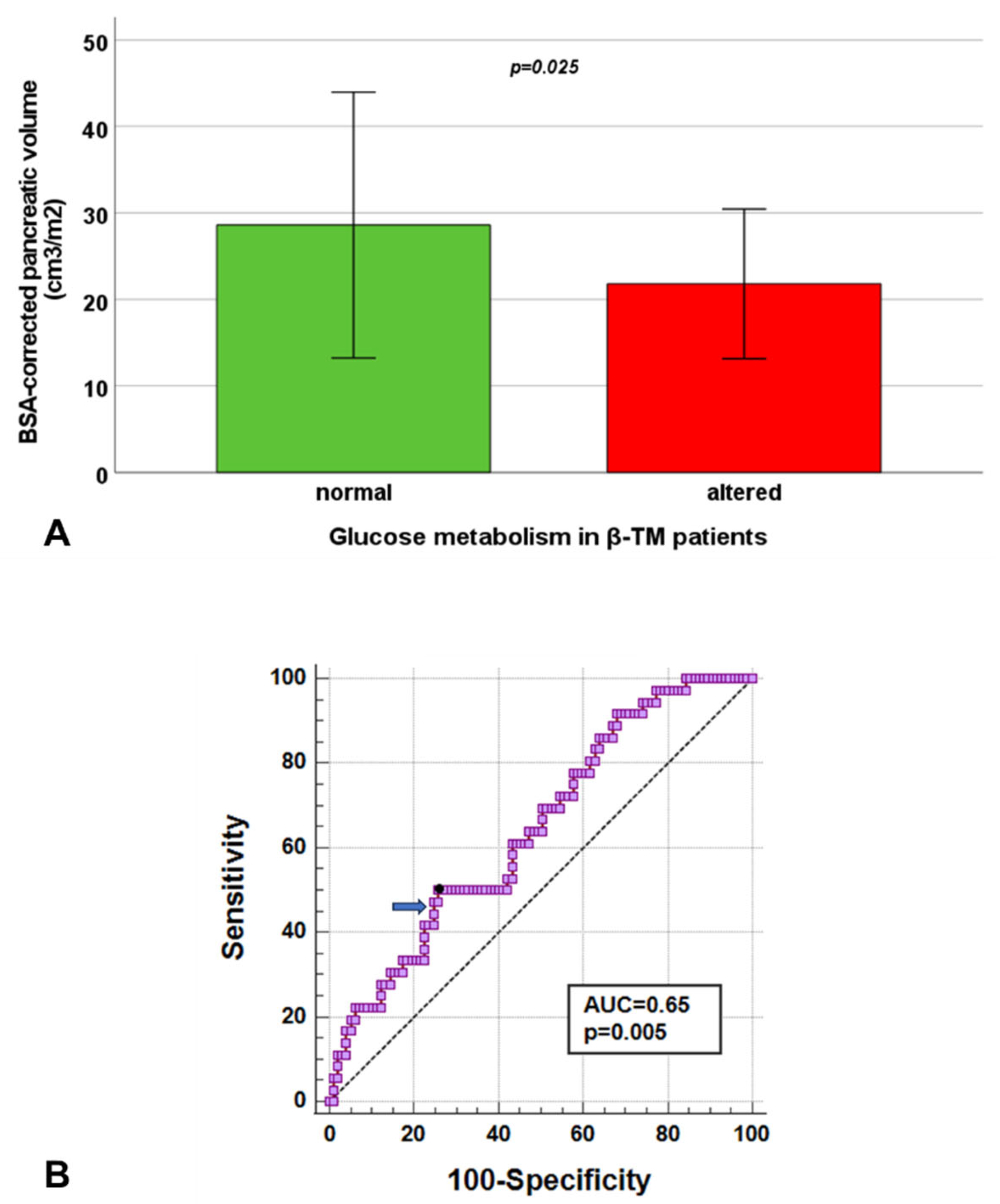
3.7. Pancreatic Volume and Glucose Metabolism in β-TM
3.8. Pancreatic Volume and Glucose Metabolism in β-TI
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rund, D.; Rachmilewitz, E. Beta-thalassemia. N. Engl. J. Med. 2005, 353, 1135–1146. [Google Scholar] [CrossRef]
- Galanello, R.; Origa, R. Beta-thalassemia. Orphanet J. Rare Dis. 2010, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Angastiniotis, M.; Lobitz, S. Thalassemias: An Overview. Int. J. Neonatal Screen. 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Porter, J.; Taher, A.; Domenica Cappellini, M.; Angastiniotis, M.; Eleftheriou, A. 2021 Thalassaemia International Federation Guidelines for the Management of Transfusion-dependent Thalassemia. Hemasphere 2022, 6, e732. [Google Scholar] [CrossRef]
- Porter, J.B. Pathophysiology of transfusional iron overload: Contrasting patterns in thalassemia major and sickle cell disease. Hemoglobin 2009, 33 (Suppl. S1), S37–S45. [Google Scholar] [CrossRef]
- Shander, A.; Cappellini, M.D.; Goodnough, L.T. Iron overload and toxicity: The hidden risk of multiple blood transfusions. Vox Sang. 2009, 97, 185–197. [Google Scholar] [CrossRef]
- Betts, M.; Flight, P.A.; Paramore, L.C.; Tian, L.; Milenković, D.; Sheth, S. Systematic Literature Review of the Burden of Disease and Treatment for Transfusion-dependent β-Thalassemia. Clin. Ther. 2020, 42, 322–337.e322. [Google Scholar] [CrossRef]
- Taher, A.T.; Musallam, K.M.; El-Beshlawy, A.; Karimi, M.; Daar, S.; Belhoul, K.; Saned, M.S.; Graziadei, G.; Cappellini, M.D. Age-related complications in treatment-naive patients with thalassaemia intermedia. Br. J. Haematol. 2010, 150, 486–489. [Google Scholar] [CrossRef]
- Taher, A.T.; Musallam, K.M.; Karimi, M.; El-Beshlawy, A.; Belhoul, K.; Daar, S.; Saned, M.S.; El-Chafic, A.H.; Fasulo, M.R.; Cappellini, M.D. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: The OPTIMAL CARE study. Blood 2010, 115, 1886–1892. [Google Scholar] [CrossRef]
- Haddad, A.; Tyan, P.; Radwan, A.; Mallat, N.; Taher, A. beta-Thalassemia Intermedia: A Bird’s-Eye View. Turk. J. Haematol. 2014, 31, 5–16. [Google Scholar] [CrossRef]
- Ricchi, P.; Meloni, A.; Pistoia, L.; Spasiano, A.; Rita Gamberini, M.; Maggio, A.; Gerardi, C.; Messina, G.; Campisi, S.; Allo, M.; et al. Longitudinal follow-up of patients with thalassaemia intermedia who started transfusion therapy in adulthood: A cohort study. Br. J. Haematol. 2020, 191, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Wong, T.; Keel, S.; Pagano, M.; Chung, J.; Kamdar, A.; Rao, L.; Ikeda, A.; Puthenveetil, G.; Shah, S.; et al. The transfusion management of beta thalassemia in the United States. Transfusion 2021, 61, 3027–3039. [Google Scholar] [CrossRef] [PubMed]
- Gardenghi, S.; Grady, R.W.; Rivella, S. Anemia, ineffective erythropoiesis, and hepcidin: Interacting factors in abnormal iron metabolism leading to iron overload in beta-thalassemia. Hematol. Oncol. Clin. N. Am. 2010, 24, 1089–1107. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, Y.; Rivella, S. beta-thalassemia: A model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood 2011, 118, 4321–4330. [Google Scholar] [CrossRef]
