Prenatal Air Pollution Exposure and Placental DNA Methylation Changes: Implications on Fetal Development and Future Disease Susceptibility
Abstract
:1. Introduction
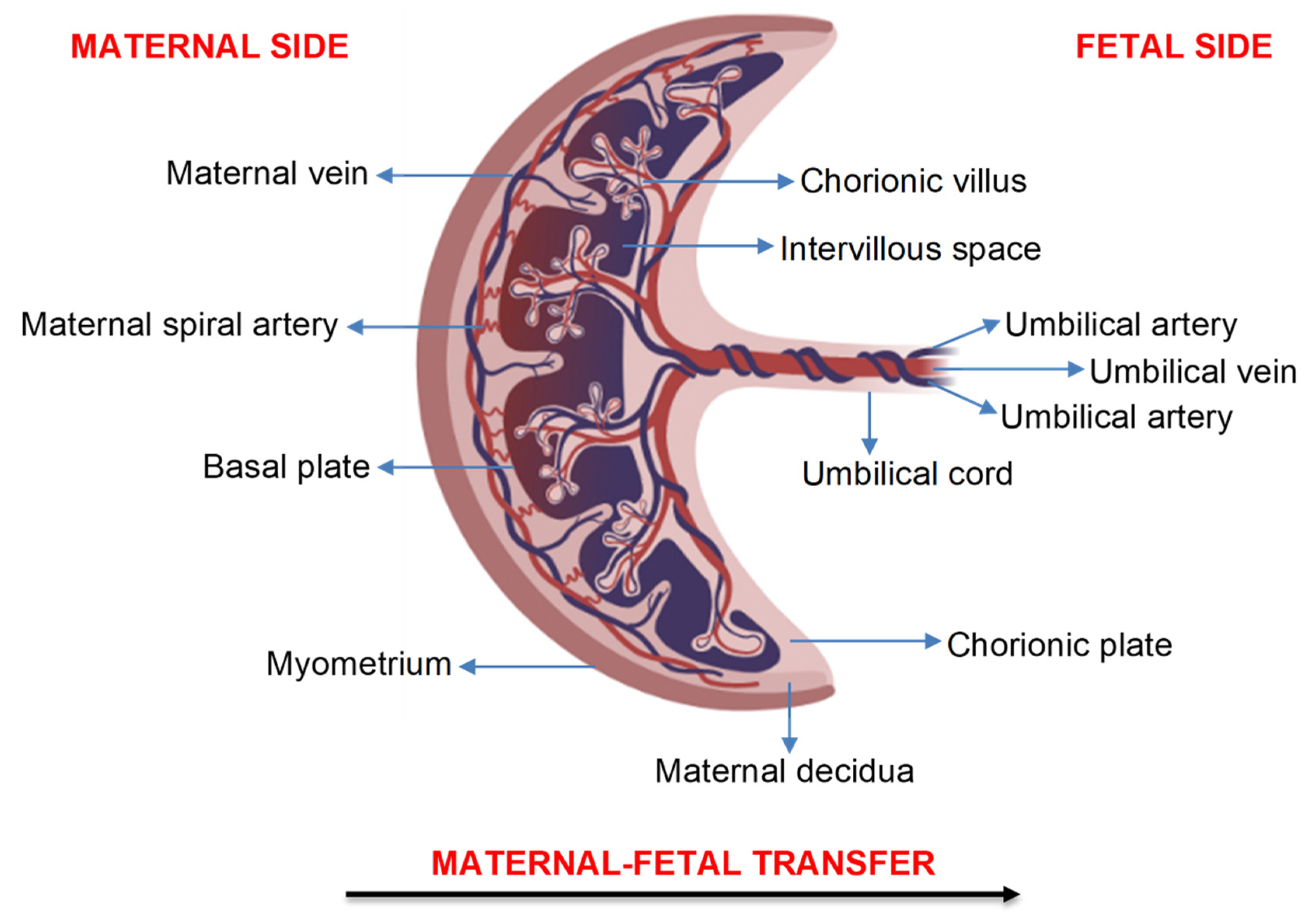
2. Transfer of Air Pollution Particles across the Human Placenta
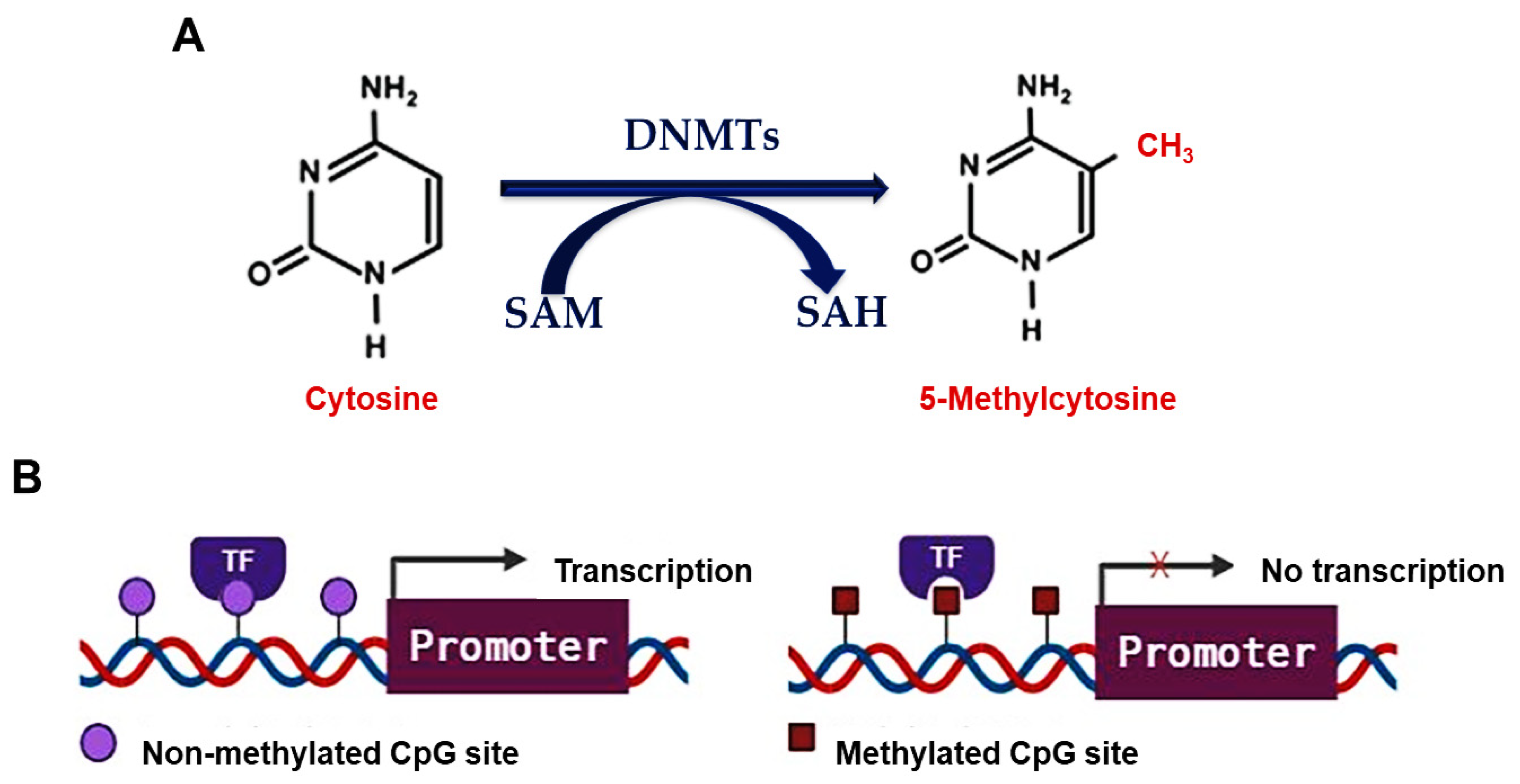
3. DNA Methylation
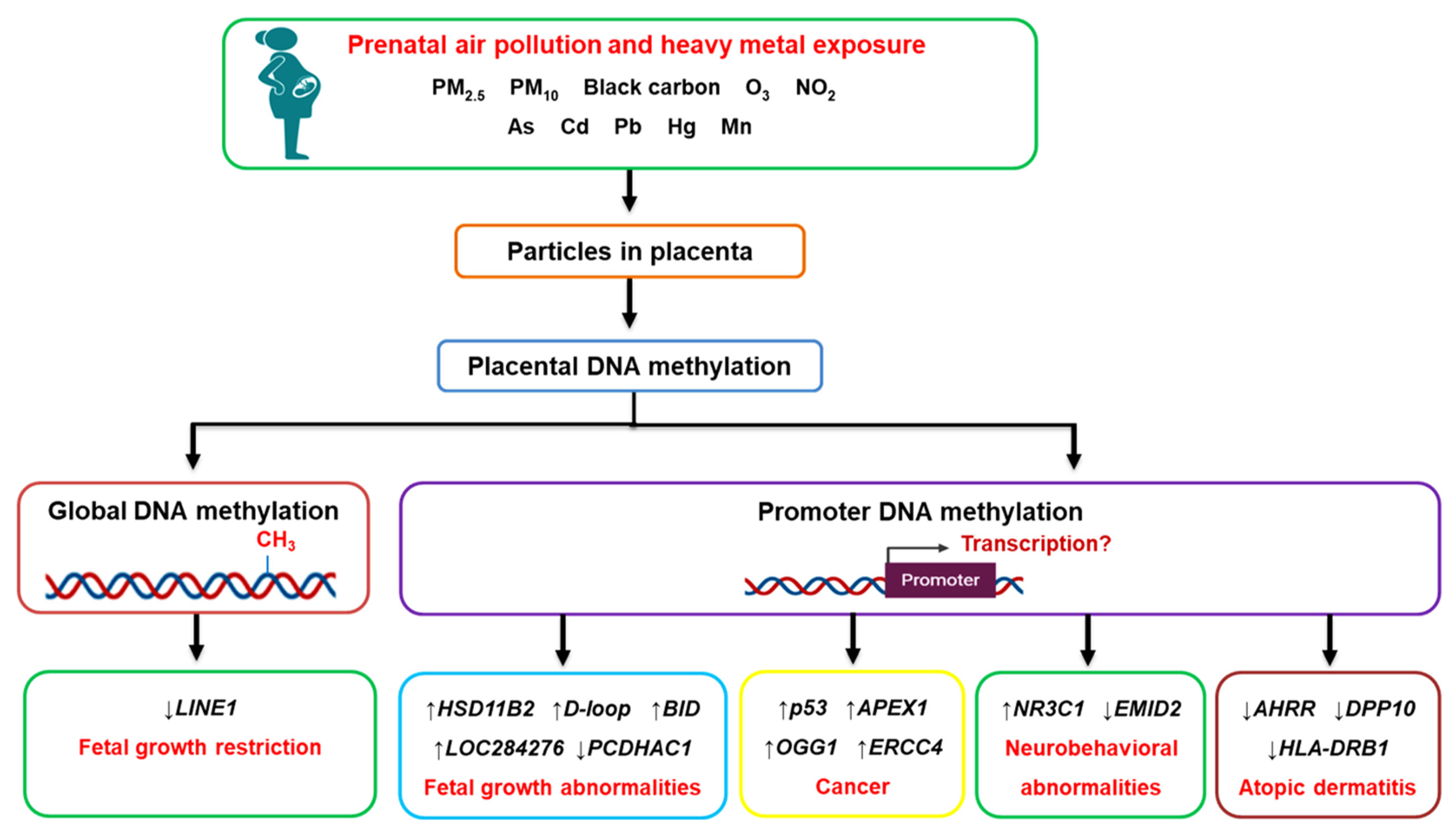
4. Prenatal Air Pollution Exposure and Placental Global DNA Methylation
5. Prenatal Air Pollution Exposure and Placental Candidate Gene Methylation
6. Prenatal Heavy Metal Exposure and Placental DNA Methylation
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Health Effects Institute. State of Global Air 2020: A Special Report on Global Exposure to Air Pollution and Its Health Impacts. Available online: https://www.stateofglobalair.org/ (accessed on 15 July 2021).
