Therapeutic Hypothermia after Cardiac Arrest Attenuates Hindlimb Paralysis and Damage of Spinal Motor Neurons and Astrocytes through Modulating Nrf2/HO-1 Signaling Pathway in Rats
Abstract
1. Introduction
2. Materials and Methods
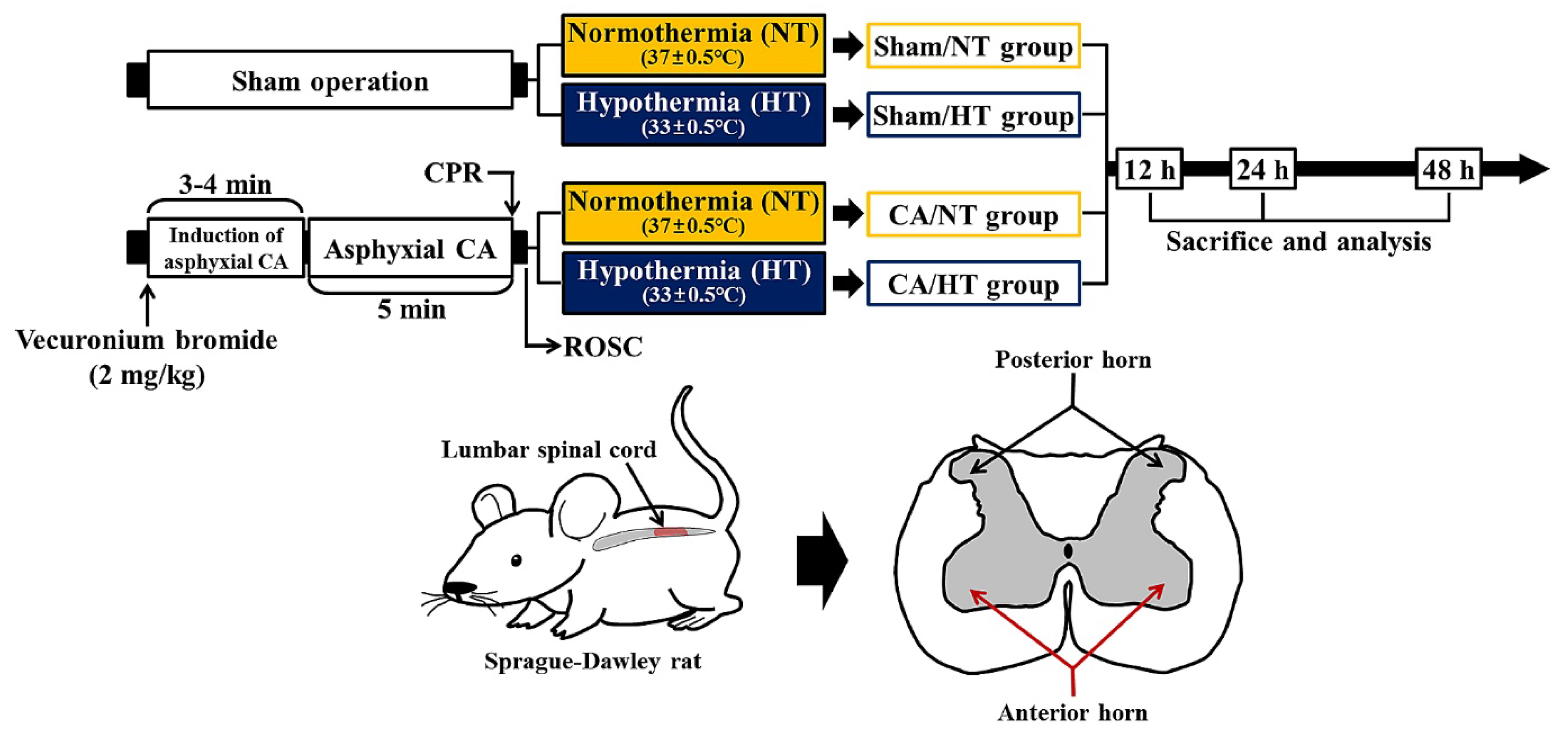
2.1. Experimental Protocol and Animals
2.2. Induction of Asphyxial CA/ROSC and Therapeutic Hypothermia
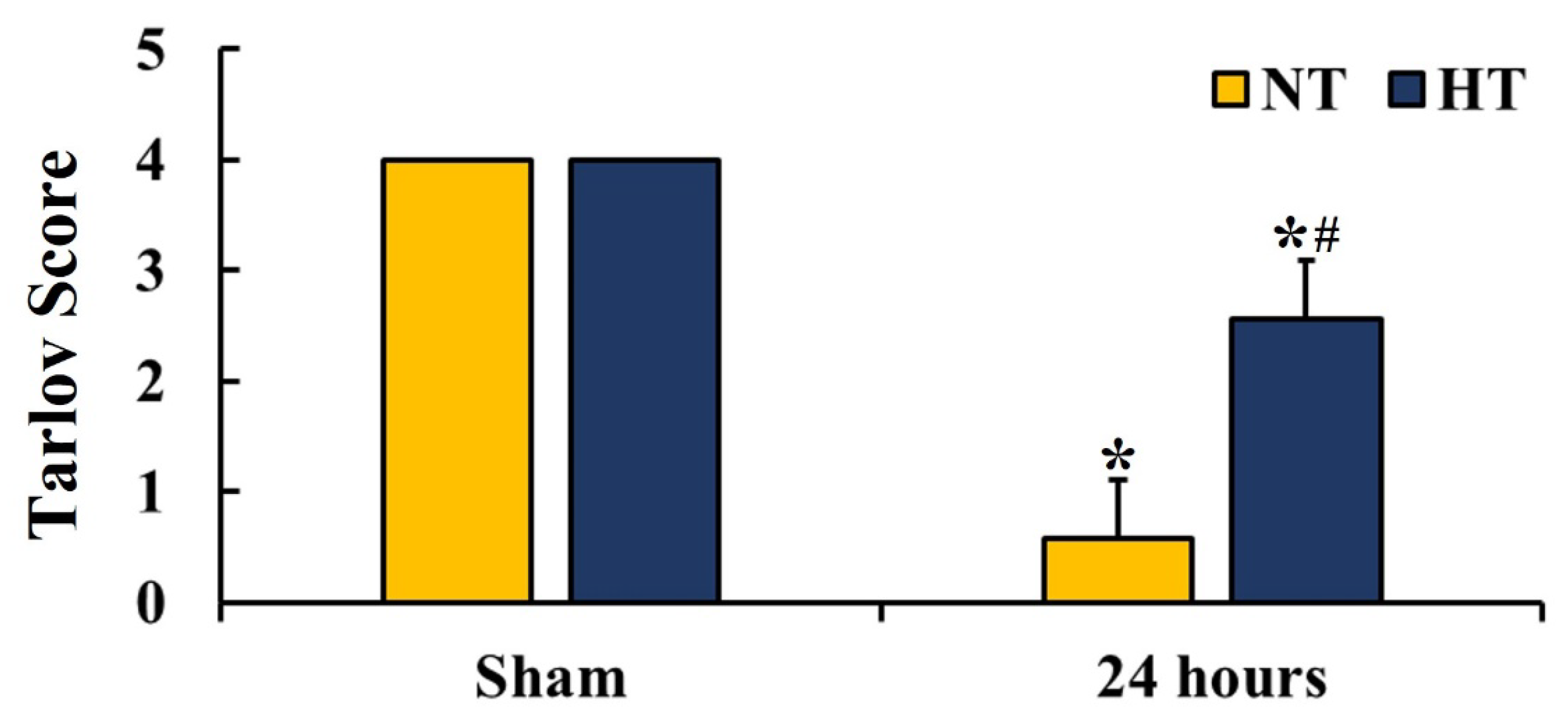
2.3. Motor Function Evaluation
2.4. Fluoro Jade B (FJB) Histofluorescence
2.5. Immunohistochemistry
2.6. Double Immunofluorescence
2.7. Western Blotting
2.8. Analyses of Data
2.9. Statistical Analyses
3. Results
3.1. Survival Rate and Physiological Characteristics
3.2. Hindlimb Motor Function
3.3. Protection of Motor Neurons
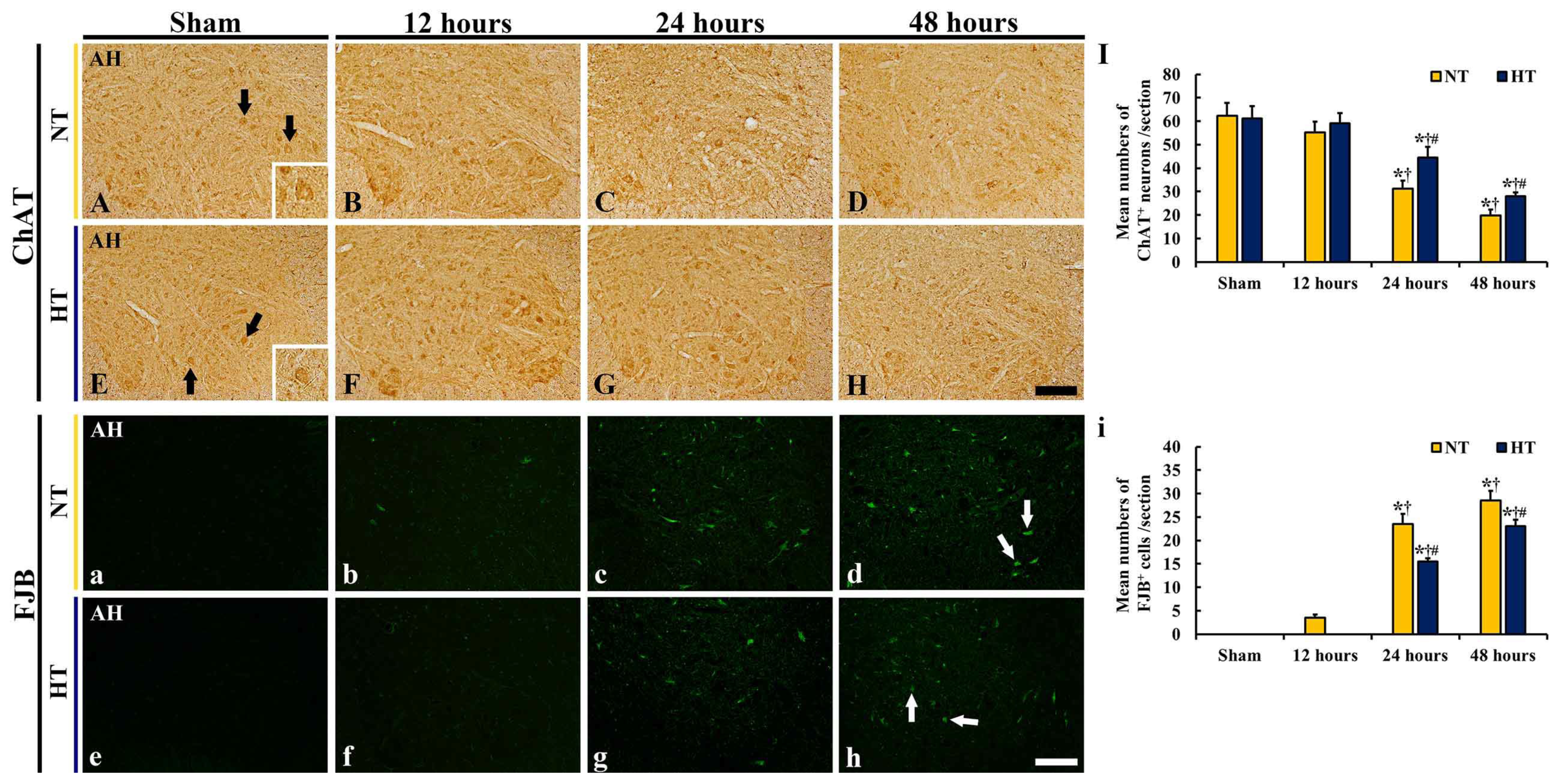
3.3.1. ChAT-Immunoreactive (ChAT+) Neurons
3.3.2. FJB-Positive (FJB+) Cells
3.4. Changes in Nrf2 and HO-1 Expressions
3.4.1. Nrf2 and HO-1 Protein Levels
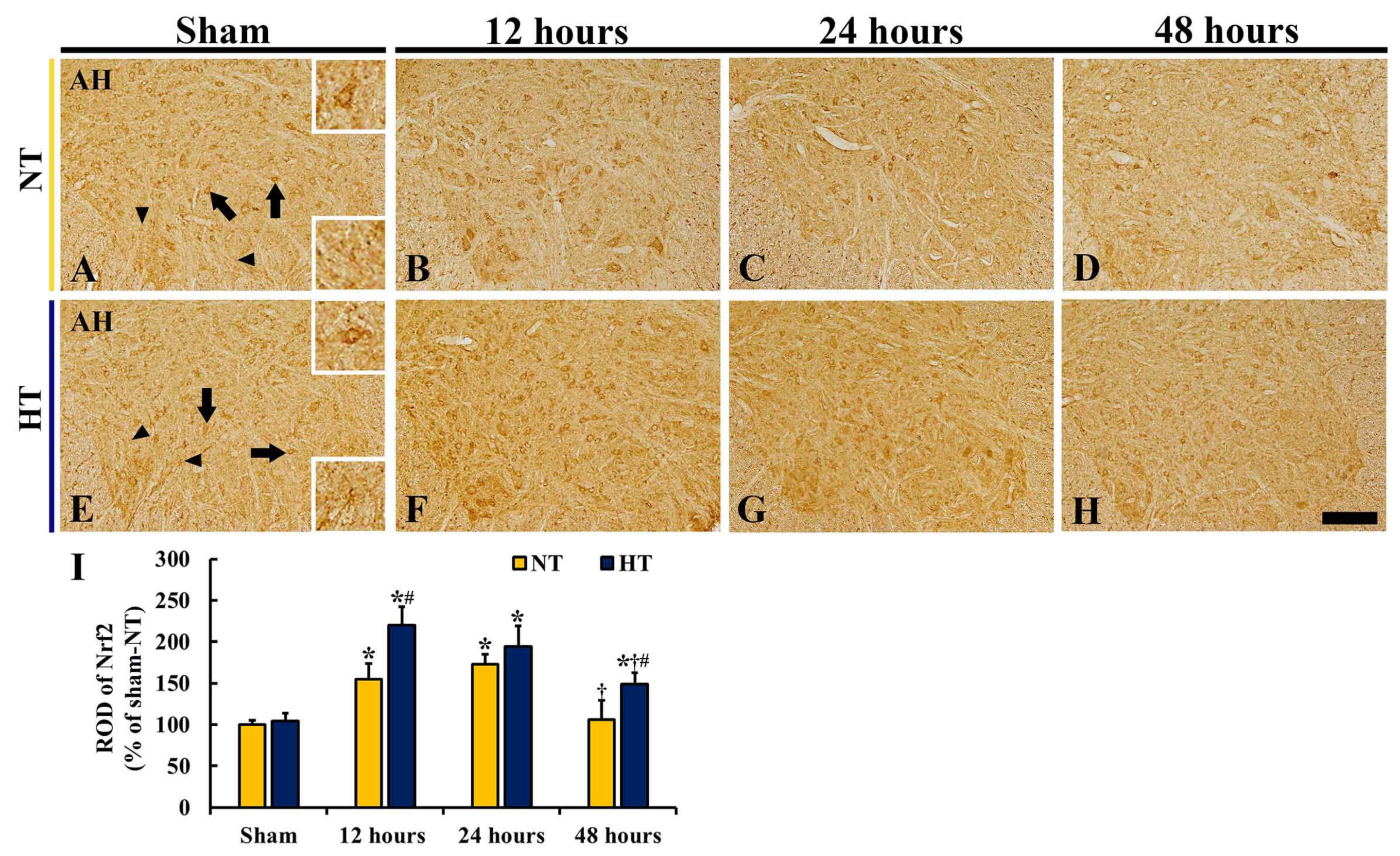
3.4.2. Nrf2 Immunoreactivity
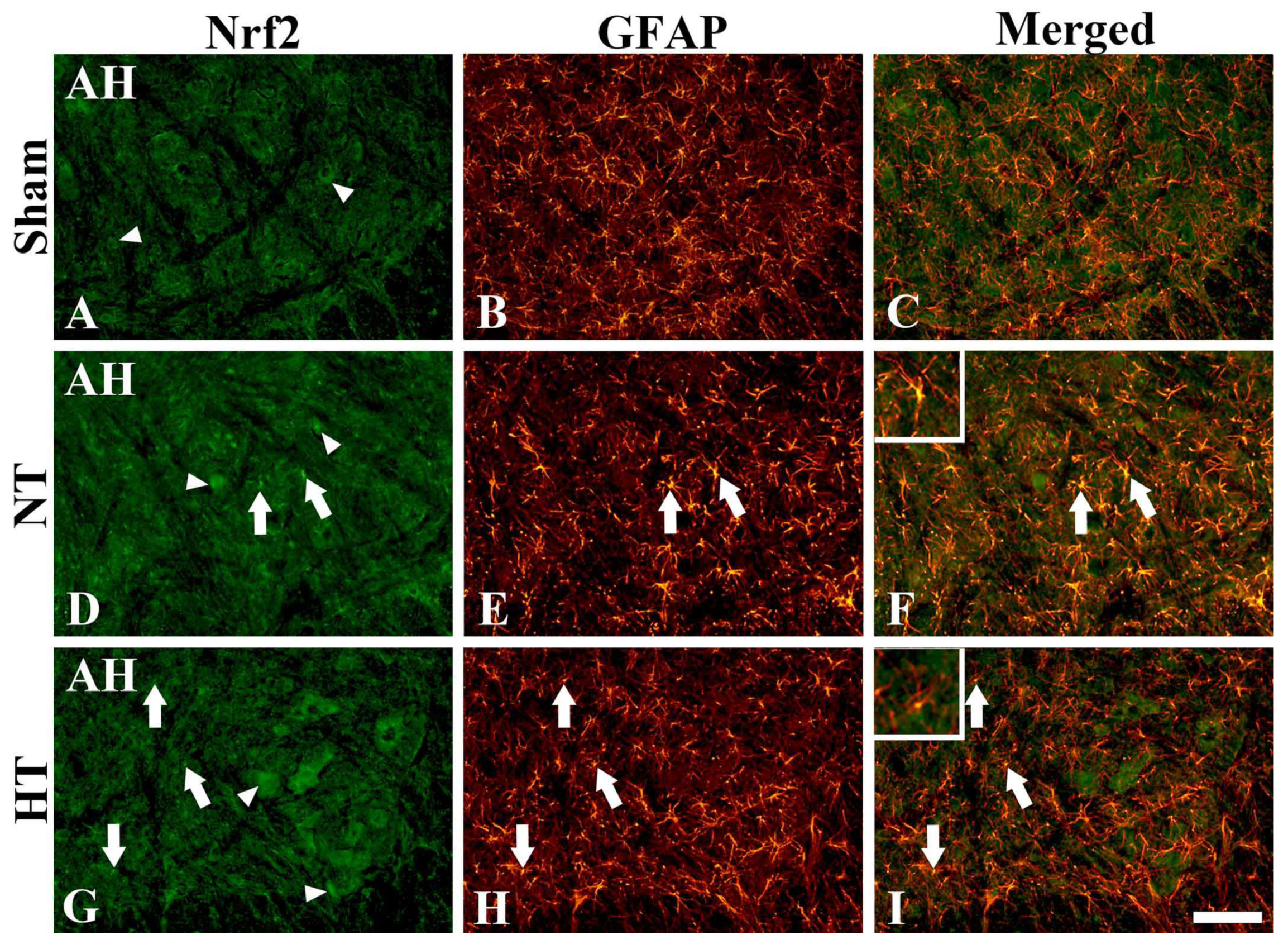
3.4.3. Nrf2 Immunofluorescence
3.4.4. HO-1 Immunoreactivity
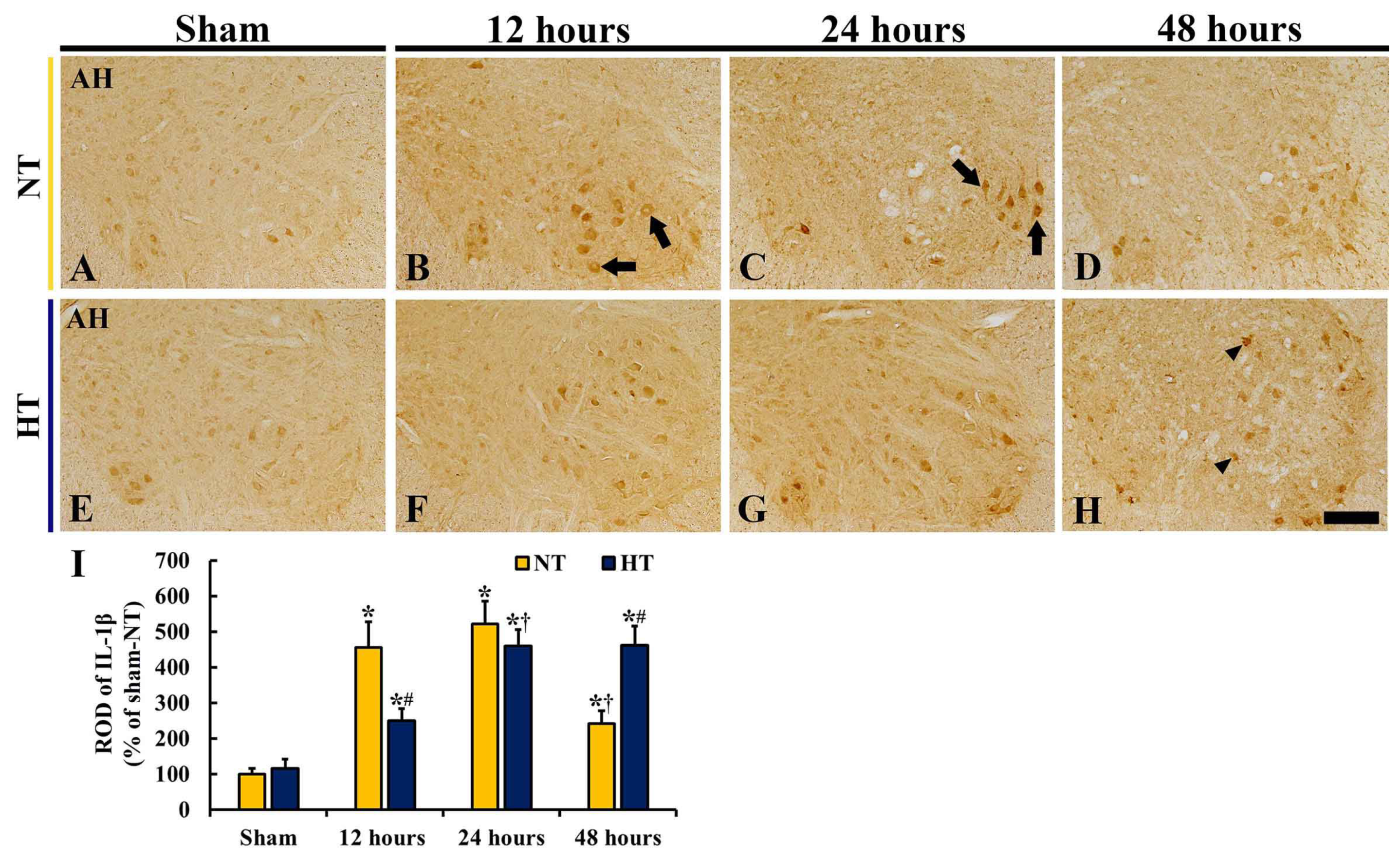
3.5. Changes in IL-1β
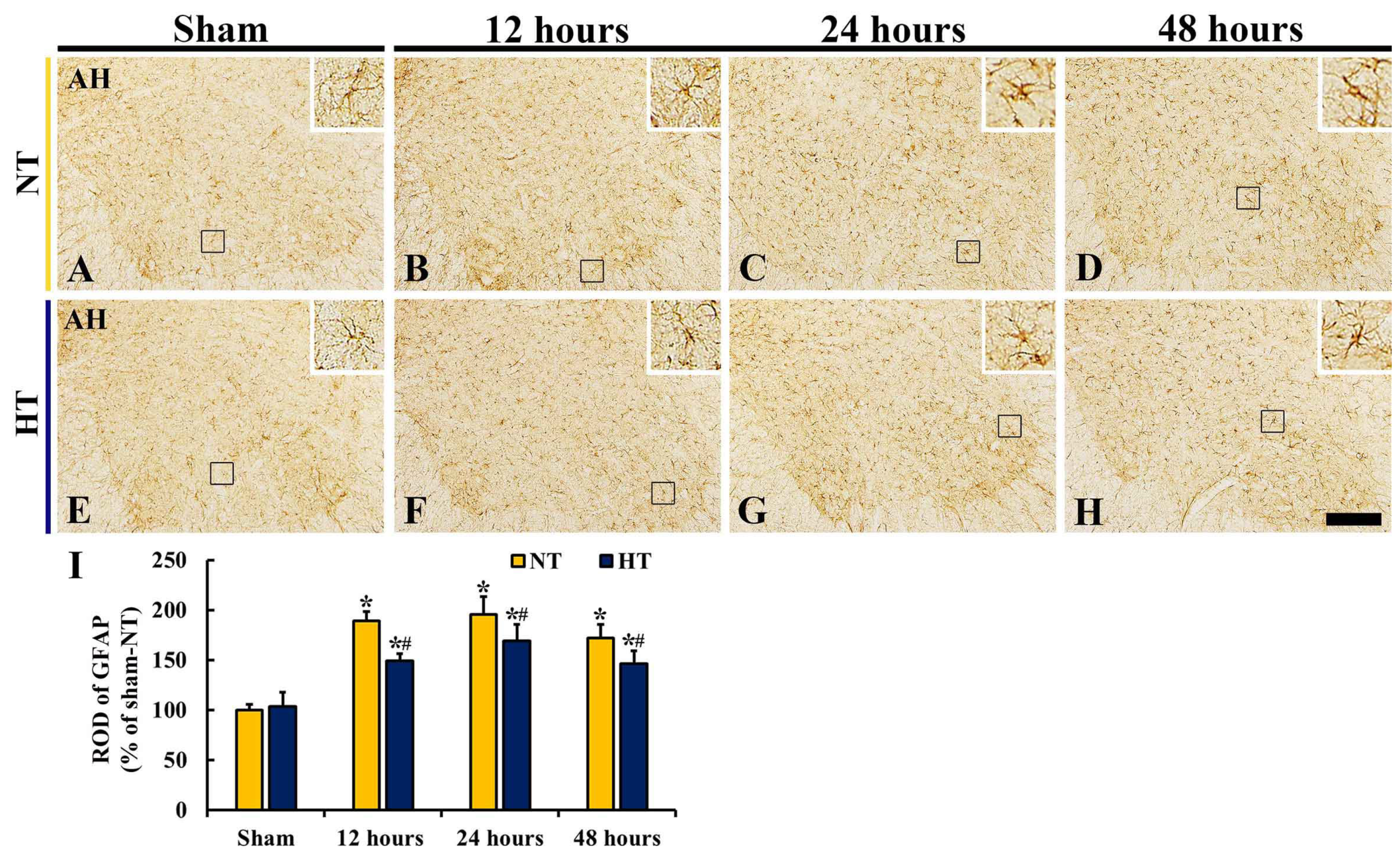
3.6. Changes in Reactive Astrocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazensky, D.; Flesarova, S.; Sulla, I. Arterial blood supply to the spinal cord in animal models of spinal cord injury. A review. Anat. Rec. 2017, 300, 2091–2106. [Google Scholar] [CrossRef]
- Imaizumi, H.; Ujike, Y.; Asai, Y.; Kaneko, M.; Chiba, S. Spinal cord ischemia after cardiac arrest. J. Emerg. Med. 1994, 12, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.; Lach, B. Selective vulnerability of the lumbosacral spinal cord after cardiac arrest and hypotension. Stroke 2002, 33, 116–121. [Google Scholar] [CrossRef]
- Drinkwater, S.L.; Goebells, A.; Haydar, A.; Bourke, P.; Brown, L.; Hamady, M.; Gibbs, R.G.; the Regional Vascular Unit, St Mary’s Hospital, Imperial College NHS Trust. The incidence of spinal cord ischaemia following thoracic and thoracoabdominal aortic endovascular intervention. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 729–735. [Google Scholar] [CrossRef]
