Effects of Oxysterols on Immune Cells and Related Diseases
Abstract
1. Introduction
2. Oxysterols and the Immune System
3. Oxysterol Receptors: LXR and EBI2
3.1. LXR
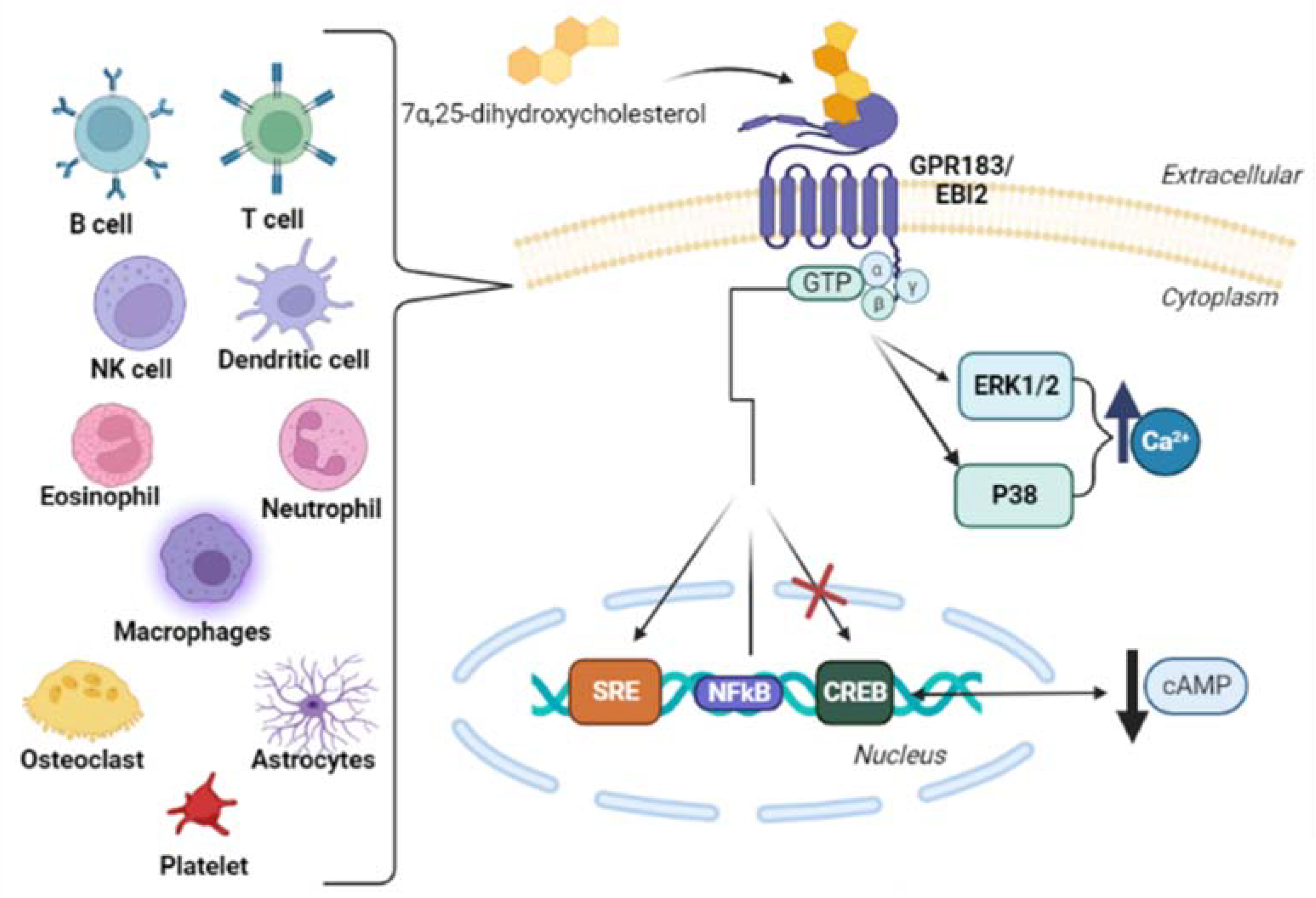
3.2. EBI2 (GPR183)
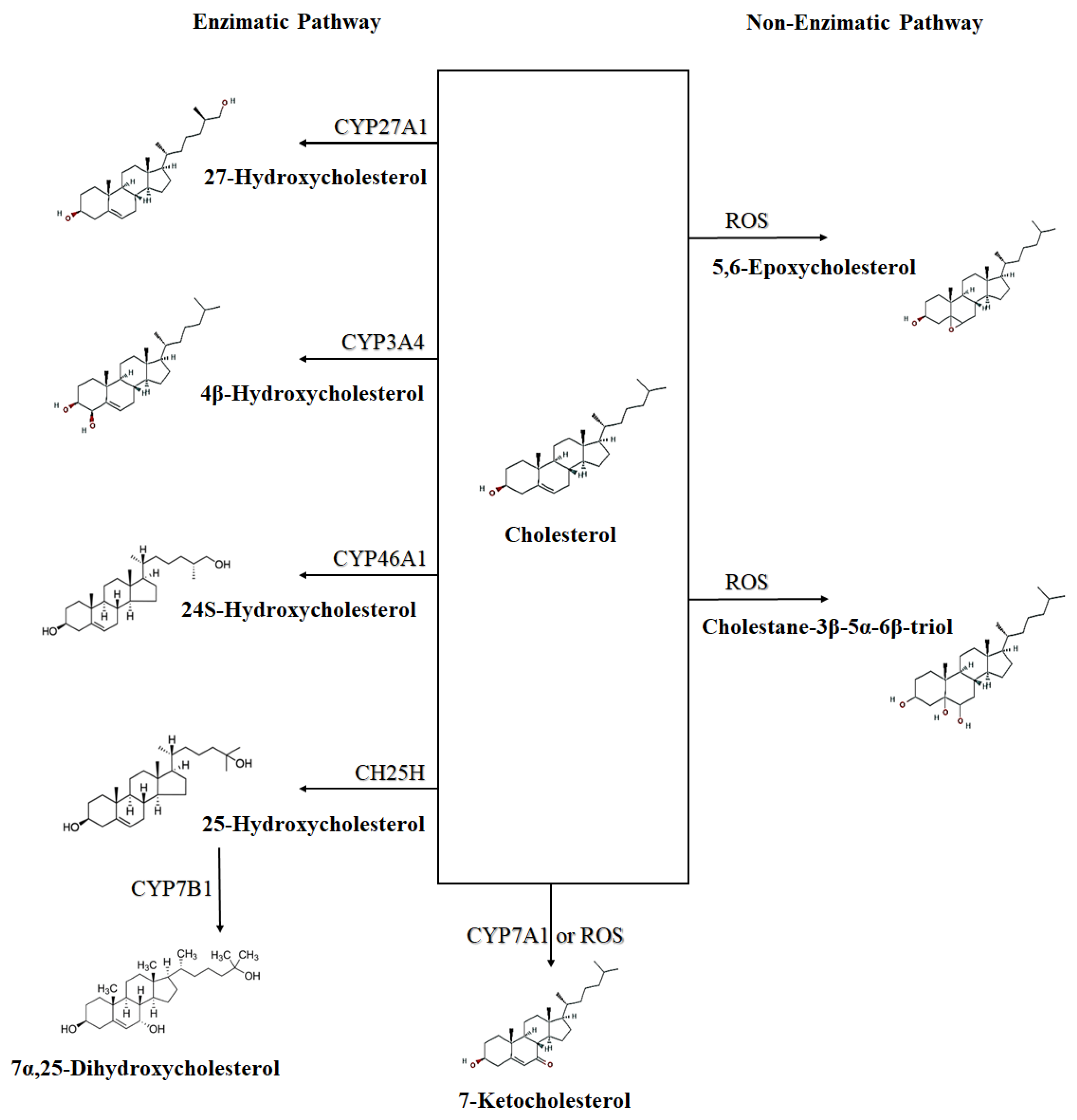
4. Main Oxysterols That Are Importance to the Immune System
4.1. 25-Hydroxycholesterol
4.2. 7α,25-Dihydroxycholesterol
5. Oxysterols and Immune System Cells
5.1. B Cells
5.2. T Cells
5.3. Macrophages
5.4. Dendritic Cells
5.5. Oligodendrocytes
5.6. Astrocytes
6. Oxysterols and Immune System-Related Diseases
6.1. Neurodegenerative Diseases
6.1.1. Multiple Sclerosis
6.1.2. Alzheimer’s Disease
6.2. Intestinal Diseases
6.3. Rheumatoid Arthritis
6.4. Cancer
Chronic Lymphocytic Leukemia
6.5. Atherosclerosis
6.6. Respiratory Diseases
6.6.1. Asthma
6.6.2. Tuberculosis
6.6.3. Chronic Obstructive Pulmonary Disease (COPD)
6.6.4. Acute Lung Injury (ALI)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. Srebps: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Sterols, oxysterols and accessible cholesterol: Signalling for homeostasis, in immunity and during development. Front. Physiol. 2021, 12, 723224. [Google Scholar] [CrossRef] [PubMed]
- Bah, S.Y.; Dickinson, P.; Forster, T.; Kampmann, B.; Ghazal, P. Immune oxysterols: Role in mycobacterial infection and inflammation. J. Steroid Biochem. Mol. Biol. 2017, 169, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Shahoei, S.H.; Nelson, E.R. Nuclear receptors, cholesterol homeostasis and the immune system. J. Steroid Biochem. Mol. Biol. 2019, 191, 105364. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Wang, Y. An update on oxysterol biochemistry: New discoveries in lipidomics. Biochem. Biophys. Res. Commun. 2018, 504, 617–622. [Google Scholar] [CrossRef] [PubMed]
- York, A.G.; Bensinger, S.J. Subverting sterols: Rerouting an oxysterol-signaling pathway to promote tumor growth. J. Exp. Med. 2013, 210, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.L.; Fernandes, L.R.; Levy, D.; Bydlowski, S.P. Interrelationship between atp-binding cassette transporters and oxysterols. Biochem. Pharmacol. 2013, 86, 80–88. [Google Scholar] [CrossRef]
- Rosa-Fernandes, L.; Maselli, L.M.F.; Maeda, N.Y.; Palmisano, G.; Bydlowski, S.P. Outside-in, inside-out: Proteomic analysis of endothelial stress mediated by 7-ketocholesterol. Chem. Phys. Lipids 2017, 207, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, W.J.; Abdel-Khalik, J.; Hearn, T.; Yutuc, E.; Morgan, A.H.; Wang, Y. Current trends in oxysterol research. Biochem. Soc. Trans. 2016, 44, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Reinmuth, L.; Hsiao, C.C.; Hamann, J.; Rosenkilde, M.; Mackrill, J. Multiple targets for oxysterols in their regulation of the immune system. Cells 2021, 10, 2078. [Google Scholar] [CrossRef] [PubMed]
- Duc, D.; Vigne, S.; Pot, C. Oxysterols in autoimmunity. Int. J. Mol. Sci. 2019, 20, 4522. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Scott, A.L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type i interferons. J. Leukoc. Biol. 2010, 88, 1081–1087. [Google Scholar] [CrossRef]
- Levy, D.; Ruiz, J.L.; Celestino, A.T.; Silva, S.F.; Ferreira, A.K.; Isaac, C.; Bydlowski, S.P. Short-term effects of 7-ketocholesterol on human adipose tissue mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun. 2014, 446, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Rosa Fernandes, L.; Stern, A.C.; Cavaglieri, R.C.; Nogueira, F.C.; Domont, G.; Palmisano, G.; Bydlowski, S.P. 7-ketocholesterol overcomes drug resistance in chronic myeloid leukemia cell lines beyond mdr1 mechanism. J. Proteom. 2017, 151, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; de Melo, T.C.; Ohira, B.Y.; Fidelis, M.L.; Ruiz, J.L.M.; Rodrigues, A.; Bydlowski, S.P. Oxysterols selectively promote short-term apoptosis in tumor cell lines. Biochem. Biophys. Res. Commun. 2018, 505, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.M.; Paz, J.L.; Otake, A.H.; Maria, D.A.; Caldini, E.G.; de Medeiros, R.S.S.; Deus, D.F.; Chammas, R.; Maranhão, R.C.; Bydlowski, S.P. Cell internalization of 7-ketocholesterol-containing nanoemulsion through ldl receptor reduces melanoma growth in vitro and in vivo: A preliminary report. Oncotarget 2018, 9, 14160–14174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silva, S.F.; Levy, D.; Ruiz, J.L.M.; de Melo, T.C.; Isaac, C.; Fidelis, M.L.; Rodrigues, A.; Bydlowski, S.P. Oxysterols in adipose tissue-derived mesenchymal stem cell proliferation and death. J. Steroid Biochem. Mol. Biol. 2017, 169, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; de Melo, T.C.; Oliveira, B.A.; Paz, J.L.; de Freitas, F.A.; Reichert, C.O.; Rodrigues, A.; Bydlowski, S.P. 7-ketocholesterol and cholestane-triol increase expression of smo and lxrα signaling pathways in a human breast cancer cell line. Biochem. Biophys. Rep. 2019, 19, 100604. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, F.A.; Levy, D.; Zarrouk, A.; Lizard, G.; Bydlowski, S.P. Impact of oxysterols on cell death, proliferation, and differentiation induction: Current status. Cells 2021, 10, 2301. [Google Scholar] [CrossRef] [PubMed]