- Chang, J.; Streitman, D. Physiologic adaptations to pregnancy. Neurol. Clin. 2012, 30, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, M.J.; Crapo, R.O. Respiratory physiology in pregnancy. Clin. Chest Med. 2011, 32, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.M.; Miyashita, L.; Maher, B.A.; McPhail, G.; Jones, C.J.P.; Barratt, B.; Thangaratinam, S.; Karloukovski, V.; Ahmed, I.A.; Aslam, Z.; et al. Evidence for the presence of air pollution nanoparticles in placental tissue cells. Sci. Total Environ. 2021, 751, 142235. [Google Scholar] [CrossRef] [PubMed]
- Reichrtová, E.; Dorociak, F.; Palkovičová, L. Sites of lead and nickel accumulation in the placental tissue. Hum. Exp. Toxicol. 1998, 17, 176–181. [Google Scholar] [CrossRef]
- Barker, D.J. In utero programming of chronic disease. Clin Sci. 1998, 95, 115–128. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Swanson, J.M.; Entringer, S.; Buss, C.; Wadhwa, P.D. Developmental origins of health and disease: Environmental exposures. Semin. Reprod. Med. 2009, 27, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; He, S.; Wu, K.; Ren, M.; Dong, H.; Di, J.; Yu, Z.; Huang, C. Ambient air pollution and gestational diabetes mellitus: A review of evidence from biological mechanisms to population epidemiology. Sci. Total Environ. 2020, 719, 137349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Liang, Q.; Knibbs, L.D.; Ren, M.; Li, C.; Bao, J.; Wang, S.; He, Y.; Zhu, L.; et al. Effects of prenatal exposure to air pollution on preeclampsia in Shenzhen, China. Environ. Pollut. 2018, 237, 18–27. [Google Scholar] [CrossRef]
- Pereira, G.; Haggar, F.; Shand, A.W.; Bower, C.; Cook, A.; Nassar, N. Association between pre-eclampsia and locally derived traffic-related air pollution: A retrospective cohort study. J. Epidemiol. Community Health 2013, 67, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Gaskins, A.J.; Hart, J.E.; Chavarro, J.E.; Missmer, S.A.; Rich-Edwards, J.W.; Laden, F.; Mahalingaiah, S. Air pollution exposure and risk of spontaneous abortion in the Nurses’ Health Study II. Hum. Reprod. 2019, 34, 1809–1817. [Google Scholar] [CrossRef]
- Kioumourtzoglou, M.-A.; Raz, R.; Wilson, A.; Fluss, R.; Nirel, R.; Broday, D.M. Traffic-related air pollution and pregnancy loss. Epidemiology 2019, 30, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Meng, X.; Liu, C.; Chen, R.; Ge, Y.; Kan, L.; Fu, Q.; Li, W.; Tse, L.A.; Kan, H. Nitrogen dioxide air pollution and preterm birth in Shanghai, China. Environ. Res. 2019, 169, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, Y.; Chen, Y.; Li, X.; Feng, B.; Cao, G.; Xiao, J.; Zeng, W.; Li, X.; Sun, J.; et al. Effects of prenatal exposure to air particulate matter on the risk of preterm birth and roles of maternal and cord blood LINE-1 methylation: A birth cohort study in Guangzhou, China. Environ. Int. 2019, 133, 105177. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Lin, Y.; Zhan, F.B. Industrial air pollution and low birth weight: A case-control study in Texas, USA. Environ. Sci. Pollut. Res. Int. 2018, 25, 30375–30389. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, S.; Li, T.; Zhu, H.; Liang, S.; Xu, K.; Zhang, Y.; Yuan, X.; Yang, Y.; Pan, H.; et al. Effect of PM2.5 on macrosomia in China: A nationwide prospective cohort study. Pediatr. Obes. 2020, 15, e12584. [Google Scholar] [CrossRef]
- Zang, H.; Cheng, H.; Song, W.; Yang, M.; Han, P.; Chen, C.; Ding, R. Ambient air pollution and the risk of stillbirth: A population-based prospective birth cohort study in the coastal area of China. Environ. Sci. Pollut. Res. Int. 2019, 26, 6717–6724. [Google Scholar] [CrossRef]
- Breton, C.V.; Mack, W.J.; Yao, J.; Berhane, K.; Amadeus, M.; Lurmann, F.; Gilliland, F.; McConnell, R.; Hodis, H.N.; Kunzli, N.; et al. Prenatal air pollution exposure and early cardiovascular phenotypes in young adults. PLoS ONE 2016, 11, e0150825. [Google Scholar] [CrossRef]
- Guxens, M.; Lubczynska, M.J.; Muetzel, R.L.; Dalmau-Bueno, A.; Jaddoe, V.W.V.; Hoek, G.; van der Lugt, A.; Verhulst, F.C.; White, T.; Brunekreef, B.; et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol. Psychiatry 2018, 84, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hehua, Z.; Qing, C.; Shanyan, G.; Qijun, W.; Yuhong, Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environ. Res. 2017, 159, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Sbihi, H.; Tamburic, L.; Koehoorn, M.; Brauer, M. Perinatal air pollution exposure and development of asthma from birth to age 10 years. Eur. Respir. J. 2016, 47, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, J.K.; Heck, J.E.; Cockburn, M.; Su, J.; Jerrett, M.; Ritz, B. Prenatal exposure to traffic-related air pollution and risk of early childhood cancers. Am. J. Epidemiol. 2013, 178, 1233–1239. [Google Scholar] [CrossRef]