- Bisdas, T.; Panuccio, G.; Sugimoto, M.; Torsello, G.; Austermann, M. Risk factors for spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. J. Vasc. Surg. 2015, 61, 1408–1416. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Feuerstein, J.S.; Theodore, N.; Cavalcanti, D.D.; Spetzler, R.F.; Preul, M.C. Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions: A review. J. Neurosurg. Spine 2011, 15, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Hayashi, T.; Abe, K.; Sadahiro, M.; Tabayashi, K. Delayed and selective motor neuron death after transient spinal cord ischemia: A role of apoptosis? J. Thorac. Cardiovasc. Surg. 1998, 115, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.D.; Puskas, F.; Meng, X.; Cho, D.; Cleveland, J.C., Jr.; Weyant, M.J.; Watkins, M.T.; Fullerton, D.A.; Reece, T.B. Ischemic dose-response in the spinal cord: Both immediate and delayed paraplegia. J. Surg. Res. 2012, 174, 238–244. [Google Scholar] [CrossRef]
- Lee, J.C.; Tae, H.J.; Cho, J.H.; Kim, I.S.; Lee, T.K.; Park, C.W.; Park, Y.E.; Ahn, J.H.; Park, J.H.; Yan, B.C.; et al. Therapeutic hypothermia attenuates paraplegia and neuronal damage in the lumbar spinal cord in a rat model of asphyxial cardiac arrest. J. Therm. Biol. 2019, 83, 1–7. [Google Scholar] [CrossRef]
- Lin, Y.; Vreman, H.J.; Wong, R.J.; Tjoa, T.; Yamauchi, T.; Noble-Haeusslein, L.J. Heme oxygenase-1 stabilizes the blood—Spinal cord barrier and limits oxidative stress and white matter damage in the acutely injured murine spinal cord. J. Cereb. Blood Flow Metab. 2007, 27, 1010–1021. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, F.; Cheng, F.; Ying, L.; Wang, C.; Shi, K.; Wang, J.; Xia, K.; Gong, Z.; Huang, X. Strategies and prospects of effective neural circuits reconstruction after spinal cord injury. Cell Death Dis. 2020, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Lee, T.-K.; Kim, B.; Lee, J.-C.; Tae, H.