- Lemaire-Ewing, S.; Prunet, C.; Montange, T.; Vejux, A.; Berthier, A.; Bessède, G.; Corcos, L.; Gambert, P.; Néel, D.; Lizard, G. Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol. Toxicol. 2005, 21, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Prunet, C.; Montange, T.; Véjux, A.; Laubriet, A.; Rohmer, J.F.; Riedinger, J.M.; Athias, A.; Lemaire-Ewing, S.; Néel, D.; Petit, J.M.; et al. Multiplexed flow cytometric analyses of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2006, 69, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, E.K.; Salomonsson, L.; Hultén, L.M.; Norén, K.; Bondjers, G.; Wiklund, O.; Björnheden, T.; Ohlsson, B.G. Hypoxia increases 25-hydroxycholesterol-induced interleukin-8 protein secretion in human macrophages. Atherosclerosis 2003, 170, 245–252. [Google Scholar] [CrossRef]
- Zarrouk, A.; Vejux, A.; Mackrill, J.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.; Lizard, G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 2014, 18, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.H.K.; Milic, I.; Heiss, C.; Ademowo, O.S.; Polidori, M.C.; Devitt, A.; Griffiths, H.R. Inflammation, lipid (per)oxidation, and redox regulation. Antioxid. Redox Signal. 2020, 33, 166–190. [Google Scholar] [CrossRef] [PubMed]
- Galea, E.; Launay, N.; Portero-Otin, M.; Ruiz, M.; Pamplona, R.; Aubourg, P.; Ferrer, I.; Pujol, A. Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: A paradigm for multifactorial neurodegenerative diseases? Biochim. Biophys. Acta 2012, 1822, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Ragot, K.; Delmas, D.; Athias, A.; Nury, T.; Baarine, M.; Lizard, G. A-tocopherol impairs 7-ketocholesterol-induced caspase-3-dependent apoptosis involving gsk-3 activation and mcl-1 degradation on 158n murine oligodendrocytes. Chem. Phys. Lipids 2011, 164, 469–478. [Google Scholar] [CrossRef]
- Ragot, K.; Mackrill, J.J.; Zarrouk, A.; Nury, T.; Aires, V.; Jacquin, A.; Athias, A.; Pais de Barros, J.P.; Véjux, A.; Riedinger, J.M.; et al. Absence of correlation between oxysterol accumulation in lipid raft microdomains, calcium increase, and apoptosis induction on 158n murine oligodendrocytes. Biochem. Pharmacol. 2013, 86, 67–79. [Google Scholar] [CrossRef]
- Hubler, M.J.; Kennedy, A.J. Role of lipids in the metabolism and activation of immune cells. J. Nutr. Biochem. 2016, 34, 1–7. [Google Scholar] [CrossRef]
- Lathe, R.; Sapronova, A.; Kotelevtsev, Y. Atherosclerosis and alzheimer—Diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr. 2014, 14, 36. [Google Scholar] [CrossRef]
- Cyster, J.G.; Dang, E.V.; Reboldi, A.; Yi, T. 25-hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol. 2014, 14, 731–743. [Google Scholar] [CrossRef]
- Varaksa, T.; Bukhdruker, S.; Grabovec, I.; Marin, E.; Kavaleuski, A.; Gusach, A.; Kovalev, K.; Maslov, I.; Luginina, A.; Zabelskii, D.; et al. Metabolic fate of human immunoactive sterols in mycobacterium tuberculosis. J. Mol. Biol. 2021, 433, 166763. [Google Scholar] [CrossRef] [PubMed]
- Traversari, C.; Russo, V. Control of the immune system by oxysterols and cancer development. Curr. Opin. Pharmacol. 2012, 12, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Traversari, C.; Sozzani, S.; Steffensen, K.R.; Russo, V. Lxr-dependent and -independent effects of oxysterols on immunity and tumor growth. Eur. J. Immunol. 2014, 44, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcer, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D.; et al. Lxr signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 2008, 134, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Li, D.; Zhao, L.; Li, Y.; Zhang, T.; Cui, B. Expression of nr1h3 in endometrial carcinoma and its effect on the proliferation of ishikawa cells in vitro. OncoTargets Ther. 2019, 12, 685–697. [Google Scholar] [CrossRef]
- Yang, X.; Lamia, K.A.; Evans, R.M. Nuclear receptors, metabolism, and the circadian clock. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 387–394. [Google Scholar] [CrossRef]
- Jarvis, S.; Williamson, C.; Bevan, C.L. Liver x receptors and male (in)fertility. Int. J. Mol. Sci. 2019, 20, 5379. [Google Scholar] [CrossRef]
- Glass, C.K.; Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000, 14, 121–141. [Google Scholar] [CrossRef]
- Villablanca, E.J.; Raccosta, L.; Zhou, D.; Fontana, R.; Maggioni, D.; Negro, A.; Sanvito, F.; Ponzoni, M.; Valentinis, B.; Bregni, M.; et al. Tumor-mediated liver x receptor-alpha activation inhibits cc chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat. Med. 2010, 16, 98–105. [Google Scholar] [CrossRef]
- Abildayeva, K.; Jansen, P.J.; Hirsch-Reinshagen, V.; Bloks, V.W.; Bakker, A.H.; Ramaekers, F.C.; de Vente, J.; Groen, A.K.; Wellington, C.L.; Kuipers, F.; et al. 24(s)-hydroxycholesterol participates in a liver x receptor-controlled pathway in astrocytes that regulates apolipoprotein e-mediated cholesterol efflux. J. Biol. Chem. 2006, 281, 12799–12808. [Google Scholar] [CrossRef]
