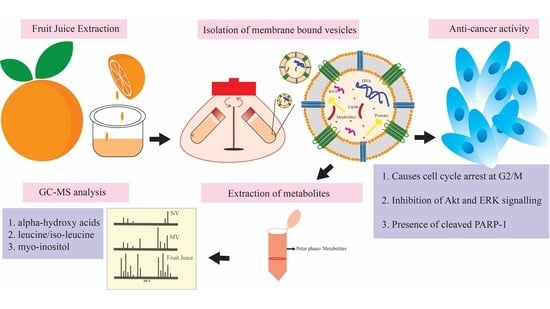
Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Vesicle Isolation
2.2. Metabolite Extraction and Derivatization
2.3. GC-MS Analysis and Data Elaboration
2.4. Cell Cultures
2.5. Cell Proliferation and Viability
2.6. Analysis of Cell Cycle by Flow Cytometry
2.7. Western Blotting
2.8. RNA Extraction and qRT-PCR Analyses
3. Results
3.1. Impact of Citrus-Derived Vesicles on Tumour Cell Lines
3.2. Metabolome Profiles of Grapefruit-Derived Micro and Nanovesicles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- De la Canal, L.; Pinedo, M. Extracellular vesicles: A missing component in plant cell wall remodeling. J. Exp. Bot. 2018, 69, 4655–4658. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles-Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef] [PubMed]
- Bleackley, M.R.; Dawson, C.S.; Anderson, M.A. Fungal Extracellular Vesicles with a Focus on Proteomic Analysis. Proteomics 2019, 19, 1800232. [Google Scholar] [CrossRef]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs. Plant Cell. 2019, 31, 315–324. [Google Scholar] [CrossRef]
- Stanly, C.; Fiume, I.; Capasso, G.; Pocsfalvi, G. Isolation of exosome-like vesicles from plants by ultracentrifugation on sucrose/deuterium oxide (D2O) density cushions. Methods Mol. Biol. 2016, 259–269. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov Identifier: NCT03493984. Plant Exosomes and Patients Diagnosed With Polycystic Ovary Syndrome (PCOS). Available online: https://clinicaltrials.gov/ct2/show/NCT03493984 (accessed on 27 September 2019).
- ClinicalTrials.gov Identifier: NCT01668849. Edible Plant Exosome Ability to Prevent Oral Mucositis Associated with Chemoradiation Treatment of Head and Neck Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01668849 (accessed on 31 August 2020).
- Hadacek, F.; Bachmann, G. Low-molecular-weight metabolite systems chemistry. Front. Environ. Sci. 2015, 3, 12. Available online: https://www.frontiersin.org/article/10.3389/fenvs.2015.00012 (accessed on 4 March 2015). [CrossRef]
- Tebani, A.; Bekri, S. Paving the Way to Precision Nutrition Through Metabolomics. Front. Nutr. 2019, 6, 41. Available online: https://www.frontiersin.org/article/10.3389/fnut.2019.00041 (accessed on 9 April 2019). [CrossRef] [PubMed]
- Berk, Z. Nutritional and health-promoting aspects of citrus consumption. In Citrus Fruit Processing; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2016; pp. 261–279. [Google Scholar] [CrossRef]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.L.; Willmitzer, N. Simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.F.; Grignani, C.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- King, R.W.; Jackson, P.K.; Kirschner, M.W. Mitosis in transition. Cell 1994, 79, 563–571. [Google Scholar] [CrossRef]
- Lei, C.; Wang, W.; Zhu, Y.; Fang, W.; Tan, W. The decrease of cyclin B2 expression inhibits invasion and metastasis of bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2016, 34, e1–e237. [Google Scholar] [CrossRef]
- El-Deiry, W.S. p21(WAF1) Mediates Cell-Cycle Inhibition, Relevant to Cancer Suppression and Therapy. Cancer Res. 2016, 76, 5189–5191. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Turiel, E. Improved molecularly imprinted polymer grafted to porous polyethylene frits for the solid-phase extraction of thiabendazole from citrus sample extracts. Mol. Impr. 2015, 1–7. [Google Scholar] [CrossRef]
- Karbowniczek, M.; Spittle, C.T.; Morrison, S.; Wu, H.; Henske, E.P. mTOR Is Activated in the Majority of Malignant Melanomas. J. Investig. Dermatol. 2008, 128, 980–987. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.C.; Cox, R.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.W.; Bottomley, J.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Slipicevic, A.; Holm, R.; Nguyen, M.P.J.; Bøhler, T.B.; Davidson, P.; Flørenes, V.A. Expression of Activated Akt and PTEN in Malignant Melanomas: Relationship With Clinical Outcome. Am. J. Clin. Pathol. 2005, 124, 528–536. [Google Scholar] [CrossRef]
- Smalley, K.S.M. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int. J. Cancer. 2003, 104, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J.; Leong, S.P. From Melanocytes to Melanoma: The Progression to Malignancy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Lassen, A.; Atefi, M.; Robert, L.; Wong, D.J.; Cerniglia, M.; Comin-Anduix, B.; Ribas, A. Effects of AKT inhibitor therapy in response and resistance to BRAF inhibition in melanoma. Mol. Cancer. 2014, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Kwong, L.N.; Davies, M.A. Navigating the Therapeutic Complexity of PI3K Pathway Inhibition in Melanoma. Clin. Cancer Res. 2013, 19, 5310–5319. [Google Scholar] [CrossRef]