- Meloni, A.; Pistoia, L.; Gamberini, M.R.; Ricchi, P.; Cecinati, V.; Sorrentino, F.; Cuccia, L.; Allo, M.; Righi, R.; Fina, P.; et al. The Link of Pancreatic Iron with Glucose Metabolism and Cardiac Iron in Thalassemia Intermedia: A Large, Multicenter Observational Study. J. Clin. Med. 2021, 10, 5561. [Google Scholar] [CrossRef]
- Meloni, A.; Pistoia, L.; Ricchi, P.; Longo, F.; Cecinati, V.; Sorrentino, F.; Cuccia, L.; Corigliano, E.; Rossi, V.; Righi, R.; et al. Multiparametric cardiac magnetic resonance in patients with thalassemia intermedia: New insights from the E-MIOT network. Radiol. Med. 2024, 129, 879–889. [Google Scholar] [CrossRef]
- Noetzli, L.J.; Papudesi, J.; Coates, T.D.; Wood, J.C. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood 2009, 114, 4021–4026. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Peluso, A.; Messina, G.; Spasiano, A.; Allo, M.; Bisconte, M.G.; Putti, M.C.; et al. The Close Link of Pancreatic Iron with Glucose Metabolism and with Cardiac Complications in Thalassemia Major: A Large, Multicenter Observational Study. Diabetes Care 2020, 43, 2830–2839. [Google Scholar] [CrossRef]
- Harrison, A.V.; Lorenzo, F.R.; McClain, D.A. Iron and the Pathophysiology of Diabetes. Annu. Rev. Physiol. 2023, 85, 339–362. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Marku, A.; Galli, A.; Marciani, P.; Dule, N.; Perego, C.; Castagna, M. Iron Metabolism in Pancreatic Beta-Cell Function and Dysfunction. Cells 2021, 10, 2841. [Google Scholar] [CrossRef] [PubMed]
- Baldini, M.; Marcon, A.; Cassin, R.; Ulivieri, F.M.; Spinelli, D.; Cappellini, M.D.; Graziadei, G. Beta-thalassaemia intermedia: Evaluation of endocrine and bone complications. Biomed. Res. Int. 2014, 2014, 174581. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Soliman, A.; Tzoulis, P.; Daar, S.; Kattamis, A.; Delaporta, P.; Yassin, M.A.; Karimi, M.; Canatan, D.; Al Jaouni, S.; et al. The Prevalence of glucose dysregulations (GDs) in patients with β-thalassemias in different countries: A preliminary ICET-A survey. Acta Biomed. 2021, 92, e2021240. [Google Scholar] [CrossRef]
- Merkel, P.A.; Simonson, D.C.; Amiel, S.A.; Plewe, G.; Sherwin, R.S.; Pearson, H.A.; Tamborlane, W.V. Insulin resistance and hyperinsulinemia in patients with thalassemia major treated by hypertransfusion. N. Engl. J. Med. 1988, 318, 809–814. [Google Scholar] [CrossRef]
- Williams, A.J.; Thrower, S.L.; Sequeiros, I.M.; Ward, A.; Bickerton, A.S.; Triay, J.M.; Callaway, M.P.; Dayan, C.M. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J. Clin. Endocrinol. Metab. 2012, 97, E2109–E2113. [Google Scholar] [CrossRef]
- Goda, K.; Sasaki, E.; Nagata, K.; Fukai, M.; Ohsawa, N.; Hahafusa, T. Pancreatic volume in type 1 and type 2 diabetes mellitus. Acta Diabetol. 2001, 38, 145–149. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Chun, E.J.; Kim, H.; Kim, S.Y.; Kim, K.M.; Choi, S.H.; Park, K.S.; Florez, J.C.; Jang, H.C. Differences in pancreatic volume, fat content, and fat density measured by multidetector-row computed tomography according to the duration of diabetes. Acta Diabetol. 2014, 51, 739–748. [Google Scholar] [CrossRef]