- Mukhutdinova, K.A.; Kasimov, M.R.; Zakyrjanova, G.F.; Gumerova, M.R.; Petrov, A.M. Oxysterol modulates neurotransmission via liver-x receptor/no synthase-dependent pathway at the mouse neuromuscular junctions. Neuropharmacology 2019, 150, 70–79. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yuhanna, I.S.; Umetani, J.; Lee, W.R.; Korach, K.S.; Shaul, P.W.; Umetani, M. Lxrβ/estrogen receptor-α signaling in lipid rafts preserves endothelial integrity. J. Clin. Investig. 2013, 123, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, A.J.; Flora, G.D.; Gibbins, J.M. Non-genomic effects of nuclear receptors: Insights from the anucleate platelet. Cardiovasc. Res. 2018, 114, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Zakyrjanova, G.F.; Tsentsevitsky, A.N.; Kuznetsova, E.A.; Petrov, A.M. Immune-related oxysterol modulates neuromuscular transmission via non-genomic liver x receptor-dependent mechanism. Free Radic. Biol. Med. 2021, 174, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.P.; Kelly, L.M.; Xu, Y.; Cyster, J.G. Ebi2 mediates b cell segregation between the outer and centre follicle. Nature 2009, 460, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Barington, L.; Wanke, F.; Niss Arfelt, K.; Holst, P.J.; Kurschus, F.C.; Rosenkilde, M.M. Ebi2 in splenic and local immune responses and in autoimmunity. J. Leukoc. Biol. 2018, 104, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Benned-Jensen, T.; Smethurst, C.; Holst, P.J.; Page, K.R.; Sauls, H.; Sivertsen, B.; Schwartz, T.W.; Blanchard, A.; Jepras, R.; Rosenkilde, M.M. Ligand modulation of the epstein-barr virus-induced seven-transmembrane receptor ebi2: Identification of a potent and efficacious inverse agonist. J. Biol. Chem. 2011, 286, 29292–29302. [Google Scholar] [CrossRef] [PubMed]
- Daugvilaite, V.; Arfelt, K.N.; Benned-Jensen, T.; Sailer, A.W.; Rosenkilde, M.M. Oxysterol-ebi2 signaling in immune regulation and viral infection. Eur. J. Immunol. 2014, 44, 1904–1912. [Google Scholar] [CrossRef]
- Birkenbach, M.; Josefsen, K.; Yalamanchili, R.; Lenoir, G.; Kieff, E. Epstein-barr virus-induced genes: First lymphocyte-specific g protein-coupled peptide receptors. J. Virol. 1993, 67, 2209–2220. [Google Scholar] [CrossRef]
- Hannedouche, S.; Zhang, J.; Yi, T.; Shen, W.; Nguyen, D.; Pereira, J.P.; Guerini, D.; Baumgarten, B.U.; Roggo, S.; Wen, B.; et al. Oxysterols direct immune cell migration via ebi2. Nature 2011, 475, 524–527. [Google Scholar] [CrossRef]
- Liu, C.; Yang, X.V.; Wu, J.; Kuei, C.; Mani, N.S.; Zhang, L.; Yu, J.; Sutton, S.W.; Qin, N.; Banie, H.; et al. Oxysterols direct b-cell migration through ebi2. Nature 2011, 475, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Rosenkilde, M.M.; Benned-Jensen, T.; Andersen, H.; Holst, P.J.; Kledal, T.N.; Lüttichau, H.R.; Larsen, J.K.; Christensen, J.P.; Schwartz, T.W. Molecular pharmacological phenotyping of ebi2. An orphan seven-transmembrane receptor with constitutive activity. J. Biol. Chem. 2006, 281, 13199–13208. [Google Scholar] [CrossRef] [PubMed]
- Heinig, M.; Petretto, E.; Wallace, C.; Bottolo, L.; Rotival, M.; Lu, H.; Li, Y.; Sarwar, R.; Langley, S.R.; Bauerfeind, A.; et al. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature 2010, 467, 460–464. [Google Scholar] [CrossRef]
- Nevius, E.; Pinho, F.; Dhodapkar, M.; Jin, H.; Nadrah, K.; Horowitz, M.C.; Kikuta, J.; Ishii, M.; Pereira, J.P. Oxysterols and ebi2 promote osteoclast precursor migration to bone surfaces and regulate bone mass homeostasis. J. Exp. Med. 2015, 212, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-J.; Hu, J.; Kashi, V.P.; Kelly, E.A.; Denlinger, L.C.; Lutchman, K.; McDonald, J.G.; Jarjour, N.N.; Malter, J.S. Epstein-barr virus-induced gene 2 mediates allergen-induced leukocyte migration into airways. Am. J. Respir. Crit. Care Med. 2017, 195, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Amisten, S.; Braun, O.O.; Bengtsson, A.; Erlinge, D. Gene expression profiling for the identification of g-protein coupled receptors in human platelets. Thromb. Res. 2008, 122, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, A.; Preuss, I.; Gessier, F.; Sailer, A.W.; Dev, K.K. Ebi2 regulates intracellular signaling and migration in human astrocyte. Glia 2015, 63, 341–351. [Google Scholar] [CrossRef]
- Zhang, P.; He, Q.; Chen, D.; Liu, W.; Wang, L.; Zhang, C.; Ma, D.; Li, W.; Liu, B.; Liu, F. G protein-coupled receptor 183 facilitates endothelial-to-hematopoietic transition via notch1 inhibition. Cell Res. 2015, 25, 1093–1107. [Google Scholar] [CrossRef]
- Ki, S.; Thyagarajan, H.M.; Hu, Z.; Lancaster, J.N.; Ehrlich, L.I.R. Ebi2 contributes to the induction of thymic central tolerance in mice by promoting rapid motility of medullary thymocytes. Eur. J. Immunol. 2017, 47, 1906–1917. [Google Scholar] [CrossRef]
- Benned-Jensen, T.; Madsen, C.M.; Arfelt, K.N.; Smethurts, C.; Blanchard, A.; Jepras, R.; Rosenkilde, M.M. Small molecule antagonism of oxysterol-induced epstein-barr virus induced gene 2 (ebi2) activation. FEBS Open Bio 2013, 3, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, C. 7α, 25-dihydroxycholesterol-mediated activation of ebi2 in immune regulation and diseases. Front. Pharmacol. 2015, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, S.; Gemiarto, A.T.; Ngo, M.D.; Sajiir, H.; Hailu, S.; Sinha, R.; Foo, C.X.; Kleynhans, L.; Tshivhula, H.; Webber, T.; et al. Gpr183 regulates interferons, autophagy, and bacterial growth during mycobacterium tuberculosis infection and is associated with tb disease severity. Front. Immunol. 2020, 11, 601534. [Google Scholar] [CrossRef] [PubMed]
- Gatto, D.; Brink, R. B cell localization: Regulation by ebi2 and its oxysterol ligand. Trends Immunol. 2013, 34, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Miyazaki, T.; Ikegami, T.; Iwamoto, J.; Maeda, T.; Hirayama, T.; Saito, Y.; Teramoto, T.; Matsuzaki, Y. Cholesterol 25-hydroxylation activity of cyp3a. J. Lipid Res. 2011, 52, 1509–1516. [Google Scholar] [CrossRef]
- Diczfalusy, U.; Olofsson, K.E.; Carlsson, A.M.; Gong, M.; Golenbock, D.T.; Rooyackers, O.; Fläring, U.; Björkbacka, H. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 2009, 50, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.; Gale, M., Jr. Sterol-izing innate immunity. Immunity 2013, 38, 3–5. [Google Scholar] [CrossRef][Green Version]