- Lavigne, E.; Belair, M.A.; Do, M.T.; Stieb, D.M.; Hystad, P.; van Donkelaar, A.; Martin, R.V.; Crouse, D.L.; Crighton, E.; Chen, H.; et al. Maternal exposure to ambient air pollution and risk of early childhood cancers: A population-based study in Ontario, Canada. Environ. Int. 2017, 100, 139–147. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [Green Version]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.-A.; Zisch, A.; Krug, H.F.; von Mandach, U. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef]
- Bongaerts, E.; Nawrot, T.S.; Van Pee, T.; Ameloot, M.; Bove, H. Translocation of (ultra)fine particles and nanoparticles across the placenta; a systematic review on the evidence of in vitro, ex vivo, and in vivo studies. Part. Fibre Toxicol. 2020, 17, 56. [Google Scholar] [CrossRef]
- Ghasemi-Tehrani, H.; Fallah, S.; Mozafarian, N.; Miranzadeh, S.; Sadeghi, S.; Azidhak, A. Effect of exposure to air pollution on placental weight in Isfahan-Iran. J. Family Reprod. Health 2017, 11, 90–96. [Google Scholar] [PubMed]
- Soto, S.F.; Melo, J.O.; Marchesi, G.D.; Lopes, K.L.; Veras, M.M.; Oliveira, I.B.; Souza, R.M.; de Castro, I.; Furukawa, L.N.S.; Saldiva, P.H.N.; et al. Exposure to fine particulate matter in the air alters placental structure and the renin-angiotensin system. PLoS ONE 2017, 12, e0183314. [Google Scholar] [CrossRef]
- Yue, H.; Ji, X.; Zhang, Y.; Li, G.; Sang, N. Gestational exposure to PM2.5 impairs vascularization of the placenta. Sci. Total Environ. 2019, 665, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.G.; Godderis, L.; Pieters, N.; Poels, K.; Kicinski, M.; Cuypers, A.; Fierens, F.; Penders, J.; Plusquin, M.; Gyselaers, W.; et al. Placental DNA hypomethylation in association with particulate air pollution in early life. Part. Fibre Toxicol. 2013, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Kingsley, S.L.; Eliot, M.N.; Whitsel, E.A.; Huang, Y.T.; Kelsey, K.T.; Marsit, C.J.; Wellenius, G.A. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ. Int. 2016, 92, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zhao, Y.; Liu, P.; Xia, B.; Zhu, Q.; Wang, X.; Song, Q.; Kan, H.; Zhang, Y. Exposure to particulate air pollution during early pregnancy is associated with placental DNA methylation. Sci. Total Environ. 2017, 607, 1103–1108. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Hossein-Nezhad, A.; Adabi, E.; Asadollah-Pour, E.; Sadeghi, M.; Mohammad-Nabi, S.; Zakeri Rad, L.; Malek Hosseini, A.A.; Radmehr, M.; Faghihi, F.; et al. Air pollution during pregnancy and placental adaptation in the levels of global DNA methylation. PLoS ONE 2018, 13, e0199772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, E.; Rousseaux, S.; Agier, L.; Giorgis-Allemand, L.; Tost, J.; Galineau, J.; Hulin, A.; Siroux, V.; Vaiman, D.; Charles, M.A.; et al. Pregnancy exposure to atmospheric pollution and meteorological conditions and placental DNA methylation. Environ. Int. 2018, 118, 334–347. [Google Scholar] [CrossRef] [Green Version]
- Ladd-Acosta, C.; Feinberg, J.I.; Brown, S.C.; Lurmann, F.W.; Croen, L.A.; Hertz-Picciotto, I.; Newschaffer, C.J.; Feinberg, A.P.; Fallin, M.D.; Volk, H.E. Epigenetic marks of prenatal air pollution exposure found in multiple tissues relevant for child health. Environ. Int. 2019, 126, 363–376. [Google Scholar] [CrossRef]
- Neven, K.Y.; Saenen, N.D.; Tarantini, L.; Janssen, B.G.; Lefebvre, W.; Vanpoucke, C.; Bollati, V.; Nawrot, T.S. Placental promoter methylation of DNA repair genes and prenatal exposure to particulate air pollution: An ENVIRONAGE cohort study. Lancet Planet. Health 2018, 2, e174–e183. [Google Scholar] [CrossRef] [Green Version]
- Nawrot, T.S.; Saenen, N.D.; Schenk, J.; Janssen, B.G.; Motta, V.; Tarantini, L.; Cox, B.; Lefebvre, W.; Vanpoucke, C.; Maggioni, C.; et al. Placental circadian pathway methylation and in utero exposure to fine particle air pollution. Environ. Int. 2018, 114, 231–241. [Google Scholar] [CrossRef]
- Saenen, N.D.; Vrijens, K.; Janssen, B.G.; Roels, H.A.; Neven, K.Y.; Vanden Berghe, W.; Gyselaers, W.; Vanpoucke, C.; Lefebvre, W.; De Boever, P. Lower placental leptin promoter methylation in association with fine particulate matter air pollution during pregnancy and placental nitrosative stress at birth in the ENVIRONAGE cohort. Environ. Health Perspect 2017, 125, 262–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Wang, P.; Zhou, Y.; Xia, B.; Zhu, Q.; Ge, W.; Li, J.; Shi, H.; Xiao, X.; Zhang, Y. Prenatal fine particulate matter exposure, placental DNA methylation changes, and fetal growth. Environ. Int. 2021, 147, 106313. [Google Scholar] [CrossRef]