-J.; Cho, J.H.; Park, Y.; Shin, M.C.; Ohk, T.G.; Park, C.W. Therapeutic hypothermia improves hind limb motor outcome and attenuates oxidative stress and neuronal damage in the lumbar spinal cord following cardiac arrest. Antioxidants 2020, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, J.; Wang, Y.; Qi, T.; Wang, Z.; Chen, L.; Suo, N. Imatinib inhibits oxidative stress response in spinal cord injury rats by activating nrf2/ho-1 signaling pathway. Exp. Ther. Med. 2020, 19, 597–602. [Google Scholar] [CrossRef]
- Gong, P.; Li, C.S.; Hua, R.; Zhao, H.; Tang, Z.R.; Mei, X.; Zhang, M.Y.; Cui, J. Mild hypothermia attenuates mitochondrial oxidative stress by protecting respiratory enzymes and upregulating mnsod in a pig model of cardiac arrest. PLoS ONE 2012, 7, e35313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, C.S.; Gong, P.; Tang, Z.R.; Hua, R.; Mei, X.; Zhang, M.Y.; Cui, J. Molecular mechanisms of therapeutic hypothermia on neurological function in a swine model of cardiopulmonary resuscitation. Resuscitation 2012, 83, 913–920. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional regulation by nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Diao, M.-Y.; Zheng, J.; Shan, Y.; Xi, S.; Zhu, Y.; Hu, W.; Lin, Z. Hypothermia prevents hippocampal oxidative stress and apoptosis via the gsk-3β/nrf2/ho-1 signaling pathway in a rat model of cardiac arrest-induced brain damage. Neurol. Res. 2020, 42, 773–782. [Google Scholar] [CrossRef]
- Jawad, A.; Yoo, Y.-J.; Cho, J.-H.; Yoon, J.C.; Tian, W.; Islam, M.S.; Lee, E.-Y.; Shin, H.-Y.; Kim, S.E.; Kim, K. Therapeutic hypothermia effect on asphyxial cardiac arrest-induced renal ischemia/reperfusion injury via change of nrf2/ho-1 levels. Exp. Ther. Med. 2021, 22, 1031. [Google Scholar] [CrossRef]
- Tae, H.J.; Kang, I.J.; Lee, T.K.; Cho, J.H.; Lee, J.C.; Shin, M.C.; Kim, Y.S.; Kim, J.D.; Ahn, J.H.; Park, J.H.; et al. Neuronal injury and tumor necrosis factor-alpha immunoreactivity in the rat hippocampus in the early period of asphyxia-induced cardiac arrest under normothermia. Neural Regen. Res. 2017, 12, 2007–2013. [Google Scholar]
- Lee, J.H.; Lee, T.K.; Kim, I.H.; Lee, J.C.; Won, M.H.; Park, J.H.; Ahn, J.H.; Shin, M.C.; Ohk, T.G.; Moon, J.B.; et al. Changes in histopathology and tumor necrosis factor-alpha levels in the hearts of rats following asphyxial cardiac arrest. Clin. Exp. Emerg. Med. 2017, 4, 160–167. [Google Scholar] [CrossRef]