- Bauman, D.R.; Bitmansour, A.D.; McDonald, J.G.; Thompson, B.M.; Liang, G.; Russell, D.W. 25-hydroxycholesterol secreted by macrophages in response to toll-like receptor activation suppresses immunoglobulin a production. Proc. Natl. Acad. Sci. USA 2009, 106, 16764–16769. [Google Scholar] [CrossRef]
- Blanc, M.; Hsieh, W.Y.; Robertson, K.A.; Kropp, K.A.; Forster, T.; Shui, G.; Lacaze, P.; Watterson, S.; Griffiths, S.J.; Spann, N.J.; et al. The transcription factor stat-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 2013, 38, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Ngo, M.D.; Bartlett, S.; Bielefeldt-Ohmann, H.; Foo, C.X.; Sinha, R.; Arachige, B.J.; Reed, S.; Mandrup-Poulsen, T.; Rosenkilde, M.M.; Ronacher, K. A blunted gpr183/oxysterol axis during dysglycemia results in delayed recruitment of macrophages to the lung during m. Tuberculosis infection. J. Infect. Dis. 2022, jiac102. [Google Scholar] [CrossRef]
- Reboldi, A.; Dang, E.V.; McDonald, J.G.; Liang, G.; Russell, D.W.; Cyster, J.G. 25-hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type i interferon. Science 2014, 345, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Bottemanne, P.; Paquot, A.; Ameraoui, H.; Guillemot-Legris, O.; Alhouayek, M.; Muccioli, G.G. 25-hydroxycholesterol metabolism is altered by lung inflammation, and its local administration modulates lung inflammation in mice. FASEB J. 2021, 35, e21514. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Wang, X.; Kelly, L.M.; An, J.; Xu, Y.; Sailer, A.W.; Gustafsson, J.A.; Russell, D.W.; Cyster, J.G. Oxysterol gradient generation by lymphoid stromal cells guides activated b cell movement during humoral responses. Immunity 2012, 37, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Wanke, F.; Moos, S.; Croxford, A.L.; Heinen, A.P.; Gräf, S.; Kalt, B.; Tischner, D.; Zhang, J.; Christen, I.; Bruttger, J.; et al. Ebi2 is highly expressed in multiple sclerosis lesions and promotes early cns migration of encephalitogenic cd4t cells. Cell Rep. 2017, 18, 1270–1284. [Google Scholar] [CrossRef]
- Raccosta, L.; Fontana, R.; Maggioni, D.; Lanterna, C.; Villablanca, E.J.; Paniccia, A.; Musumeci, A.; Chiricozzi, E.; Trincavelli, M.L.; Daniele, S.; et al. The oxysterol-cxcr2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J. Exp. Med. 2013, 210, 1711–1728. [Google Scholar] [CrossRef]
- Sensi, C.; Daniele, S.; Parravicini, C.; Zappelli, E.; Russo, V.; Trincavelli, M.L.; Martini, C.; Abbracchio, M.P.; Eberini, I. Oxysterols act as promiscuous ligands of class-a gpcrs: In silico molecular modeling and in vitro validation. Cell. Signal. 2014, 26, 2614–2620. [Google Scholar] [CrossRef]
- Nachtergaele, S.; Mydock, L.K.; Krishnan, K.; Rammohan, J.; Schlesinger, P.H.; Covey, D.F.; Rohatgi, R. Oxysterols are allosteric activators of the oncoprotein smoothened. Nat. Chem. Biol. 2012, 8, 211–220. [Google Scholar] [CrossRef]
- Myers, B.R.; Sever, N.; Chong, Y.C.; Kim, J.; Belani, J.D.; Rychnovsky, S.; Bazan, J.F.; Beachy, P.A. Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev. Cell 2013, 26, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Spann, N.J.; Glass, C.K. Sterols and oxysterols in immune cell function. Nat. Immunol. 2013, 14, 893–900. [Google Scholar] [CrossRef]
- Gatto, D.; Paus, D.; Basten, A.; Mackay, C.R.; Brink, R. Guidance of b cells by the orphan g protein-coupled receptor ebi2 shapes humoral immune responses. Immunity 2009, 31, 259–269. [Google Scholar] [CrossRef]
- Chalmin, F.; Rochemont, V.; Lippens, C.; Clottu, A.; Sailer, A.W.; Merkler, D.; Hugues, S.; Pot, C. Oxysterols regulate encephalitogenic cd4(+) t cell trafficking during central nervous system autoimmunity. J. Autoimmun. 2015, 56, 45–55. [Google Scholar] [CrossRef]
- Soroosh, P.; Wu, J.; Xue, X.; Song, J.; Sutton, S.W.; Sablad, M.; Yu, J.; Nelen, M.I.; Liu, X.; Castro, G.; et al. Oxysterols are agonist ligands of rorγt and drive th17 cell differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 12163–12168. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Qin, X.; Wu, L.; Zhang, Y.; Sheng, X.; Yu, Q.; Sheng, H.; Xi, B.; Zhang, J.Z.; Zang, Y.Q. Liver x receptor (lxr) mediates negative regulation of mouse and human th17 differentiation. J. Clin. Investig. 2011, 121, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Olkkonen, V.M. Macrophage oxysterols and their binding proteins: Roles in atherosclerosis. Curr. Opin. Lipidol. 2012, 23, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Glass, C.K. Macrophages, oxysterols and atherosclerosis. Circ. J. 2010, 74, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Gordon, S. Macrophages and the nervous system. Int. Rev. Cytol. 1991, 125, 203–244. [Google Scholar]
- Kimura, T.; Nada, S.; Takegahara, N.; Okuno, T.; Nojima, S.; Kang, S.; Ito, D.; Morimoto, K.; Hosokawa, T.; Hayama, Y.; et al. Polarization of m2 macrophages requires lamtor1 that integrates cytokine and amino-acid signals. Nat. Commun. 2016, 7, 13130. [Google Scholar] [CrossRef]
- Li, Z.; Martin, M.; Zhang, J.; Huang, H.Y.; Bai, L.; Zhang, J.; Kang, J.; He, M.; Li, J.; Maurya, M.R.; et al. Krüppel-like factor 4 regulation of cholesterol-25-hydroxylase and liver x receptor mitigates atherosclerosis susceptibility. Circulation 2017, 136, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Bellora, F.; Ricciarelli, R.; De Ciucis, C.; Furfaro, A.; Leardi, R.; Colla, R.; Pacini, D.; Traverso, N.; Moretta, A.; et al. Oxysterol mixture and, in particular, 27-hydroxycholesterol drive m2 polarization of human macrophages. BioFactors 2016, 42, 80–92. [Google Scholar]