- Yang, S.-I.; Lee, S.-H.; Lee, S.-Y.; Kim, H.-C.; Kim, H.-B.; Kim, J.-H.; Lim, H.; Park, M.J.; Cho, H.-J.; Yoon, J. Prenatal PM2.5 exposure and vitamin D–associated early persistent atopic dermatitis via placental methylation. Ann. Allergy Asthma Immunol. 2020, 125, 665–673. [Google Scholar] [CrossRef]
- Janssen, B.G.; Byun, H.M.; Gyselaers, W.; Lefebvre, W.; Baccarelli, A.A.; Nawrot, T.S. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics 2015, 10, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Vos, S.; Nawrot, T.S.; Martens, D.S.; Byun, H.M.; Janssen, B.G. Mitochondrial DNA methylation in placental tissue: A proof of concept study by means of prenatal environmental stressors. Epigenetics 2021, 16, 121–131. [Google Scholar] [CrossRef]
- Appleton, A.A.; Jackson, B.P.; Karagas, M.; Marsit, C.J. Prenatal exposure to neurotoxic metals is associated with increased placental glucocorticoid receptor DNA methylation. Epigenetics 2017, 12, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardenas, A.; Houseman, E.A.; Baccarelli, A.A.; Quamruzzaman, Q.; Rahman, M.; Mostofa, G.; Wright, R.O.; Christiani, D.C.; Kile, M.L. In utero arsenic exposure and epigenome-wide associations in placenta, umbilical artery, and human umbilical vein endothelial cells. Epigenetics 2015, 10, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, B.B.; Karagas, M.R.; Punshon, T.; Jackson, B.P.; Robbins, D.J.; Houseman, E.A.; Marsit, C.J. Epigenome-wide assessment of DNA methylation in the placenta and arsenic exposure in the New Hampshire Birth Cohort Study (USA). Environ. Health Perspect. 2016, 124, 1253–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, A.F.; Farin, F.M.; Bammler, T.K.; MacDonald, J.W.; Afsharinejad, Z.; Burbacher, T.M.; Siscovick, D.S.; Williams, M.A.; Enquobahrie, D.A. Infant sex-specific placental cadmium and DNA methylation associations. Environ. Res. 2015, 138, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Everson, T.M.; Armstrong, D.A.; Jackson, B.P.; Green, B.B.; Karagas, M.R.; Marsit, C.J. Maternal cadmium, placental PCDHAC1, and fetal development. Reprod. Toxicol. 2016, 65, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Everson, T.M.; Punshon, T.; Jackson, B.P.; Hao, K.; Lambertini, L.; Chen, J.; Karagas, M.R.; Marsit, C.J. Cadmium-associated differential methylation throughout the placental genome: Epigenome-wide association study of two US birth cohorts. Environ. Health Perspect. 2018, 126, 017010. [Google Scholar] [CrossRef] [Green Version]
- Maccani, J.Z.; Koestler, D.C.; Lester, B.; Houseman, E.A.; Armstrong, D.A.; Kelsey, K.T.; Marsit, C.J. Placental DNA methylation related to both infant toenail mercury and adverse neurobehavioral outcomes. Environ. Health Perspect. 2015, 123, 723–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccani, J.Z.; Koestler, D.C.; Houseman, E.A.; Armstrong, D.A.; Marsit, C.J.; Kelsey, K.T. DNA methylation changes in the placenta are associated with fetal manganese exposure. Reprod. Toxicol. 2015, 57, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koukoura, O.; Sifakis, S.; Spandidos, D.A. DNA methylation in the human placenta and fetal growth. Mol. Med. Rep. 2012, 5, 883–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, R.-Z.; Zhang, X.; Hu, P.; Liu, X.-M.; Hua, X.-D.; Wang, X.; Ding, H.-J. Screening for differential methylation status in human placenta in preeclampsia using a CpG island plus promoter microarray. Int. J. Mol. Med. 2012, 30, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acurio, J.; Troncoso, F.; Bertoglia, P.; Salomon, C.; Aguayo, C.; Sobrevia, L.; Escudero, C. Potential role of A2B adenosine receptors on proliferation/migration of fetal endothelium derived from preeclamptic pregnancies. Biomed. Res. Int. 2014, 2014, 274507. [Google Scholar] [CrossRef] [Green Version]
- Marsit, C.J.; Maccani, M.A.; Padbury, J.F.; Lester, B.M. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS ONE 2012, 7, e33794. [Google Scholar] [CrossRef] [Green Version]
- Van den Berg, C.B.; Chaves, I.; Herzog, E.M.; Willemsen, S.P.; van der Horst, G.T.J.; Steegers-Theunissen, R.P.M. Early- and late-onset preeclampsia and the DNA methylation of circadian clock and clock-controlled genes in placental and newborn tissues. Chronobiol. Int. 2017, 34, 921–932. [Google Scholar] [CrossRef]