- Macauley, M.; Percival, K.; Thelwall, P.E.; Hollingsworth, K.G.; Taylor, R. Altered volume, morphology and composition of the pancreas in type 2 diabetes. PLoS ONE 2015, 10, e0126825. [Google Scholar] [CrossRef]
- Garcia, T.S.; Rech, T.H.; Leitao, C.B. Pancreatic size and fat content in diabetes: A systematic review and meta-analysis of imaging studies. PLoS ONE 2017, 12, e0180911. [Google Scholar] [CrossRef]
- Donath, M.Y.; Schumann, D.M.; Faulenbach, M.; Ellingsgaard, H.; Perren, A.; Ehses, J.A. Islet Inflammation in Type 2 Diabetes: From metabolic stress to therapy. Diabetes Care 2008, 31, S161–S164. [Google Scholar] [CrossRef]
- Philippe, M.F.; Benabadji, S.; Barbot-Trystram, L.; Vadrot, D.; Boitard, C.; Larger, E. Pancreatic volume and endocrine and exocrine functions in patients with diabetes. Pancreas 2011, 40, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Bagust, A.; Beale, S. Deteriorating beta-cell function in type 2 diabetes: A long-term model. Qjm 2003, 96, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Knop, M.R.; Geng, T.T.; Gorny, A.W.; Ding, R.; Li, C.; Ley, S.H.; Huang, T. Birth Weight and Risk of Type 2 Diabetes Mellitus, Cardiovascular Disease, and Hypertension in Adults: A Meta-Analysis of 7 646 267 Participants From 135 Studies. J. Am. Heart Assoc. 2018, 7, e008870. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Lam, W.W.; Chu, W.; Tam, S.; Wong, W.K.; Liang, R.; Ha, S.Y. A T2* magnetic resonance imaging study of pancreatic iron overload in thalassemia major. Haematologica 2008, 93, 116–119. [Google Scholar] [CrossRef]
- Ramazzotti, A.; Pepe, A.; Positano, V.; Rossi, G.; De Marchi, D.; Brizi, M.G.; Luciani, A.; Midiri, M.; Sallustio, G.; Valeri, G.; et al. Multicenter validation of the magnetic resonance t2* technique for segmental and global quantification of myocardial iron. J. Magn. Reson. Imaging 2009, 30, 62–68. [Google Scholar] [CrossRef]
- Meloni, A.; De Marchi, D.; Pistoia, L.; Grassedonio, E.; Peritore, G.; Preziosi, P.; Restaino, G.; Righi, R.; Riva, A.; Renne, S.; et al. Multicenter validation of the magnetic resonance T2* technique for quantification of pancreatic iron. Eur. Radiol. 2019, 29, 2246–2252. [Google Scholar] [CrossRef]
- Meloni, A.; Luciani, A.; Positano, V.; De Marchi, D.; Valeri, G.; Restaino, G.; Cracolici, E.; Caruso, V.; Dell’amico, M.C.; Favilli, B.; et al. Single region of interest versus multislice T2* MRI approach for the quantification of hepatic iron overload. J. Magn. Reson. Imaging 2011, 33, 348–355. [Google Scholar] [CrossRef]
- Restaino, G.; Meloni, A.; Positano, V.; Missere, M.; Rossi, G.; Calandriello, L.; Keilberg, P.; Mattioni, O.; Maggio, A.; Lombardi, M.; et al. Regional and global pancreatic T*2 MRI for iron overload assessment in a large cohort of healthy subjects: Normal values and correlation with age and gender. Magn. Reson. Med. 2011, 65, 764–769. [Google Scholar] [CrossRef]