- Dennis, E.A.; Deems, R.A.; Harkewicz, R.; Quehenberger, O.; Brown, H.A.; Milne, S.B.; Myers, D.S.; Glass, C.K.; Hardiman, G.; Reichart, D.; et al. A mouse macrophage lipidome. J. Biol. Chem. 2010, 285, 39976–39985. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor lxr alpha. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Ghisletti, S.; Huang, W.; Ogawa, S.; Pascual, G.; Lin, M.E.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. Parallel sumoylation-dependent pathways mediate gene- and signal-specific transrepression by lxrs and ppargamma. Mol. Cell 2007, 25, 57–70. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.G.; Thompson, B.M.; McCrum, E.C.; Russell, D.W. Extraction and analysis of sterols in biological matrices by high performance liquid chromatography electrospray ionization mass spectrometry. Methods Enzymol. 2007, 432, 145–170. [Google Scholar]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. Srebp activity is regulated by mtorc1 and contributes to akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mtor complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, A.; Joseph, S.B.; Vaidya, S.A.; Haberland, M.; Fogelman, A.M.; Cheng, G.; Tontonoz, P. Crosstalk between lxr and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell 2003, 12, 805–816. [Google Scholar] [CrossRef]
- Joseph, S.B.; Bradley, M.N.; Castrillo, A.; Bruhn, K.W.; Mak, P.A.; Pei, L.; Hogenesch, J.; O’Connell R, M.; Cheng, G.; Saez, E.; et al. Lxr-dependent gene expression is important for macrophage survival and the innate immune response. Cell 2004, 119, 299–309. [Google Scholar] [CrossRef]
- Dang, E.V.; McDonald, J.G.; Russell, D.W.; Cyster, J.G. Oxysterol restraint of cholesterol synthesis prevents aim2 inflammasome activation. Cell 2017, 171, 1057–1071.e1011. [Google Scholar] [CrossRef]
- Geyeregger, R.; Zeyda, M.; Bauer, W.; Kriehuber, E.; Säemann, M.D.; Zlabinger, G.J.; Maurer, D.; Stulnig, T.M. Liver x receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood 2007, 109, 4288–4295. [Google Scholar] [CrossRef]
- Trousson, A.; Bernard, S.; Petit, P.X.; Liere, P.; Pianos, A.; El Hadri, K.; Lobaccaro, J.M.; Ghandour, M.S.; Raymondjean, M.; Schumacher, M.; et al. 25-hydroxycholesterol provokes oligodendrocyte cell line apoptosis and stimulates the secreted phospholipase a2 type iia via lxr beta and pxr. J. Neurochem. 2009, 109, 945–958. [Google Scholar] [CrossRef]
- Rutkowska, A.; Dev, K.K.; Sailer, A.W. The role of the oxysterol/ebi2 pathway in the immune and central nervous systems. Curr. Drug Targets 2016, 17, 1851–1860. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Dijkstra, C.D.; Polman, C.H.; Hoogervorst, E.L.; von Bergmann, K.; Lütjohann, D. Decreased levels of the brain specific 24s-hydroxycholesterol and cholesterol precursors in serum of multiple sclerosis patients. Neurosci. Lett. 2003, 347, 159–162. [Google Scholar] [CrossRef]
- Odnoshivkina, U.G.; Sytchev, V.I.; Starostin, O.; Petrov, A.M. Brain cholesterol metabolite 24-hydroxycholesterol modulates inotropic responses to β-adrenoceptor stimulation: The role of no and phosphodiesterase. Life Sci. 2019, 220, 117–126. [Google Scholar] [CrossRef]
- Linsenbardt, A.J.; Taylor, A.; Emnett, C.M.; Doherty, J.J.; Krishnan, K.; Covey, D.F.; Paul, S.M.; Zorumski, C.F.; Mennerick, S. Different oxysterols have opposing actions at n-methyl-d-aspartate receptors. Neuropharmacology 2014, 85, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.C.; Yu, J.; Sardi, S.P.; Shihabuddin, L.S. Sterol auto-oxidation adversely affects human motor neuron viability and is a neuropathological feature of amyotrophic lateral sclerosis. Sci. Rep. 2021, 11, 803. [Google Scholar] [CrossRef] [PubMed]
- Zakyrjanova, G.F.; Giniatullin, A.R.; Mukhutdinova, K.A.; Kuznetsova, E.A.; Petrov, A.M. Early differences in membrane properties at the neuromuscular junctions of als model mice: Effects of 25-hydroxycholesterol. Life Sci. 2021, 273, 119300. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Noh, M.Y.; Kim, H.; Cheon, S.Y.; Lee, K.M.; Lee, J.; Cha, E.; Park, K.S.; Lee, K.W.; Sung, J.J.; et al. 25-hydroxycholesterol is involved in the pathogenesis of amyotrophic lateral sclerosis. Oncotarget 2017, 8, 11855–11867. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Namsi, A.; Nury, T.; Moreau, T.; Lizard, G. Biomarkers of amyotrophic lateral sclerosis: Current status and interest of oxysterols and phytosterols. Front. Mol. Neurosci. 2018, 11, 12. [Google Scholar] [CrossRef]
- Abdel-Khalik, J.; Yutuc, E.; Crick, P.J.; Gustafsson, J.; Warner, M.; Roman, G.; Talbot, K.; Gray, E.; Griffiths, W.J.; Turner, M.R.; et al. Defective cholesterol metabolism in amyotrophic lateral sclerosis. J. Lipid Res. 2017, 58, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Mukhutdinova, K.A.; Kasimov, M.R.; Giniatullin, A.R.; Zakyrjanova, G.F.; Petrov, A.M. 24s-hydroxycholesterol suppresses neuromuscular transmission in sod1(g93a) mice: A possible role of no and lipid rafts. Mol. Cell. Neurosci. 2018, 88, 308–318. [Google Scholar] [CrossRef]
