Prognostic Significance of PTEN Loss in Prostate Cancer: A Meta-Analysis of Gleason Grade and Clinical Outcomes
Simple Summary
Abstract
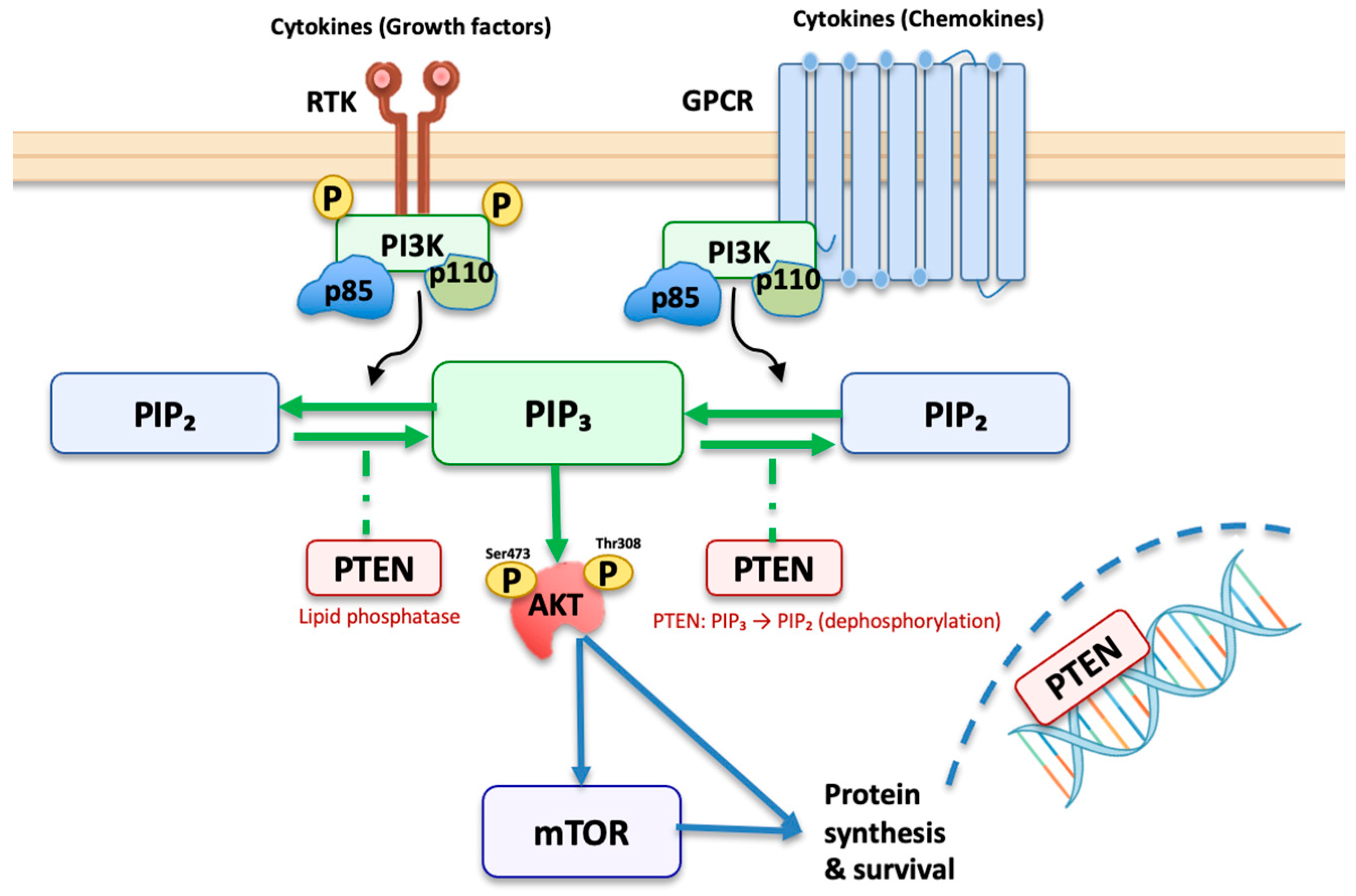