- Williams, J.M.; Hilmes, M.A.; Archer, B.; Dulaney, A.; Du, L.; Kang, H.; Russell, W.E.; Powers, A.C.; Moore, D.J.; Virostko, J. Repeatability and Reproducibility of Pancreas Volume Measurements Using MRI. Sci. Rep. 2020, 10, 4767. [Google Scholar] [CrossRef]
- Wang, Y.; Moss, J.; Thisted, R. Predictors of body surface area. J. Clin. Anesth. 1992, 4, 4–10. [Google Scholar] [CrossRef]
- Meloni, A.; De Marchi, D.; Positano, V.; Neri, M.G.; Mangione, M.; Keilberg, P.; Lendini, M.; Cirotto, C.; Pepe, A. Accurate estimate of pancreatic T2* values: How to deal with fat infiltration. Abdom. Imaging 2015, 40, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T.D. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef]
- Meloni, A.; Rienhoff, H.Y., Jr.; Jones, A.; Pepe, A.; Lombardi, M.; Wood, J.C. The use of appropriate calibration curves corrects for systematic differences in liver R2* values measured using different software packages. Br. J. Haematol. 2013, 161, 888–891. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Yaarubi, S.A.; Skordis, N.; Khater, D.; El Kholy, M.; Stoeva, I.; Fiscina, B.; Angastiniotis, M.; et al. The ICET-A Recommendations for the Diagnosis and Management of Disturbances of Glucose Homeostasis in Thalassemia Major Patients. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016058. [Google Scholar] [CrossRef]
- Hershko, C. Pathogenesis and management of iron toxicity in thalassemia. Ann. N. Y. Acad. Sci. 2010, 1202, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pilo, F.; Cilloni, D.; Della Porta, M.G.; Forni, G.L.; Piperno, A.; Santini, V.; Angelucci, E. Iron-mediated tissue damage in acquired ineffective erythropoiesis disease: It’s more a matter of burden or more of exposure to toxic iron form? Leuk. Res. 2022, 114, 106792. [Google Scholar] [CrossRef] [PubMed]
- Brissot, P.; Ropert, M.; Le Lan, C.; Loreal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta 2011, 1820, 403–410. [Google Scholar] [CrossRef]
- Cabantchik, Z.I.; Breuer, W.; Zanninelli, G.; Cianciulli, P. LPI-labile plasma iron in iron overload. Best. Pract. Res. Clin. Haematol. 2005, 18, 277–287. [Google Scholar] [CrossRef]
- Cooksey, R.C.; Jouihan, H.A.; Ajioka, R.S.; Hazel, M.W.; Jones, D.L.; Kushner, J.P.; McClain, D.A. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 2004, 145, 5305–5312. [Google Scholar] [CrossRef]
- Midiri, M.; Lo Casto, A.; Sparacia, G.; D’Angelo, P.; Malizia, R.; Finazzo, M.; Montalto, G.; Solbiati, L.; Lagalla, R.; De Maria, M. MR imaging of pancreatic changes in patients with transfusion-dependent beta-thalassemia major. AJR Am. J. Roentgenol. 1999, 173, 187–192. [Google Scholar] [CrossRef]
- Pfeifer, C.D.; Schoennagel, B.P.; Grosse, R.; Wang, Z.J.; Graessner, J.; Nielsen, P.; Adam, G.; Fischer, R.; Yamamura, J. Pancreatic iron and fat assessment by MRI-R2* in patients with iron overload diseases. J. Magn. Reson. Imaging 2015, 42, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Nobile, M.; Keilberg, P.; Positano, V.; Santarelli, M.F.; Pistoia, L.; Spasiano, A.; Casini, T.; Putti, M.C.; Cuccia, L.; et al. Pancreatic fatty replacement as risk marker for altered glucose metabolism and cardiac iron and complications in thalassemia major. Eur. Radiol. 2023, 33, 7215–7225. [Google Scholar] [CrossRef] [PubMed]