- Mouzat, K.; Molinari, N.; Kantar, J.; Polge, A.; Corcia, P.; Couratier, P.; Clavelou, P.; Juntas-Morales, R.; Pageot, N.; Lobaccaro, J.A.; et al. Liver x receptor genes variants modulate als phenotype. Mol. Neurobiol. 2018, 55, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.M.; Pikuleva, I.A. Cholesterol 24-hydroxylation by cyp46a1: Benefits of modulation for brain diseases. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2019, 16, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.W.; Halford, R.W.; Ramirez, D.M.; Shah, R.; Kotti, T. Cholesterol 24-hydroxylase: An enzyme of cholesterol turnover in the brain. Annu. Rev. Biochem. 2009, 78, 1017–1040. [Google Scholar] [CrossRef] [PubMed]
- Sodero, A.O. 24s-hydroxycholesterol: Cellular effects and variations in brain diseases. J. Neurochem. 2021, 157, 899–918. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. Multiple sclerosis: A coordinated immunological attack against myelin in the central nervous system. Cell 1996, 85, 299–302. [Google Scholar] [CrossRef]
- Fellows Maxwell, K.; Bhattacharya, S.; Bodziak, M.L.; Jakimovski, D.; Hagemeier, J.; Browne, R.W.; Weinstock-Guttman, B.; Zivadinov, R.; Ramanathan, M. Oxysterols and apolipoproteins in multiple sclerosis: A 5 year follow-up study. J. Lipid Res. 2019, 60, 1190–1198. [Google Scholar] [CrossRef]
- Moutinho, M.; Nunes, M.J.; Rodrigues, E. Cholesterol 24-hydroxylase: Brain cholesterol metabolism and beyond. Biochim. Biophys. Acta 2016, 1861, 1911–1920. [Google Scholar] [CrossRef]
- Diestel, A.; Aktas, O.; Hackel, D.; Hake, I.; Meier, S.; Raine, C.S.; Nitsch, R.; Zipp, F.; Ullrich, O. Activation of microglial poly(adp-ribose)-polymerase-1 by cholesterol breakdown products during neuroinflammation: A link between demyelination and neuronal damage. J. Exp. Med. 2003, 198, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Crick, P.J.; Griffiths, W.J.; Zhang, J.; Beibel, M.; Abdel-Khalik, J.; Kuhle, J.; Sailer, A.W.; Wang, Y. Reduced plasma levels of 25-hydroxycholesterol and increased cerebrospinal fluid levels of bile acid precursors in multiple sclerosis patients. Mol. Neurobiol. 2017, 54, 8009–8020. [Google Scholar] [CrossRef]
- Forwell, A.L.; Bernales, C.Q.; Ross, J.P.; Yee, I.M.; Encarnacion, M.; Lee, J.D.; Sadovnick, A.D.; Traboulsee, A.L.; Vilariño-Güell, C. Analysis of ch25h in multiple sclerosis and neuromyelitis optica. J. Neuroimmunol. 2016, 291, 70–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Jia, H.; Liao, M.; Chen, X.; Xu, J.; Bao, Y.; Liu, G. Genetic variants regulate nr1h3 expression and contribute to multiple sclerosis risk. J. Neurol. Sci. 2018, 390, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of epstein-barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, K.M.; Lee, C.; Jo, Y.S.; Muradillaevna, M.S.; Kim, J.H.; Yoon, J.H.; Song, P. Pathophysiological role of 27-hydroxycholesterol in human diseases. Adv. Biol. Regul. 2021, 83, 100837. [Google Scholar] [CrossRef] [PubMed]
- Willinger, T. Metabolic control of innate lymphoid cell migration. Front. Immunol. 2019, 10, 2010. [Google Scholar] [CrossRef]
- Emgård, J.; Kammoun, H.; García-Cassani, B.; Chesné, J.; Parigi, S.M.; Jacob, J.M.; Cheng, H.W.; Evren, E.; Das, S.; Czarnewski, P.; et al. Oxysterol sensing through the receptor gpr183 promotes the lymphoid-tissue-inducing function of innate lymphoid cells and colonic inflammation. Immunity 2018, 48, 120–132.e128. [Google Scholar] [CrossRef] [PubMed]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Trindade, B.C.; Ceglia, S.; Berthelette, A.; Raso, F.; Howley, K.; Muppidi, J.R.; Reboldi, A. The cholesterol metabolite 25-hydroxycholesterol restrains the transcriptional regulator srebp2 and limits intestinal iga plasma cell differentiation. Immunity 2021, 54, 2273–2287.e2276. [Google Scholar] [CrossRef]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef]
- Vejux, A.; Malvitte, L.; Lizard, G. Side effects of oxysterols: Cytotoxicity, oxidation, inflammation, and phospholipidosis. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. E Biol. 2008, 41, 545–556. [Google Scholar] [CrossRef]
- Plat, J.; Nichols, J.A.; Mensink, R.P. Plant sterols and stanols: Effects on mixed micellar composition and lxr (target gene) activation. J. Lipid Res. 2005, 46, 2468–2476. [Google Scholar] [CrossRef]
- Mascia, C.; Maina, M.; Chiarpotto, E.; Leonarduzzi, G.; Poli, G.; Biasi, F. Proinflammatory effect of cholesterol and its oxidation products on caco-2 human enterocyte-like cells: Effective protection by epigallocatechin-3-gallate. Free Radic. Biol. Med. 2010, 49, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Mascia, C.; Poli, G. The contribution of animal fat oxidation products to colon carcinogenesis, through modulation of tgf-beta1 signaling. Carcinogenesis 2008, 29, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Rossin, D.; Calfapietra, S.; Sottero, B.; Poli, G.; Biasi, F. Hne and cholesterol oxidation products in colorectal inflammation and carcinogenesis. Free Radic. Biol. Med. 2017, 111, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Mascia, C.; Astegiano, M.; Chiarpotto, E.; Nano, M.; Vizio, B.; Leonarduzzi, G.; Poli, G. Pro-oxidant and proapoptotic effects of cholesterol oxidation products on human colonic epithelial cells: A potential mechanism of inflammatory bowel disease progression. Free Radic. Biol. Med. 2009, 47, 1731–1741. [Google Scholar] [CrossRef]
- Chalubinski, M.; Zemanek, K.; Skowron, W.; Wojdan, K.; Gorzelak, P.; Broncel, M. The effect of 7-ketocholesterol and 25-hydroxycholesterol on the integrity of the human aortic endothelial and intestinal epithelial barriers. Inflamm. Res. 2013, 62, 1015–1023. [Google Scholar] [CrossRef]