- Drabek, T.; Janata, A.; Wilson, C.D.; Stezoski, J.; Janesko-Feldman, K.; Tisherman, S.A.; Foley, L.M.; Verrier, J.D.; Kochanek, P.M. Minocycline attenuates brain tissue levels of tnf-alpha produced by neurons after prolonged hypothermic cardiac arrest in rats. Resuscitation 2014, 85, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.H.; Becker, L.B.; Ornato, J.P.; Hedges, J.R.; Bircher, N.G.; Chandra, N.C.; Cummins, R.O.; Dick, W.; Ebmeyer, U.; Halperin, H.R.; et al. Utstein-style guidelines for uniform reporting of laboratory cpr research. A statement for healthcare professionals from a task force of the american heart association, the american college of emergency physicians, the american college of cardiology, the european resuscitation council, the heart and stroke foundation of canada, the institute of critical care medicine, the safar center for resuscitation research, and the society for academic emergency medicine. Resuscitation 1996, 33, 69–84. [Google Scholar] [PubMed]
- Liachenko, S.; Tang, P.; Hamilton, R.L.; Xu, Y. A reproducible model of circulatory arrest and remote resuscitation in rats for nmr investigation. Stroke A J. Cereb. Circ. 1998, 29, 1229–1238, discussion 1238–1229. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Koenig, M.A.; Shin, H.C.; Zhen, G.; Pardo, C.A.; Hanley, D.F.; Thakor, N.V.; Geocadin, R.G. Improving neurological outcomes post-cardiac arrest in a rat model: Immediate hypothermia and quantitative eeg monitoring. Resuscitation 2008, 76, 431–442. [Google Scholar] [CrossRef]
- Tarlov, I.M. Acute spinal cord compression paralysis. J. Neurosurg. 1972, 36, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hwang, I.K.; Choi, J.H.; Yoo, K.Y.; Han, T.H.; Park, O.K.; Lee, S.Y.; Ryu, P.D.; Won, M.H. Calcium binding proteins immunoreactivity in the rat basolateral amygdala following myocardial infarction. Cell. Mol. Neurobiol. 2010, 30, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Schmued, L.C.; Hopkins, K.J. Fluoro-jade b: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]