- Li, H.; Qian, X.; Wang, Q.g. Heavy metals in atmospheric particulate matter: A comprehensive understanding is needed for monitoring and risk mitigation. Environ. Sci. Technol. 2013, 47, 13210–13211. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, X.; Ku, T.; Li, G.; Sang, N. Heavy metals bound to fine particulate matter from northern China induce season-dependent health risks: A study based on myocardial toxicity. Environ. Pollut. 2016, 216, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.H.; Torrone, D.; Lovinsky-Desir, S.; Perzanowski, M.; Bautista, J.; Jezioro, J.R.; Hoepner, L.; Ross, J.; Perera, F.P.; Chillrud, S.N. Short-term exposure to PM2.5 and vanadium and changes in asthma gene DNA methylation and lung function decrements among urban children. Respir. Res. 2017, 18, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollati, V.; Marinelli, B.; Apostoli, P.; Bonzini, M.; Nordio, F.; Hoxha, M.; Pegoraro, V.; Motta, V.; Tarantini, L.; Cantone, L.; et al. Exposure to metal-rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ. Health Perspect. 2010, 118, 763–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaevska, E.; Moccia, C.; Asta, F.; Cibella, F.; Gagliardi, L.; Ronfani, L.; Rusconi, F.; Stazi, M.A.; Richiardi, L. Exposure to ambient air pollution in the first 1000 days of life and alterations in the DNA methylome and telomere length in children: A systematic review. Environ. Res. 2021, 193, 110504. [Google Scholar] [CrossRef] [PubMed]
| Author | Study | Sample Size | Method | Air Pollutant | Duration Exposed | Findings |
|---|---|---|---|---|---|---|
| Janssen et al. [33] | ENVIRONAGE cohort, Belgium | 240 | UPLC/MS-MS | PM2.5: 5 µg/m3 increment | Implantation (6–21 days after conception) | ↓ Global DNA methylation (−1.08%, 95% CI: −1.80, −0.36%, p = 0.004) |
| PM2.5: 5 µg/m3 increment | First trimester | ↓ Global DNA methylation (−2.41%, 95% CI: −3.62, −1.20%, p = 0.0001) | ||||
| PM2.5: 5 µg/m3 increment | Second trimester | ↓ Global DNA methylation (−1.51%, 95% CI: −2.66, −0.36%, p = 0.01) | ||||
| PM2.5: 5 µg/m3 increment | Entire pregnancy | ↓ Global DNA methylation (−2.19%, 95% CI: −3.65, −0.73%, p = 0.004) | ||||
| Kingsley et al. [34] | RICHS cohort, US | 471 | Bisulfite-PCR pyrosequencing | Traffic-related air pollution: Women residing ≤ 150 m from a major roadway or ≤50 m from a secondary road | Entire pregnancy | Residing near a major roadway: ↓ LINE1 methylation (−0.82%, 95% CI: −1.57, −0.07%, p = 0.03); No significant association with AluYb8 methylation (p = 0.07) |
| Cai et al. [35] | Case-control study, China | 181 | Bisulfite-PCR pyrosequencing | PM10: 10 µg/m3 increment | First trimester | Placenta of fetal growth restricted newborns: ↓ LINE1 methylation (−1.78%, 95% CI: −3.35, −0.22%, p < 0.05) Placenta of normal growth newborns: No significant association with LINE1 methylation (p > 0.05) |
| Maghbooli et al. [36] | Nested case-control study, Iran | 92 | RP-HPLC | PM2.5: 20.43 ± 0.68 µg/m3 and 30.99 ± 0.86 µg/m3 PM10: 64.97 ± 2.52 µg/m3 and 74.34 ± 2.66 µg/m3 | First trimester | PM2.5: ↑ Global DNA methylation (r = 0.26, p = 0.01) PM10: ↑ Global DNA methylation (r = 0.38, p = 0.0001) |
| Abraham et al. [37] | EDEN cohort, France | 668 | Illumina Infinium HumanMethylation450K BeadChip | PM10: 10 µg/m3 increment | Day before birth | ↑ Alu methylation (β = 0.08, p = 0.01); No significant association with LINE1 methylation (β = 0.09, p = 0.28) |
| Ladd-Acosta et al. [38] | EARLI cohort, US | 124 | Illumina Infinium HumanMethylation450K BeadChip | NO2 and O3 | Entire pregnancy | O3: ↓ DNA methylation at shelf regions (p = 0.00028), ↑ DNA methylation at CpG islands (p = 0.00295) and shore regions (p = 0.00266) NO2: ↓ DNA methylation in CpG islands (p = 0.00359) and shore regions (p = 0.04284) |
| Author | Study | Sample Size | Technique | Air Pollutant | Duration Exposed | Findings |
|---|---|---|---|---|---|---|
| Kingsley et al. [34] | RICHS cohort, US | 215 | Illumina Infinium HumanMethylation450K BeadChip | Traffic-related air pollution: Women residing ≤150 m from a major roadway or ≤50 m from a secondary road | Entire Pregnancy | Residing near a major roadway: Differential methylation of 7 CpG sites—4 were mapped to non-genic regions and 3 were mapped to genes. ↑ PTPRN2 methylation (+0.061%, p = 2.904 × 10−6), ↓ TMEM125 methylation (−0.012%, p = 1.077 × 10−3), ↓ VPS4A methylation (−0.016%, p = 3.151 × 10−5) |
| Cai et al. [35] | Case-control study, China | 181 | Bisulfite-PCR pyrosequencing | PM10: 10 µg/m3 increment | First trimester | Placenta of fetal growth restricted newborns: ↑ HSD11B2 methylation (+1.03%, 95% CI: 0.07, 1.98%, p < 0.05) Placenta of normal growth newborns: No significant association with HSD11B2 methylation (p > 0.05) |
| PM10: 10 µg/m3 increment | Second trimester | Placenta of fetal growth restricted newborns: ↑ HSD11B2 methylation (+2.23%, 95% CI: 0.69, 3.76%, p < 0.05) Placenta of normal growth newborns: No significant association with HSD11B2 methylation (p > 0.05) Total population: ↑ HSD11B2 methylation (+1.42%, 95% CI: 0.24, 2.57%, p < 0.05) | ||||
| PM10: 10 µg/m3 increment | Entire pregnancy | Total population: ↑ HSD11B2 methylation (+1.98%, 95% CI: 0.53, 3.43%, p < 0.05) | ||||