- Raselli, T.; Wyss, A.; Gonzalez Alvarado, M.N.; Weder, B.; Mamie, C.; Spalinger, M.R.; Van Haaften, W.T.; Dijkstra, G.; Sailer, A.W.; Imenez Silva, P.H.; et al. The oxysterol synthesising enzyme ch25h contributes to the development of intestinal fibrosis. J. Crohn’s Colitis 2019, 13, 1186–1200. [Google Scholar] [CrossRef]
- Wyss, A.; Raselli, T.; Perkins, N.; Ruiz, F.; Schmelczer, G.; Klinke, G.; Moncsek, A.; Roth, R.; Spalinger, M.R.; Hering, L.; et al. The ebi2-oxysterol axis promotes the development of intestinal lymphoid structures and colitis. Mucosal Immunol. 2019, 12, 733–745. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Perucha, E.; Melchiotti, R.; Bibby, J.A.; Wu, W.; Frederiksen, K.S.; Roberts, C.A.; Hall, Z.; LeFriec, G.; Robertson, K.A.; Lavender, P.; et al. The cholesterol biosynthesis pathway regulates il-10 expression in human th1 cells. Nat. Commun. 2019, 10, 498. [Google Scholar] [CrossRef]
- Kloudova-Spalenkova, A.; Holy, P.; Soucek, P. Oxysterols in cancer management: From therapy to biomarkers. Br. J. Pharmacol. 2021, 178, 3235–3247. [Google Scholar] [CrossRef] [PubMed]
- Raccosta, L.; Fontana, R.; Corna, G.; Maggioni, D.; Moresco, M.; Russo, V. Cholesterol metabolites and tumor microenvironment: The road towards clinical translation. Cancer Immunol. Immunother. 2016, 65, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.E.; Yu, Y.A.; He, S.; Wardell, S.E.; Chang, C.Y.; Kwon, S.; Pillai, R.V.; McDowell, H.B.; Thompson, J.W.; Dubois, L.G.; et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 2017, 8, 864. [Google Scholar] [CrossRef]
- Lanterna, C.; Musumeci, A.; Raccosta, L.; Corna, G.; Moresco, M.; Maggioni, D.; Fontana, R.; Doglioni, C.; Bordignon, C.; Traversari, C.; et al. The administration of drugs inhibiting cholesterol/oxysterol synthesis is safe and increases the efficacy of immunotherapeutic regimens in tumor-bearing mice. Cancer Immunol. Immunother. 2016, 65, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.M.; Kittai, A.; Tabbara, I.A. Chronic lymphocytic leukemia: Current concepts. Anticancer Res. 2015, 35, 5149–5165. [Google Scholar] [PubMed]
- Niss Arfelt, K.; Barington, L.; Benned-Jensen, T.; Kubale, V.; Kovalchuk, A.L.; Daugvilaite, V.; Christensen, J.P.; Thomsen, A.R.; Egerod, K.L.; Bassi, M.R.; et al. Ebi2 overexpression in mice leads to b1 b-cell expansion and chronic lymphocytic leukemia-like b-cell malignancies. Blood 2017, 129, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Jessup, W.; Wilson, P.; Gaus, K.; Kritharides, L. Oxidized lipoproteins and macrophages. Vasc. Pharmacol. 2002, 38, 239–248. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Li, M.; Chen, M.; Sun, C. Multifaceted functions of ch25h and 25hc to modulate the lipid metabolism, immune responses, and broadly antiviral activities. Viruses 2020, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Zanjani, B.N.; Samadi, A.; Isikhan, S.Y.; Lay, I.; Beyaz, S.; Gelincik, A.; Buyukozturk, S.; Arda, N. Plasma levels of oxysterols 7-ketocholesterol and cholestane-3β, 5α, 6β-triol in patients with allergic asthma. J. Asthma 2022, 1–10. [Google Scholar] [CrossRef]
- Goenka, A.; Ghosh, A.; Dixon, S.; Griffiths, W.; Hughes, S.; Newman, W.; Urquhart, J.; Wang, Y.; Wynn, R.; Hussell, T. Susceptibility to Bcg Abscess Associated with Deletion of Two Cholesterol Metabolism Genes: Lysosomal Acid Lipase and Cholesterol 25-hydroxylase; UKPIN Conference: Brighton, UK, 2017. [Google Scholar]
- Korf, H.; Vander Beken, S.; Romano, M.; Steffensen, K.R.; Stijlemans, B.; Gustafsson, J.A.; Grooten, J.; Huygen, K. Liver x receptors contribute to the protective immune response against mycobacterium tuberculosis in mice. J. Clin. Investig. 2009, 119, 1626–1637. [Google Scholar] [CrossRef]
- Raherison, C.; Girodet, P.O. Epidemiology of copd. Eur. Respir. Rev. 2009, 18, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Sugiura, H.; Koarai, A.; Ichikawa, T.; Minakata, Y.; Matsunaga, K.; Nakanishi, M.; Hirano, T.; Akamatsu, K.; Yanagisawa, S.; et al. Increase of 27-hydroxycholesterol in the airways of patients with copd: Possible role of 27-hydroxycholesterol in tissue fibrosis. Chest 2012, 142, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Sugiura, H.; Togo, S.; Koarai, A.; Abe, K.; Yamada, M.; Ichikawa, T.; Kikuchi, T.; Numakura, T.; Onodera, K.; et al. 27-hydroxycholesterol accelerates cellular senescence in human lung resident cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L1028–L1041. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Conlon, T.M.; Sarker, R.S.; Taşdemir, D.; Smirnova, N.F.; Srivastava, B.; Verleden, S.E.; Güneş, G.; Wu, X.; Prehn, C.; et al. Cholesterol metabolism promotes b-cell positioning during immune pathogenesis of chronic obstructive pulmonary disease. EMBO Mol. Med. 2018, 10, e8349. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Koarai, A.; Ichikawa, T.; Minakata, Y.; Matsunaga, K.; Hirano, T.; Akamatsu, K.; Yanagisawa, S.; Furusawa, M.; Uno, Y.; et al. Increased 25-hydroxycholesterol concentrations in the lungs of patients with chronic obstructive pulmonary disease. Respirology 2012, 17, 533–540. [Google Scholar] [CrossRef]
- Parekh, D.; Dancer, R.C.; Thickett, D.R. Acute lung injury. Clin. Med. 2011, 11, 615–618. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhou, H.; Liu, C.; Wang, S.; Han, Y.; Xia, J.; Xu, F. 25-hydroxycholesterol protects against acute lung injury via targeting md-2. J. Cell. Mol. Med. 2018, 22, 5494–5503. [Google Scholar] [CrossRef]