- Park, C.W.; Ahn, J.H.; Lee, T.K.; Park, Y.E.; Kim, B.; Lee, J.C.; Kim, D.W.; Shin, M.C.; Park, Y.; Cho, J.H.; et al. Post-treatment with oxcarbazepine confers potent neuroprotection against transient global cerebral ischemic injury by activating nrf2 defense pathway. Biomed. Pharmacother. 2020, 124, 109850. [Google Scholar] [CrossRef]
- Lee, C.H.; Ahn, J.H.; Chen, B.H.; Kim, D.W.; Sim, H.; Lee, T.-K.; Park, J.H.; Won, M.-H.; Choi, S.Y. Differences in tnf-α and tnf-r1 expression in damaged neurons and activated astrocytes of the hippocampal ca1 region between young and adult gerbils following transient forebrain ischemia. Mol. Med. Rep. 2021, 24, 625. [Google Scholar] [CrossRef]
- Zhao, W.; Gasterich, N.; Clarner, T.; Voelz, C.; Behrens, V.; Beyer, C.; Fragoulis, A.; Zendedel, A. Astrocytic nrf2 expression protects spinal cord from oxidative stress following spinal cord injury in a male mouse model. J. Neuroinflamm. 2022, 19, 134. [Google Scholar] [CrossRef]
- Lee, J.C.; Park, J.H.; Kim, I.H.; Cho, G.S.; Ahn, J.H.; Tae, H.J.; Choi, S.Y.; Cho, J.H.; Kim, D.W.; Kwon, Y.G.; et al. Neuroprotection of ischemic preconditioning is mediated by thioredoxin 2 in the hippocampal ca1 region following a subsequent transient cerebral ischemia. Brain Pathol. 2017, 27, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Ahn, J.H.; Kim, H.; Kim, D.W.; Lee, T.K.; Lee, J.C.; Kim, Y.M.; Lee, C.H.; Hwang, I.K.; Yan, B.C.; et al. Chronic high-fat diet-induced obesity in gerbils increases pro-inflammatory cytokines and mtor activation, and elicits neuronal death in the striatum following brief transient ischemia. Neurochem. Int. 2018, 121, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Shin, H.-Y.; Lee, E.-Y.; Yoo, Y.-J.; Kim, R.-H.; Cho, J.-H.; Lee, T.-K.; Ahn, D.; Park, B.-Y.; Yoon, J.C. Effect of therapeutic hypothermia against renal injury in a rat model of asphyxial cardiac arrest: A focus on the survival rate, pathophysiology and antioxidant enzymes. Mol. Med. Rep. 2022, 25, 19. [Google Scholar] [CrossRef] [PubMed]