1. Introduction
1.1. Methodology
1.2. Data Extraction
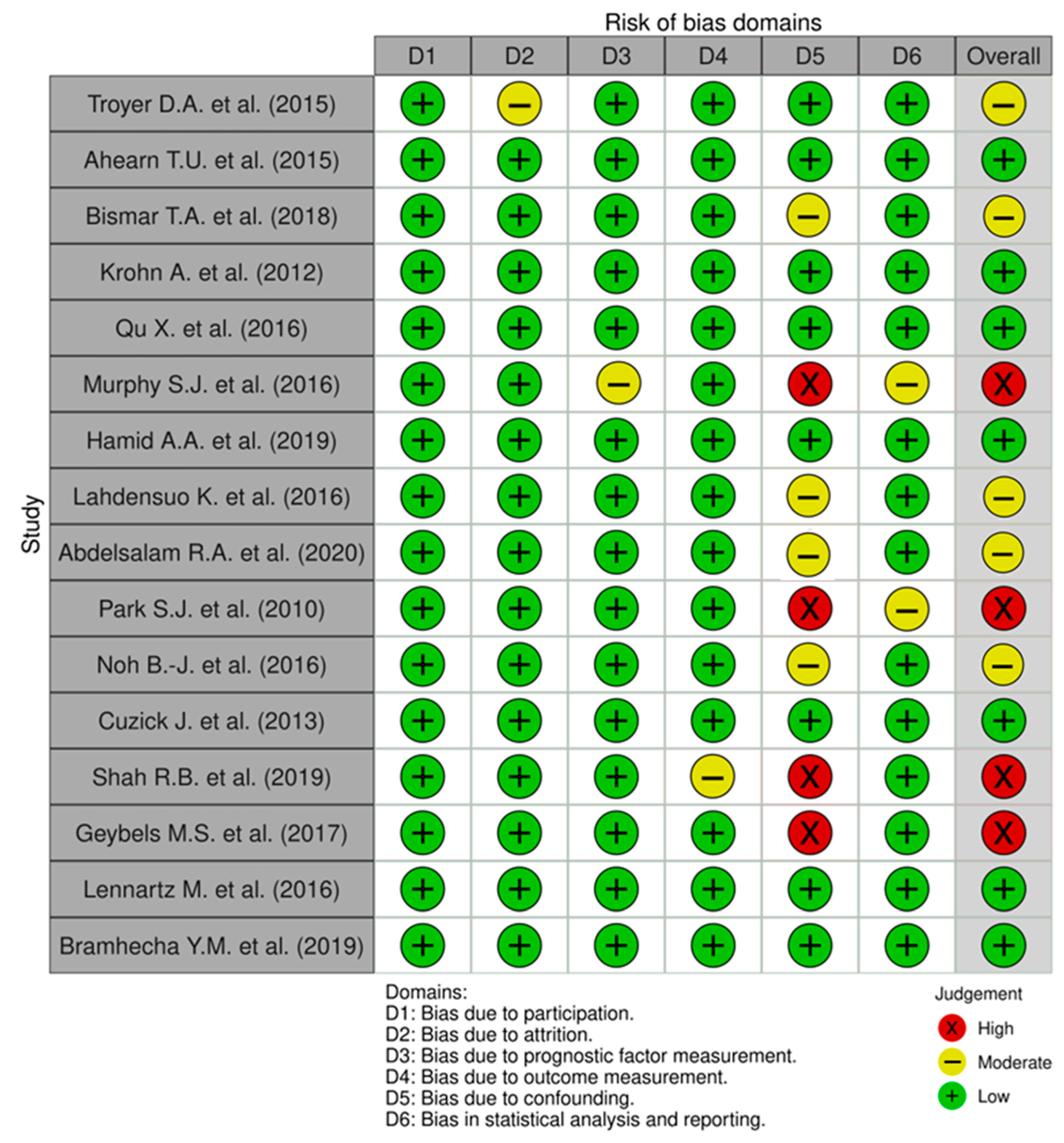
1.3. Study Quality and Risk of Bias Assessment