- Iborra, R.T.; Machado-Lima, A.; Castilho, G.; Nunes, V.S.; Abdalla, D.S.; Nakandakare, E.R.; Passarelli, M. Advanced glycation in macrophages induces intracellular accumulation of 7-ketocholesterol and total sterols by decreasing the expression of abca-1 and abcg-1. Lipids Health Dis. 2011, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Adachi, J.; Ueno, Y.; Yoshida, K. Oxysterols increase in diabetic rats. Free Radic. Res. 2005, 39, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Ferderbar, S.; Pereira, E.C.; Apolinário, E.; Bertolami, M.C.; Faludi, A.; Monte, O.; Calliari, L.E.; Sales, J.E.; Gagliardi, A.R.; Xavier, H.T.; et al. Cholesterol oxides as biomarkers of oxidative stress in type 1 and type 2 diabetes mellitus. Diabetes/Metab. Res. Rev. 2007, 23, 35–42. [Google Scholar] [CrossRef] [PubMed]

| Disease | Oxysterol, Gene or Enzyme | Reference |
|---|---|---|
| Atherosclerosis | ↑ 27-hydroxycholesterol | [29,84] |
| ↑ 7α-hydroxycholesterol | [29,84] | |
| 7-ketocholestrol | [159] | |
| 25-hidroxycholesterol in foam cell formation | [148] | |
| Alzheimer’s disease | ↑ 25-hidroxycholesterol | [29] |
| 27-hydroxycholesterol | [123] | |
| ↓ 24-hydroxycholesterol in advanced disease | [117] | |
| ↑ 24-hydroxycholesterol in early disease | [117] | |
| ↑ CH25H | [29] | |
| CH25H polymorphism | [81] | |
| Multiple sclerosis | ↓ 24-hydroxycholesterol in advanced disease | [102,116,117] |
| ↑ 24-hydroxycholesterol in early disease | [117] | |
| ↓ 25-hydroxycholesterol in plasma | [119] | |
| ↓ (25R)-26-hydroxycholesterol | [116] | |
| ↓ 7α-hydroxycholesterol | [116] | |
| 7-ketocholesterol released during inflammatory demyelination in the MS course | [118] | |
| ↑ 25-hydroxycholesterol in spinal cord | [74,119] | |
| ↑ 7α,25-dihydroxycholesterol in spinal cord | [74] | |
| ↑ 7α,25-dihydroxycholesterol in CNS | [74] | |
| ↑ 7α,26-dihydroxycholesterol in spinal cord | [74] | |
| ↑ 7α,24-dihydroxycholesterol in spinal cord | [74] | |
| ↓ 26-hydroxycholesterol in spinal cord | [74] | |
| ↑ CH25H in microglia | [74] | |
| ↑ CYP7B1 in CNS-infiltrating immune cells | [74] | |
| Genetic variants of NR1H3 (LXRα) | [121] | |
| Genetic variants of CH25H | [121] | |
| Tuberculosis | 25-hydroxycholesterol modulation | [31] |
| 3βHSD | [31] | |
| CYP125 | [31] | |
| CYP142 | [31] | |
| CYP124 | [31] | |
| CH25H | [151] | |
| LIPA | [151] | |
| Chronic obstructive pulmonary disease | ↑ 25-hydroxycholesterol | [29] |
| Intestinal diseases | High ingestion of of 25-hydroxycholesterol | [127] |
| Inflammatory bowel diseases | Oxysterols originated from diet | [128,129,130,131,132,133,134] |
| 7-ketocholesterol | [135] | |
| 25-hydroxycholesterol | [135] | |
| ↓ CH25H enzyme | [136] | |
| Ulcerative colitis | CH25H | [125] |
| CYP7B1 | [125] | |
| Rheumatoid arthritis | 25-hydroxycholesterol | [140] |
| Diabetes mellitus | ↑ 7α-hydroperoxycholest-5-en-3β-ol in the kidney, heart, and liver | [160] |
| ↑ 7β-hydroperoxycholest-5-en-3β-ol in the kidney, heart, and liver | [160] | |
| ↑ 7α-hydroxycholesterol in the kidney, heart, and liver | [160] | |
| ↑ 7β-hydroxycholesterol in the kidney, heart, and liver | [160] | |
| ↑ 7-ketocholesterol in the kidney, heart, and liver | [160] | |
| ↑ Total oxysterols in plasma | [161] | |
| Cancer | 22-hydroxycholesterol | [142] |
| 27-hydroxycholesterol | [123,142,143] | |
| LXR/oxysterols axis | [142] | |
| CYP27A1 | [143] | |
| Asthma | ↑ 7-Ketocholesterol in plasma level | [149] |
| ↑ Cholestane-3β, 5α, 6β-triol in plasma level | [149] | |
| ↑ 25-hydroxycholesterol in BLA | [56] | |
| ↑ 7β,27-dihydroxycholesterol in BLA | [56] | |
| ↑ 27-hydroxycholesterol in BLA | [56] | |
| ↑ 7α-hydroxycholesterol in BLA | [56] | |
| Chronic obstructive pulmonary disease | ↑ CYP27A1 in the lung | [153] |
| ↑ 27-Hydroxycholesterol in sputum | [153] | |
| 27-Hydroxycholesterol—differentiation of lung fibroblasts into myofibroblasts | [153] | |
| ↑ CYP27A1 in lung fibroblasts and alveolar macrophages | [154] | |
| ↑ CH25H in airway epithelial cells | [155] | |
| ↑ CYP7B1 airway epithelial cells | [155] | |
| 7α,25-dihydroxycholesterol linked with inducible bronchus-associated lymphoid tissue generation | [155] | |
| ↑ CH25H localized in alveolar macrophages and pneumocytes | [156] | |
| ↑ 25-Hidroxycholesteol in sputum | [156] | |
| Acute lung injury | 25-Hydroxycholesterol protection from ALI | [158] |
| 25-Hydroxycholesterol altered levels during lung inflammation in ALI | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas, F.A.; Levy, D.; Reichert, C.O.; Cunha-Neto, E.; Kalil, J.; Bydlowski, S.P. Effects of Oxysterols on Immune Cells and Related Diseases. Cells 2022, 11, 1251. https://doi.org/10.3390/cells11081251
de Freitas FA, Levy D, Reichert CO, Cunha-Neto E, Kalil J, Bydlowski SP. Effects of Oxysterols on Immune Cells and Related Diseases. Cells. 2022; 11(8):1251. https://doi.org/10.3390/cells11081251
Chicago/Turabian Stylede Freitas, Fábio Alessandro, Débora Levy, Cadiele Oliana Reichert, Edecio Cunha-Neto, Jorge Kalil, and Sérgio Paulo Bydlowski. 2022. "Effects of Oxysterols on Immune Cells and Related Diseases" Cells 11, no. 8: 1251. https://doi.org/10.3390/cells11081251
APA Stylede Freitas, F. A., Levy, D., Reichert, C. O., Cunha-Neto, E., Kalil, J., & Bydlowski, S. P. (2022). Effects of Oxysterols on Immune Cells and Related Diseases. Cells, 11(8), 1251. https://doi.org/10.3390/cells11081251