- Savoia, P.; Fava, P.; Casoni, F.; Cremona, O. Targeting the ERK Signaling Pathway in Melanoma. Int. J. Mol. Sci. 2019, 20, 1483. [Google Scholar] [CrossRef]
- Ciołczyk-Wierzbicka, D.; Gil, D.; Laidler, P. Treatment of melanoma with selected inhibitors of signaling kinases effectively reduces proliferation and induces expression of cell cycle inhibitors. Med. Oncol. 2017, 35, 7. [Google Scholar] [CrossRef]
- Roh, J.I.; Lee, H.W. A myo-inositol diet for lung cancer prevention and beyond. J. Thorac. Dis. 2018, 10, S3919–S3921. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Guo, F.; Ouyang, D. A review of the pharmacology and toxicology of aucubin. Fitoterapia 2020, 140, 104443. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.J.; Robinson, D.P.; Stolzenberg-Solomon, R.Z.; Bamlet, W.R.; de Andrade, M.; Oberg, A.L.; Hammer, T.J.; Rabe, K.G.; Anderson, K.E.; Olson, J.E.; et al. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes Control. 2011, 22, 1613. [Google Scholar] [CrossRef] [PubMed]
- Maserejian, N.E.; Giovannucci, N.; Rosner, B.; Zavras, A.; Joshipura, K. Prospective Study of Fruits and Vegetables and Risk of Oral Premalignant Lesions in Men. Am. J. Epidemiol. 2006, 164, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.-J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.R.; Alteri, D.; Jemal, A. Cancer treatment and survivorship statistics. CA. Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef]
- Curtin, N.J. DNA repair dysregulation from cancer driver to therapeutic target. Nat. Rev. Cancer. 2012, 12, 801–817. [Google Scholar] [CrossRef]
- Viana, L.R.; Tobar, N.; Busanello, E.N.B.; Marques, A.C.; de Oliveira, A.G.; Lima, T.I.; Machado, G.; Castelucci, B.G.; Ramos, C.D.; Brunetto, S.Q.; et al. Leucine-rich diet induces a shift in tumour metabolism from glycolytic towards oxidative phosphorylation, reducing glucose consumption and metastasis in Walker-256 tumour-bearing rats. Sci. Rep. 2019, 9, 15529. [Google Scholar] [CrossRef]
- Chhetri, D.R. Myo-Inositol and Its Derivatives: Their Emerging Role in the Treatment of Human Diseases. Front. Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Gu, M.; Roy, S.; Raina, K.; Agarwal, C.; Agarwal, R. Inositol Hexaphosphate Suppresses Growth and Induces Apoptosis in Prostate Carcinoma Cells in Culture and Nude Mouse Xenograft: PI3K-Akt Pathway as Potential Target. Cancer Res. 2009, 69, 9465–9472. [Google Scholar] [CrossRef] [PubMed]
- Kapral, M.; Wawszczyk, J.; Jesse, K.; Paul-Samojedny, M.; Kuśmierz, D.; Węglarz, L. Inositol Hexaphosphate Inhibits Proliferation and Induces Apoptosis of Colon Cancer Cells by Suppressing the AKT/mTOR Signaling Pathway. Molecules 2017, 22, 1657. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Furuse, T.; Yagasaki, K. Inhibitory effect of serum from rats administered with coffee on the proliferation and invasion of rat ascites hepatoma cells. Cytotechnology 1997, 25, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Zhang, S.Y.; Yu, Y.G.Q.; Su, Y. (-)-4-O-(4-O-β-D-glucopyranosylcaffeoyl)quinic acid presents antitumor activity in HT-29 human colon cancer in vitro and in vivo. Mol. Cell. Toxicol. 2015, 11, 457–463. [Google Scholar] [CrossRef]
- Kyoung, S.P.; Chang, I.M. Anti-inflammatory activity of aucubin by inhibition of tumor necrosis factor-α production in RAW 264.7 cells. Planta Med. 2004, 70, 778–779. [Google Scholar] [CrossRef]
- Chang, I.M. Antiviral activity of aucubin against hepatitis B virus replication. Phyther. Res. 1997, 11, 189–192. [Google Scholar] [CrossRef]
- Hung, J.Y.; Yang, C.J.; Tsai, Y.M.; Huang, H.W.; Huang, M.S. Antiproliferative Activity Of Aucubin Is Through Cell Cycle Arrest And Apoptosis In Human Non-Small Cell Lung Cancer A549 Cells. Clin. Exp. Pharmacol. Physiol. 2008, 35, 995–1001. [Google Scholar] [CrossRef]
- Shim, K.M.; Choi, S.H.; Jeong, M.J.; Kang, S.S. Effects of Aucubin on the Healing of Oral Wounds. Vivo 2007, 21, 1037–1041. Available online: http://iv.iiarjournals.org/content/21/6/1037.full.pdf+html (accessed on 30 August 2007).