- Lang-Lazdunski, L.; Matsushita, K.; Hirt, L.; Waeber, C.; Vonsattel, J.P.; Moskowitz, M.A.; Dietrich, W.D. Spinal cord ischemia. Development of a model in the mouse. Stroke 2000, 31, 208–213. [Google Scholar] [CrossRef]
- Lee, T.-K.; Lee, J.-C.; Tae, H.-J.; Kim, H.-I.; Shin, M.C.; Ahn, J.H.; Park, J.H.; Kim, D.W.; Hong, S.; Choi, S.Y. Therapeutic effects of risperidone against spinal cord injury in a rat model of asphyxial cardiac arrest: A focus on body temperature, paraplegia, motor neuron damage, and neuroinflammation. Vet. Sci. 2021, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulos, G.K.; Kato, H.; Wu, Y.; Dougenis, D.; Mackey, M.; Hsu, C.Y.; Kouchoukos, N.T. Neuronal cell death in the ischemic spinal cord: The effect of methylprednisolone. Ann. Thorac. Surg. 1997, 64, 1279–1286. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 2012, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Eguchi, S.; Alam, A.; Ma, D. The role of nuclear factor-erythroid 2 related factor 2 (nrf-2) in the protection against lung injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 312, L155–L162. [Google Scholar] [CrossRef]
- Li, D.; Tian, H.; Li, X.; Mao, L.; Zhao, X.; Lin, J.; Lin, S.; Xu, C.; Liu, Y.; Guo, Y. Zinc promotes functional recovery after spinal cord injury by activating nrf2/ho-1 defense pathway and inhibiting inflammation of nlrp3 in nerve cells. Life Sci. 2020, 245, 117351. [Google Scholar] [CrossRef]
- Wei, W.; Shurui, C.; Zipeng, Z.; Hongliang, D.; Hongyu, W.; Yuanlong, L.; Kang, Z.; Zhaoliang, S.; Yue, G.; Chang, L. Aspirin suppresses neuronal apoptosis, reduces tissue inflammation, and restrains astrocyte activation by activating the nrf2/ho-1 signaling pathway. Neuroreport 2018, 29, 524–531. [Google Scholar] [CrossRef]
- Zhu, P.; Li, J.-x.; Fujino, M.; Zhuang, J.; Li, X.-K. Development and treatments of inflammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediat. Inflamm. 2013, 2013, 701970. [Google Scholar] [CrossRef] [PubMed]