- Tushuizen, M.E.; Bunck, M.C.; Pouwels, P.J.; Bontemps, S.; van Waesberghe, J.H.; Schindhelm, R.K.; Mari, A.; Heine, R.J.; Diamant, M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007, 30, 2916–2921. [Google Scholar] [CrossRef]
- Ou, H.Y.; Wang, C.Y.; Yang, Y.C.; Chen, M.F.; Chang, C.J. The association between nonalcoholic fatty pancreas disease and diabetes. PLoS ONE 2013, 8, e62561. [Google Scholar] [CrossRef]
- Smits, M.M.; van Geenen, E.J. The clinical significance of pancreatic steatosis. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 169–177. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, C.; Kong, X.; Xia, Z.; Kong, W.; Si, K.; Han, P.; Vivian Liu, W.; Li, X. Pancreatic fat infiltration, β-cell function and insulin resistance: A study of the young patients with obesity. Diabetes Res. Clin. Pract. 2022, 187, 109860. [Google Scholar] [CrossRef]
- Kim, D.; Jun, H.-S. In Vivo Imaging of Transplanted Pancreatic Islets. Front. Endocrinol. 2018, 8, 382. [Google Scholar] [CrossRef]
- Valente, R.; Coppola, A.; Scandavini, C.M.; Halimi, A.; Magnusson, A.; Lauro, A.; Sotirova, I.; Arnelo, U.; Franklin, O. Interactions between the Exocrine and the Endocrine Pancreas. J. Clin. Med. 2024, 13, 1179. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Amin, A.; Jalali, S.; Amin, R.; Aale-yasin, S.; Jamalian, N.; Karimi, M. Evaluation of the Serum Levels of Immunoglobulin and Complement Factors in b-thalassemia Major Patients in Southern Iran. Iran. J. Immunol. 2005, 2, 220–225. [Google Scholar]
- Nakanishi, K.; Kobayashi, T.; Miyashita, H.; Okubo, M.; Sugimoto, T.; Murase, T.; Kosaka, K.; Hara, M. Relationships among residual beta cells, exocrine pancreas, and islet cell antibodies in insulin-dependent diabetes mellitus. Metabolism 1993, 42, 196–203. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin—A regulator of intestinal iron absorption and iron recycling by macrophages. Best. Pract. Res. Clin. Haematol. 2005, 18, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Kautz, L.; Rodriguez, R.; Hansen, M.; Gabayan, V.; Ginzburg, Y.; Roth, M.P.; Nemeth, E.; Ganz, T. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology 2011, 53, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, Y.Z. Hepcidin-ferroportin axis in health and disease. Vitam. Horm. 2019, 110, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Kulaksiz, H.; Fein, E.; Redecker, P.; Stremmel, W.; Adler, G.; Cetin, Y. Pancreatic β-cells express hepcidin, an iron-uptake regulatory peptide. J. Endocrinol. 2008, 197, 241–249. [Google Scholar] [CrossRef]
- Kaddah, A.M.; Abdel-Salam, A.; Farhan, M.S.; Ragab, R. Serum Hepcidin as a Diagnostic Marker of Severe Iron Overload in Beta-thalassemia Major. Indian J. Pediatr. 2017, 84, 745–750. [Google Scholar] [CrossRef]