- Benito, P.B.; Lanza, A.M.D.; Sen, A.M.S.; Galindez, J.D.S.; Matellano, L.F.; Gómez, A.S.; Martínez, M.J. Effects of some iridoids from plant origin on arachidonic acid metabolism in cellular systems. Planta Med. 2000, 66, 324–328. [Google Scholar] [CrossRef]
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. Critical Review on the Effect of Docosahexaenoic Acid (DHA) on Cancer Cell Cycle Progression. Int. J. Mol. Sci. 2017, 18, 1784. [Google Scholar] [CrossRef]
- Shaikh, I.I.; Brown, A.A.; Schofield, A.C.; Wahle, K.S.D.; Heys, W. Docosahexaenoic acid enhances the efficacy of docetaxel in prostate cancer cells by modulation of apoptosis: The role of genes associated with the NF-κB pathway. Prostate J. 2008, 68, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Plant-Derived Edible Nanoparticles and miRNAs: Emerging Frontier for Therapeutics and Targeted Drug-Delivery. ACS Sustain. Chem. Eng. 2019, 7, 8055–8069. [Google Scholar] [CrossRef]




| A | ||
| N° | Area Counts × Min | Name |
| 1 | 8.4 × 108 | D-(−)-Fructose, pentakis(trimethylsilyl) ether, methyloxime (syn) |
| 2 | 4.2 × 108 | Citric acid, 4TMS derivative |
| 3 | 4.2 × 108 | d-Glucose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 4 | 3.7 × 108 | Sucrose, 8TMS derivative |
| 5 | 2.7 × 108 | Myo-Inositol, 6TMS derivative |
| 6 | 1.7 × 108 | d-Galactose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 7 | 6.5 × 107 | Lactulose, octakis(trimethylsilyl) ether, methyloxime (isomer 1) |
| 8 | 6.2 × 107 | Quininic acid (5TMS) |
| 9 | 4.8 × 107 | L-Aspartic acid, 2TMS derivative+L-Aspartic acid, 3TMS derivative |
| 10 | 3.1 × 107 | Malic acid, 3TMS derivative |
| 11 | 2.3 × 107 | Serine, 3TMS derivative |
| 12 | 2.1 × 107 | Glycolic acid, 2TMS derivative |
| 13 | 1.9 × 107 | L-Proline, 2TMS derivative |
| 14 | 1.7 × 107 | d-Ribose, 2,3,4,5-tetrakis-O-(trimethylsilyl)-, O-methyloxime |
| 15 | 1.6 × 107 | 4-Aminobutanoic acid, 3TMS derivative |
| 16 | 1.4 × 107 | N-Acetyl glucosamine methoxime, tetrakis(trimethylsilyl) |
| 17 | 1.3 × 107 | D-(+)-Turanose, octakis(trimethylsilyl) ether |
| 18 | 9.9 × 106 | L-Alanine, 2TMS derivative |
| 19 | 9.1 × 106 | L-Glutamic acid, 3TMS derivative |
| 20 | 7.2 × 106 | α-D-Glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate |
| B | ||
| N° | Area Counts × Min | Name |
| 1 | 6.0 × 108 | D-Fructose, 1,3,4,5,6-pentakis-O-(trimethylsilyl)-, O-methyloxime |
| 2 | 4.1 × 108 | Sucrose, 8TMS derivative |
| 3 | 3.7 × 108 | d-Glucose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 4 | 2.9 × 108 | Citric acid, 3TMS derivative+Citric acid, 4TMS derivative |
| 5 | 2.1 × 108 | Myo-Inositol, 6TMS derivative |
| 6 | 1.7 × 108 | d-Galactose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 7 | 1.4 × 108 | Oxalic acid, 2TMS derivative |
| 8 | 6.9 × 107 | D-(+)-Turanose, octakis(trimethylsilyl) ether |