- Vila, N.; Castillo, J.; Dávalos, A.; Chamorro, A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 2000, 31, 2325–2329. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Matsuura, N.; Shozuhara, H.; Onodera, H.; Itoyama, Y.; Kogure, K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke 1995, 26, 676–681. [Google Scholar] [CrossRef]
- Satoh, T.; Otsuka, A.; Contassot, E.; French, L.E. The inflammasome and il-1β: Implications for the treatment of inflammatory diseases. Immunotherapy 2015, 7, 243–254. [Google Scholar] [CrossRef]
- Boutin, H.; LeFeuvre, R.A.; Horai, R.; Asano, M.; Iwakura, Y.; Rothwell, N.J. Role of il-1alpha and il-1beta in ischemic brain damage. J. Neurosci. 2001, 21, 5528–5534. [Google Scholar] [CrossRef]
- Huang, L.-Q.; Zhu, G.-F.; Deng, Y.-Y.; Jiang, W.-Q.; Fang, M.; Chen, C.-B.; Cao, W.; Wen, M.-Y.; Han, Y.-L.; Zeng, H.-K. Hypertonic saline alleviates cerebral edema by inhibiting microglia-derived tnf-α and il-1β-induced na-k-cl cotransporter up-regulation. J. Neuroinflamm. 2014, 11, 102. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2014, 7, a020420. [Google Scholar] [CrossRef]
- Shen, X.-Y.; Gao, Z.-K.; Han, Y.; Yuan, M.; Guo, Y.-S.; Bi, X. Activation and role of astrocytes in ischemic stroke. Front. Cell. Neurosci. 2021, 15, 755955. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lee, T.-K.; Kim, D.W.; Lim, S.S.; Kang, I.J.; Ahn, J.H.; Park, J.H.; Lee, J.-C.; Kim, C.-H.; Park, Y. Relationship between neuronal damage/death and astrogliosis in the cerebral motor cortex of gerbil models of mild and severe ischemia and reperfusion injury. Int. J. Mol. Sci. 2022, 23, 5096. [Google Scholar] [CrossRef]
- Radenovic, L.; Selakovic, V.; Olivan, S.; Calvo, A.C.; Rando, A.; Janac, B.; Osta, R. Neuroprotective efficiency of tetanus toxin c fragment in model of global cerebral ischemia in mongolian gerbils. Brain Res. Bull. 2014, 101, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ito, U.; Hakamata, Y.; Kawakami, E.; Oyanagi, K. Degeneration of astrocytic processes and their mitochondria in cerebral cortical regions peripheral to the cortical infarction: Heterogeneity of their disintegration is closely associated with disseminated selective neuronal necrosis and maturation of injury. Stroke 2009, 40, 2173–2181. [Google Scholar]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Rothhammer, V. Protective functions of reactive astrocytes following central nervous system insult. Front. Immunol. 