| Abraham et al. [37] | EDEN cohort, France | 668 | Illumina Infinium HumanMethylation450K BeadChip | NO2: 10 µg/m3 increment | First trimester | ↓ ADORA2B methylation at 2 CpG sites (cg17580614: β = −0.037, p < 0.001; cg07563400: β = −0.042, p < 0.001) |
| NO2: 10 µg/m3 increment | Second trimester | ↓ ADORA2B methylation at 2 CpG sites (cg17580614: β = −0.044, p < 0.0001; cg07563400: β = −0.047, p < 0.0001), ↑ PXT1/KCTD20 methylation (cg10984505: β = 0.002, p = 0.02 | ||||
| NO2: 10 µg/m3 increment | Third trimester | ↓ CAPN10 methylation (cg01712700: β = −0.004, p = 0.02) | ||||
| PM10: 10 µg/m3 increment | One month before birth | ↑ SLC44A5 methylation (cg12659128: β = 0.037, p = 0.03), ↑ ADCK5 methylation (cg23075260: β = 0.018, p = 0.03), ↑ TGM6 methylation (cg06967014: β = 0.007, p = 0.03), ↓ TUBGCP2 methylation (cg05142592: β = −0.008, p = 0.03) | ||||
| PM10: 10 µg/m3 increment | 3 days before birth | ↓ KYNU methylation (cg04112100: β = −0.012, p = 0.04) | ||||
| Ladd-Acosta et al. [38] | EARLI cohort, US | 124 | Illumina Infinium HumanMethylation450K BeadChip | NO2 and O3 | Entire pregnancy | Differentially methylated regions in 5 genes that seemed to be specific to placental tissue: ZNF442, PTPRH, SLC25A44, F11R, and STK38 Differentially methylated regions in 3 genes found in cord blood and showed similar methylation patterns in placental tissue: RNF39, CYP2E1, and PM20D1 |
| Neven et al. [39] | ENVIRONAGE cohort, Belgium | 463 | Bisulfite-PCR pyrosequencing | PM2.5: 3.84 µg/m3 increment | Entire pregnancy | ↑ APEX1 methylation (+7.34%, 95% CI: 0.52, 14.16%, p = 0.0089), ↑ OGG1 methylation (+13.06%, 95% CI: 3.88, 22.24%, p = 0.0054), ↑ ERCC4 methylation (+ 16.31%, 95% CI: 5.43, 27.18%, p = 0.0034), ↑ p53 methylation (+10.60%, 95% CI: 4.46, 16.74%, p = 0.0008), ↓ DAPK1 methylation (−12.92%, 95% CI: −22.35, −3.49%, p = 0.0073) |
| Black carbon: 0.36 µg/m3 increment | Entire pregnancy | ↑ APEX1 methylation (+9.16%, 95% CI: 4.06, 14.25%, p = 0.005), ↑ ERCC4 methylation (+27.56%, 95% CI: 17.58, 37.55%, p < 0.0001) | ||||
| Nawrot et al. [40] | ENVIRONAGE cohort, Belgium | 407 | Bisulfite-PCR pyrosequencing | PM2.5: 7.9 µg/m3 increment | First trimester | ↓ CLOCK methylation (−0.59 Log (fold-change), 95% CI: −0.93, −0.25, p = 0.0007) |
| PM2.5: 8.9 µg/m3 increment | Third trimester | ↑ NPAS2 methylation (+0.16 Log (fold-change), 95% CI: 0.06, 0.27, p = 0.002), ↑ CRY1 methylation (+0.59 Log (fold-change), 95% CI: 0.22, 0.95, p = 0.002), ↑ PER2 methylation (+0.36 Log (fold-change), 95% CI: 0.16, 0.57, p = 0.0005), ↑ PER3 methylation (+0.42 Log (fold-change), 95% CI: 0.18, 0.67, p = 0.0008), ↓ PER1 methylation (−0.51 Log (fold-change), 95% CI: −0.90, −0.13, p = 0.01) | ||||
| PM2.5: 9.7 µg/m3 increment | Last month of pregnancy | ↑ CRY1 methylation (p = 0.01), ↑ PER2 methylation (p = 0.0003), ↑ PER3 methylation (p = 0.02) | ||||
| Saenen et al. [41] | ENVIRONAGE cohort, Belgium | 361 | Bisulfite-PCR pyrosequencing | PM2.5: 7.5 µg/m3 increment | Second trimester | ↓ Lep methylation (−1.4%, 95% CI: −2.7, −0.19%, p = 0.02), ↑ Lep methylation in placentae from male neonates compared to placentae from female neonates (+1.33%, 95% CI: 0.40, 2.27%, p = 0.005) |
| Zhao et al. [42] | Shanghai MCPC, China | 287 | Bisulfite-PCR pyrosequencing | PM2.5: 1 µg/m3 increment | Second trimester | ↓ IGF2 methylation (−0.135%, 95% CI: −0.236, −0.034), ↑ BID methylation (+ 0.132%, 95% CI: 0.047, 0.217), ↑ FOXN3 methylation (position 1, +0.091, 95% CI: 0.008, 0.174) |
| PM2.5: 1 µg/m3 increment | Third trimester | ↓ IGF2 methylation (−0.229%, 95% CI: −0.384, −0.073), ↑ BID methylation (+ 0.209%, 95% CI: 0.072, 0.346) | ||||
| PM2.5: 1 µg/m3 increment | Entire pregnancy | ↓ IGF2 methylation (−0.297%, 95% CI: −0.489, −0.105), ↑ BID methylation (+ 0.209%, 95% CI: 0.039, 0.380) | ||||
| Yang et al. [43] | COCOA study, Korea | 1180 | Illumina Infinium HumanMethylationEPIC BeadChip | PM2.5 | First trimester (3–7 weeks of pregnancy) | Placenta of children with high PM2.5 exposure, low cord blood vitamin D levels, and atopic dermatitis: ↓ AHRR methylation (cg16371648: β = −0.367, p = 0.026), ↓ DPP10 methylation (cg19211931: β = −0.263, p = 0.013), ↓ HLA-DRB1 methylation (cg10632894: β = −0.318, p = 0.026) |
| Janssen et al. [44] | ENVIRONAGE cohort, Belgium | 381 | Bisulfite-PCR pyrosequencing | PM2.5: 7.8 µg/m3 increment | First trimester | ↑ D-loop methylation (+0.44%, 95% CI: 0.12, 0.75%, p < 0.05), ↑ MT-RNR1 methylation (+1.27%, 95% CI: 0.23, 2.32%, p < 0.05) |
| PM2.5: 3.0 µg/m3 increment | Entire pregnancy | ↑ D-loop methylation (+0.21%, 95% CI: -0.003, 1.02%, p > 0.05), ↑ MT-RNR1 methylation (+0.91, 95% CI: 0.56, 4.18%, p < 0.05) | ||||