- Caglar, V.; Songur, A.; Yagmurca, M.; Acar, M.; Toktas, M.; Gonul, Y. Age-related volumetric changes in pancreas: A stereological study on computed tomography. Surg. Radiol. Anat. 2012, 34, 935–941. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, K.G.; Kim, Y.J.; Lim, S.; Park, Y.H.; Kim, D.; Kang, H.T.; Lee, D.H. Distribution and Characteristics of Pancreatic Volume Using Computed Tomography Volumetry. Healthc. Inform. Res. 2020, 26, 321–327. [Google Scholar] [CrossRef]
- Djuric-Stefanovic, A.; Masulovic, D.; Kostic, J.; Randjic, K.; Saranovic, D. CT volumetry of normal pancreas: Correlation with the pancreatic diameters measurable by the cross-sectional imaging, and relationship with the gender, age, and body constitution. Surg. Radiol. Anat. 2012, 34, 811–817. [Google Scholar] [CrossRef]
- Saisho, Y.; Butler, A.E.; Meier, J.J.; Monchamp, T.; Allen-Auerbach, M.; Rizza, R.A.; Butler, P.C. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin. Anat. 2007, 20, 933–942. [Google Scholar] [CrossRef]
- Statsenko, Y.; Kuznetsov, N.V.; Morozova, D.; Liaonchyk, K.; Simiyu, G.L.; Smetanina, D.; Kashapov, A.; Meribout, S.; Gorkom, K.N.-V.; Hamoudi, R.; et al. Reappraisal of the Concept of Accelerated Aging in Neurodegeneration and Beyond. Cells 2023, 12, 2451. [Google Scholar] [CrossRef] [PubMed]
- Murea, M.; Ma, L.; Freedman, B.I. Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev. Diabet. Stud. 2012, 9, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Pistoia, L.; Ricchi, P.; Allo, M.; Rosso, R.; Cuccia, L.; Casini, T.; Cecinati, V.; Serra, M.; Rossi, V.; et al. Prospective changes of pancreatic iron in patients with thalassemia major and association with chelation therapy. Blood Adv. 2023, 7, 2237–2240. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, N.G.; Zervas, A.; Livadas, S.; Adamopoulos, I.; Giannopoulos, D.; Goula, A.; Tolis, G. Reduced insulin secretion in normoglycaemic patients with beta-thalassaemia major. Diabet. Med. 2006, 23, 1327–1331. [Google Scholar] [CrossRef]
- Gluvic, Z.; Obradovic, M.; Lackovic, M.; Samardzic, V.; Tica Jevtic, J.; Essack, M.; Bajic, V.B.; Isenovic, E.R. HbA1C as a marker of retrograde glycaemic control in diabetes patient with co-existed beta-thalassaemia: A case report and a literature review. J. Clin. Pharm. Ther. 2020, 45, 379–383. [Google Scholar] [CrossRef]

| β-TM Group (N = 145) | β-TI Group (N = 19) | p-Value | |
|---|---|---|---|
| Age (years) | 38.16 ± 10.08 | 39.63 ± 12.67 | 0.470 |
| Females, N (%) | 83 (57.2) | 10 (52.6) | 0.703 |
| Splenectomy, N (%) | 63 (43.4) | 12 (63.2) | 0.105 |
| Regular transfusions, N (%) | 145 (100) | 11 (57.9) | <0.0001 |
| Age at start of regular transfusions (years) | 1.06 ± 0.82 | 23.08 ± 21.24 | <0.0001 |
| Duration of regular transfusions (years) | 35.90 ± 10.55 | 20.61 ± 18.00 | 0.036 |
| Patients in chelation therapy, N (%) | 145 (100) | 15 (78.9) | <0.0001 |
| Age at start of chelation therapy (years) | 5.34 ± 7.35 | 27.83 ± 19.01 | 0.001 |
| Mean pre-transfusion hemoglobin (g/dL) | 9.55 ± 0.51 | 8.85 ± 1.20 | 0.018 |