2020, 11, 573256. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.R.; Pehar, M.; Cassina, P.; Martínez-Palma, L.; Thompson, J.A.; Beckman, J.S.; Barbeito, L. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factorerythroid 2-related factor 2 (nrf2) in spinal cord astrocytes: Consequencesfor motor neuronsurvival. J. Biol. Chem. 2005, 280, 25571–25579. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.H.; Lee, T.-K.; Kim, D.W.; Shin, M.C.; Cho, J.H.; Lee, J.-C.; Tae, H.-J.; Park, J.H.; Hong, S.; Lee, C.-H.; et al. Therapeutic Hypothermia after Cardiac Arrest Attenuates Hindlimb Paralysis and Damage of Spinal Motor Neurons and Astrocytes through Modulating Nrf2/HO-1 Signaling Pathway in Rats. Cells 2023, 12, 414. https://doi.org/10.3390/cells12030414
Ahn JH, Lee T-K, Kim DW, Shin MC, Cho JH, Lee J-C, Tae H-J, Park JH, Hong S, Lee C-H, et al. Therapeutic Hypothermia after Cardiac Arrest Attenuates Hindlimb Paralysis and Damage of Spinal Motor Neurons and Astrocytes through Modulating Nrf2/HO-1 Signaling Pathway in Rats. Cells. 2023; 12(3):414. https://doi.org/10.3390/cells12030414
Chicago/Turabian StyleAhn, Ji Hyeon, Tae-Kyeong Lee, Dae Won Kim, Myoung Cheol Shin, Jun Hwi Cho, Jae-Chul Lee, Hyun-Jin Tae, Joon Ha Park, Seongkweon Hong, Choong-Hyun Lee, and et al. 2023. "Therapeutic Hypothermia after Cardiac Arrest Attenuates Hindlimb Paralysis and Damage of Spinal Motor Neurons and Astrocytes through Modulating Nrf2/HO-1 Signaling Pathway in Rats" Cells 12, no. 3: 414. https://doi.org/10.3390/cells12030414
APA StyleAhn, J. H., Lee, T.-K., Kim, D. W., Shin, M. C., Cho, J. H., Lee, J.-C., Tae, H.-J., Park, J. H., Hong, S., Lee, C.-H., Won, M.-H., & Kim, Y. H. (2023). Therapeutic Hypothermia after Cardiac Arrest Attenuates Hindlimb Paralysis and Damage of Spinal Motor Neurons and Astrocytes through Modulating Nrf2/HO-1 Signaling Pathway in Rats. Cells, 12(3), 414. https://doi.org/10.3390/cells12030414