| Vos et al. [45] | ENVIRONAGE cohort, Belgium | 60 | Bisulfite-PCR pyrosequencing | PM2.5 (5.4 µg/m3 increment) and black carbon (0.9 µg/m3 increment) | Entire pregnancy | ↑ D-loop methylation (+0.47%, 95% CI: 0.20, 0.73%, p = 0.61), ↑ LDLR2 methylation (+0.81%, 95% CI: −0.17, 1.78, p = 0.09), ↓ PINK1 methylation (−0.42%, 95% CI: −0.60, −0.24%, p < 0.05) |
| Author | Study | Sample Size | Method | Heavy Metal | Findings |
|---|---|---|---|---|---|
| Appleton et al. [46] | RICHS cohort, US | 222 | Bisulfite-PCR pyrosequencing | Arsenic (0.14 µg/g; measured in toenail clippings) | ↑ NR3C1 methylation (+0.71, p = 0.0002) |
| Cadmium (0.17 µg/g; measured in toenail clippings) | ↑ NR3C1 methylation (+0.74, p < 0.001) | ||||
| Lead (2.3 µg/g; measured in toenail clippings) | ↑ NR3C1 methylation (+0.77, p = 0.004) | ||||
| Mercury (0.17 µg/g; measured in toenail clippings) | ↑ NR3C1 methylation (+1.41, p < 0.001) | ||||
| Manganese (2.2 µg/g; measured in toenail clippings) | ↑ NR3C1 methylation (+0.80, p = 0.02) | ||||
| Cardenas et al. [47] | Nested cohort, Bangladesh | 37 | Illumina Infinium HumanMethylation450 BeadChip | Arsenic (63.7 ± 116.5 µg/L; measured in maternal drinking water by ICP-MS) | CpG methylation at 3 genes—TRA2B, PLCE1, and CD36; hypermethylation of open sea regions |
| Green et al. [48] | NHBCS, US | 285 | Illumina Infinium HumanMethylation450K BeadChip | Arsenic (0.82 µg/kg; measured in placental tissue by ICP-MS) | Differential methylation at 163 CpG sites (q < 0.05). Of these, 13 attained genome-wide significance and were tracked to LYRM2 (11 CpG sites), CAMTA1 (1 CpG site), and CCDC57 (1 CpG site) genes |
| Mohanty et al. [49] | Omega cohort, Pacific Northwest Placenta MicroArray Study (pilot case-control study) | 24 | Illumina Infinium HumanMethylation450K BeadChip | Cadmium (5 ng/g in placental tissue from female neonates and 2 ng/g in placental tissue from male neonates; measured by ICP-MS) | Placenta of female neonates: hypomethylation of 3 CpG sites located near ARL9 (p = 0.01), SIAH3 (p = 0.08), and HS3ST4 (p = 0.08) genes; hypomethylation of 1 genomic region on chromosome 7 (region 86974674 to 86975244, including CROT and TP53TG1 genes; p = 0.06) Placenta of male neonates: hypomethylation of 2 CpG sites located near MECOM (p < 0.01); hypermethylation of 1 CpG site located near SALL1 (p = 0.08); hypomethylation of 2 genomic regions (region 169379554 to 169380078 on chromosome 3, including the MECOM gene (p = 0.03) and region 1792758 to 1792758 on chromosome 8, including the ARHGEF10 gene (p = 0.07)) |
| Everson et al. [50] | RICHS cohort, US | 94 | Illumina Infinium HumanMethylation450K BeadArray | Cadmium (0.01 µg/g; measured in maternal toenail clippings by ICP-MS) | ↓ PCDHAC1 methylation (TSS200 and TSS1500) |
| Everson et al. [51] | NHBCS and RICHS cohort, US | 343 (NHBCS) 141 (RICHS cohort) | Illumina Infinium HumanMethylation450K BeadArray | Cadmium (3.13 ng/g (NHBCS) and 4.37 ng/g (RICHS cohort); measured in placental tissue by ICP-MS) | Differential methylation of 17 CpG sites (p < 1 × 10−5); DNA methylation at 9 of these 17 CpG sites were associated with ↑ expression of TNFAIP2, EXOC3L4, GAS7, SREBF1, ACOT7, and RORA |
| Maccani et al. [52] | RICHS, US | 41 | Illumina Infinium HumanMethylation450 BeadArray | Mercury (0.077–0.425 μg/g; measured in infant toenail clippings) | Differential methylation at 339 loci; 10 loci residing in CPLX1, TTC23, and EMID2 were associated with a high risk for adverse neurobehavioral profiles (p < 0.01) |
| Maccani et al. [53] | RICHS cohort, US | 61 | Illumina Infinium HumanMethylation450 BeadChip | Manganese (0.858–5.666 μg/g; measured in infant toenail clippings) | Differential methylation at 5 CpG loci: EMX2OS (cg16063747; p = 3.15 × 10−8), ATAD2B (cg08192560; p = 3.48 × 10−8), FTO/RPGRIP1L (cg26692097; p = 8.69 × 10−8), EN1 (cg07419575; p = 1.26 × 10−7), and LOC284276 (cg22284422; p = 1.29 × 10−7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazi, T.; Naidoo, P.; Naidoo, R.N.; Chuturgoon, A.A. Prenatal Air Pollution Exposure and Placental DNA Methylation Changes: Implications on Fetal Development and Future Disease Susceptibility. Cells 2021, 10, 3025. https://doi.org/10.3390/cells10113025
Ghazi T, Naidoo P, Naidoo RN, Chuturgoon AA. Prenatal Air Pollution Exposure and Placental DNA Methylation Changes: Implications on Fetal Development and Future Disease Susceptibility. Cells. 2021; 10(11):3025. https://doi.org/10.3390/cells10113025
Chicago/Turabian StyleGhazi, Terisha, Pragalathan Naidoo, Rajen N. Naidoo, and Anil A. Chuturgoon. 2021. "Prenatal Air Pollution Exposure and Placental DNA Methylation Changes: Implications on Fetal Development and Future Disease Susceptibility" Cells 10, no. 11: 3025. https://doi.org/10.3390/cells10113025