| Mean serum ferritin (ng/mL) | 1012.63 ± 865.07 | 615.75 ± 541.77 | 0.018 |
| Altered glucose metabolism, N (%) | 35/116 (30.2) | 1/17 (5.9) | 0.040 |
| MRI LIC (mg/g dw) | 6.12 ± 9.47 | 5.39 ± 6.34 | 0.522 |
| Global pancreas T2* (ms) | 11.86 ± 9.08 | 23.78 ± 9.69 | <0.0001 |
| Pancreatic iron overload, N (%) | 130 (89.7) | 10 (52.6) | <0.0001 |
| BSA-indexed pancreatic volume (cm3/m2) | 25.48 ± 13.26 | 32.40 ± 11.87 | 0.007 |
| Reduced pancreatic volume, N (%) | 10 (52.6) | 130 (89.7) | <0.0001 |
| β-TM Group | β-TI Group | |
|---|---|---|
| Categorical Variables | Difference of BSA-Corrected Pancreatic Volume Between Two Groups (Absent vs. Present) | |
| Females | 27.55 ± 14.94 cm3/m2 vs. 23.94 ± 11.72 cm3/m2 (p = 0.197) | 36.63 ± 11.73 cm3/m2 vs. 28.60 ± 11.21 cm3/m2 (p = 0.146) |
| Splenectomy | 24.37 ± 11.42 cm3/m2 vs. 26.92 ± 15.31 cm3/m2 (p = 0.441) | 29.39 ± 11.53 cm3/m2 vs. 34.16 ± 12.21 cm3/m2 (p = 0.414) |
| Pancreatic iron overload | 36.57 ± 19.77 cm3/m2 vs. 24.20 ± 11.74 cm3/m2 (p = 0.004) | 35.13 ± 11.21 cm3/m2 vs. 29.37 ± 12.49 cm3/m2 (p = 0.304) |
| Continuous Variables | Correlation (R, p-Value) with BSA-Corrected Pancreatic Volume | |
| Age | R = −0.268, p = 0.001 | R = 0.285, p = 0.237 |
| Duration of regular transfusions (for regularly transfused patients) | R = −0.288, p = 0.008 | R = −0.314, p = 0.544 |
| Mean serum hemoglobin | R = −0.132, p = 0.198 | R = 0.577, p = 0.019 |
| Mean serum ferritin | R = −0.056, p = 0.585 | R = −0.297, p = 0.264 |
| MRI LIC | R = 0.067, p = 0.429 | R = −0.635, p = 0.003 |
| Global pancreas T2* | R = 0.221, p = 0.008 | R = 0.296, p = 0.218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, A.; Restaino, G.; Positano, V.; Pistoia, L.; Keilberg, P.; Santodirocco, M.; Spasiano, A.; Casini, T.; Serra, M.; De Marco, E.; et al. Pancreatic Volume in Thalassemia: Determinants and Association with Alterations of Glucose Metabolism. Diagnostics 2025, 15, 568. https://doi.org/10.3390/diagnostics15050568
Meloni A, Restaino G, Positano V, Pistoia L, Keilberg P, Santodirocco M, Spasiano A, Casini T, Serra M, De Marco E, et al. Pancreatic Volume in Thalassemia: Determinants and Association with Alterations of Glucose Metabolism. Diagnostics. 2025; 15(5):568. https://doi.org/10.3390/diagnostics15050568
Chicago/Turabian StyleMeloni, Antonella, Gennaro Restaino, Vincenzo Positano, Laura Pistoia, Petra Keilberg, Michele Santodirocco, Anna Spasiano, Tommaso Casini, Marilena Serra, Emanuela De Marco, and et al. 2025. "Pancreatic Volume in Thalassemia: Determinants and Association with Alterations of Glucose Metabolism" Diagnostics 15, no. 5: 568. https://doi.org/10.3390/diagnostics15050568
APA StyleMeloni, A., Restaino, G., Positano, V., Pistoia, L., Keilberg, P., Santodirocco, M., Spasiano, A., Casini, T., Serra, M., De Marco, E., Roberti, M. G., Bagnato, S., Pepe, A., Clemente, A., & Missere, M. (2025). Pancreatic Volume in Thalassemia: Determinants and Association with Alterations of Glucose Metabolism. Diagnostics, 15(5), 568. https://doi.org/10.3390/diagnostics15050568