| 9 | 4.0 × 107 | Quininic acid (5TMS) |
| 10 | 3.9 × 107 | β-D-Galactopyranoside, methyl 2,3-bis-O-(trimethylsilyl)-, cyclic methylboronate |
| 11 | 3.4 × 107 | α-D-Glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate |
| 12 | 3.2 × 107 | Lactulose, octakis(trimethylsilyl) ether (isomer 1) |
| 13 | 2.8 × 107 | (2S,3R)-3-[(4E,7E)-Nona-4,7-dienoyl]-N,N-bis(trimethylsilyl)oxirane-2-carboxamide |
| 14 | 2.1 × 107 | N-Acetyl glucosamine methoxime, tetrakis(trimethylsilyl) |
| 15 | 2.0 × 107 | D-Mannitol, 6TMS derivative |
| 16 | 1.6 × 107 | D-(−)-Tagatose, pentakis(trimethylsilyl) ether, methyloxime (anti) |
| 17 | 1.5 × 107 | L-Aspartic acid, 2TMS derivative+L-Aspartic acid, 3TMS derivative |
| 18 | 1.4 × 107 | Aucubin, hexakis(trimethylsilyl) ether |
| 19 | 1.0 × 106 | Malic acid, 3TMS derivative |
| 20 | 8.1 × 106 | Myo-Inositol, pentakis-O-(trimethylsilyl)-, bis(trimethylsilyl) phosphate |
| C | ||
| N° | Area Counts × Min | Name |
| 1 | 1.1 × 108 | Glycolic acid, 2TMS derivative |
| 2 | 3.1 × 107 | (2S,3R)-3-[(4E,7E)-Nona-4,7-dienoyl]-N,N-bis(trimethylsilyl)oxirane-2-carboxamide |
| 3 | 4.7 × 107 | L-Isoleucine, 2TMS derivative |
| 4 | 4.3 × 106 | L-Leucine, TMS derivative+Leucine, 2TMS derivative |
| 5 | 3.9 × 106 | d-Glucose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 6 | 3.6 × 106 | D-(−)-Tagatose, pentakis(trimethylsilyl) ether, methyloxime (anti) |
| 7 | 2.1 × 106 | D-Psicose, pentakis(trimethylsilyl) ether, methyloxime (syn) |
| 8 | 1.3 × 106 | Urea, 2TMS derivative |
| 9 | 1.2 × 106 | D-(+)-Turanose, octakis(trimethylsilyl) ether |
| 10 | 1.1 × 106 | Lactic Acid, 2TMS derivative |
| 11 | 9.4 × 105 | d-Mannose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- |
| 12 | 7.6 × 105 | L-(−)-Sorbose, pentakis(trimethylsilyl) ether, methyloxime (anti) |
| 13 | 5.9 × 105 | Citric acid, 4TMS derivative |
| 14 | 4.5 × 105 | Sucrose, 8TMS derivative |
| 15 | 1.5 × 105 | Myo-Inositol, 6TMS derivative |
| 16 | 1.3 × 105 | Palmitic Acid, TMS derivative |
| 17 | 7.5 × 104 | 2-Hydroxycyclohexane-1-carboxylic acid, bis(trimethylsilyl) deriv. |
| 18 | 4.7 × 104 | Doconexent, TMS derivative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. https://doi.org/10.3390/cells9122722
Stanly C, Alfieri M, Ambrosone A, Leone A, Fiume I, Pocsfalvi G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells. 2020; 9(12):2722. https://doi.org/10.3390/cells9122722
Chicago/Turabian StyleStanly, Christopher, Mariaevelina Alfieri, Alfredo Ambrosone, Antonietta Leone, Immacolata Fiume, and Gabriella Pocsfalvi. 2020. "Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line" Cells 9, no. 12: 2722. https://doi.org/10.3390/cells9122722
APA StyleStanly, C., Alfieri, M., Ambrosone, A., Leone, A., Fiume, I., & Pocsfalvi, G. (2020). Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells, 9(12), 2722. https://doi.org/10.3390/cells9122722