1.4. Statistical Analysis, Heterogeneity Assessment, Publication Bias
2. Results
2.1. Quality Assessment
2.2. Characteristics of the Included Studies
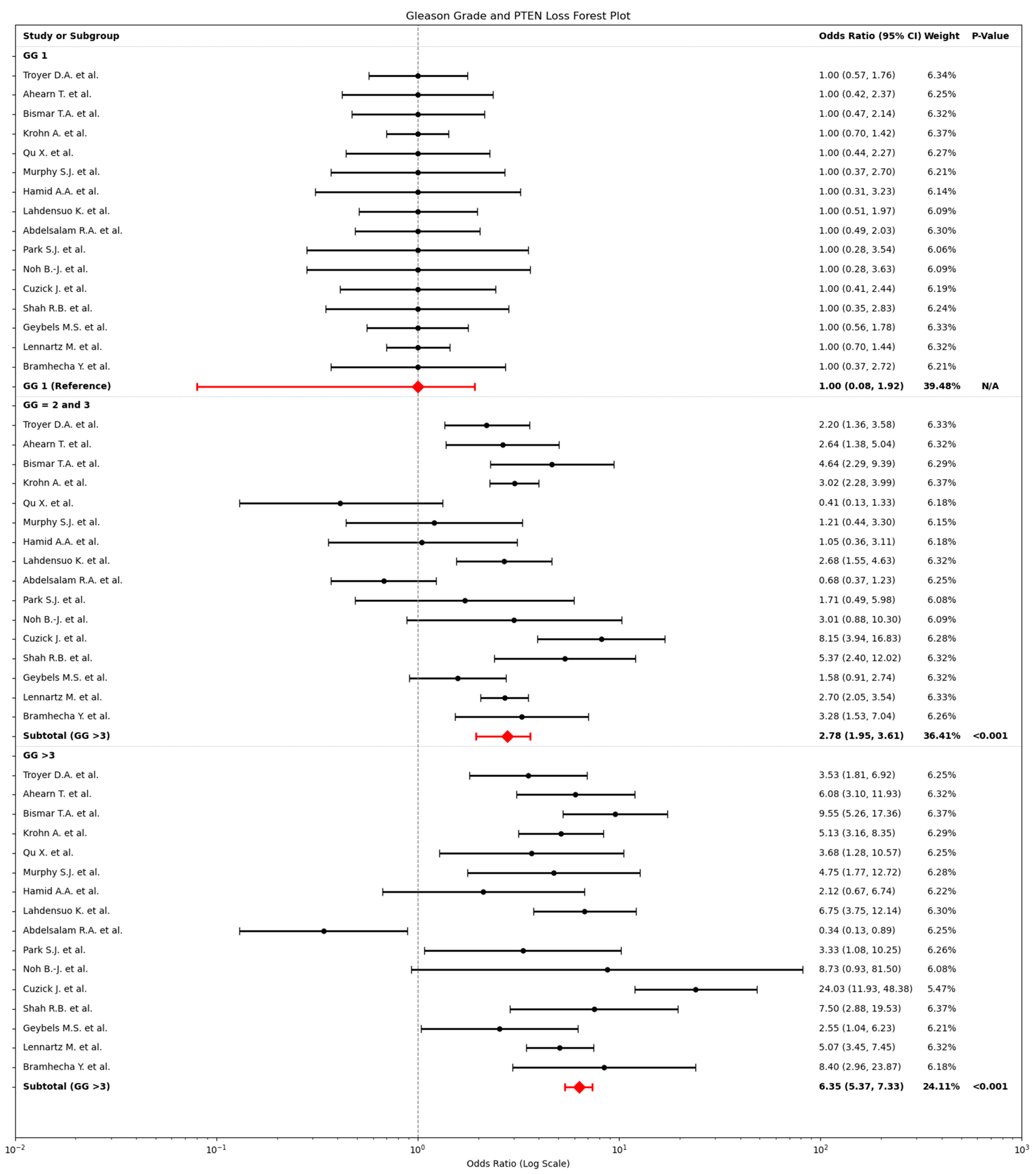
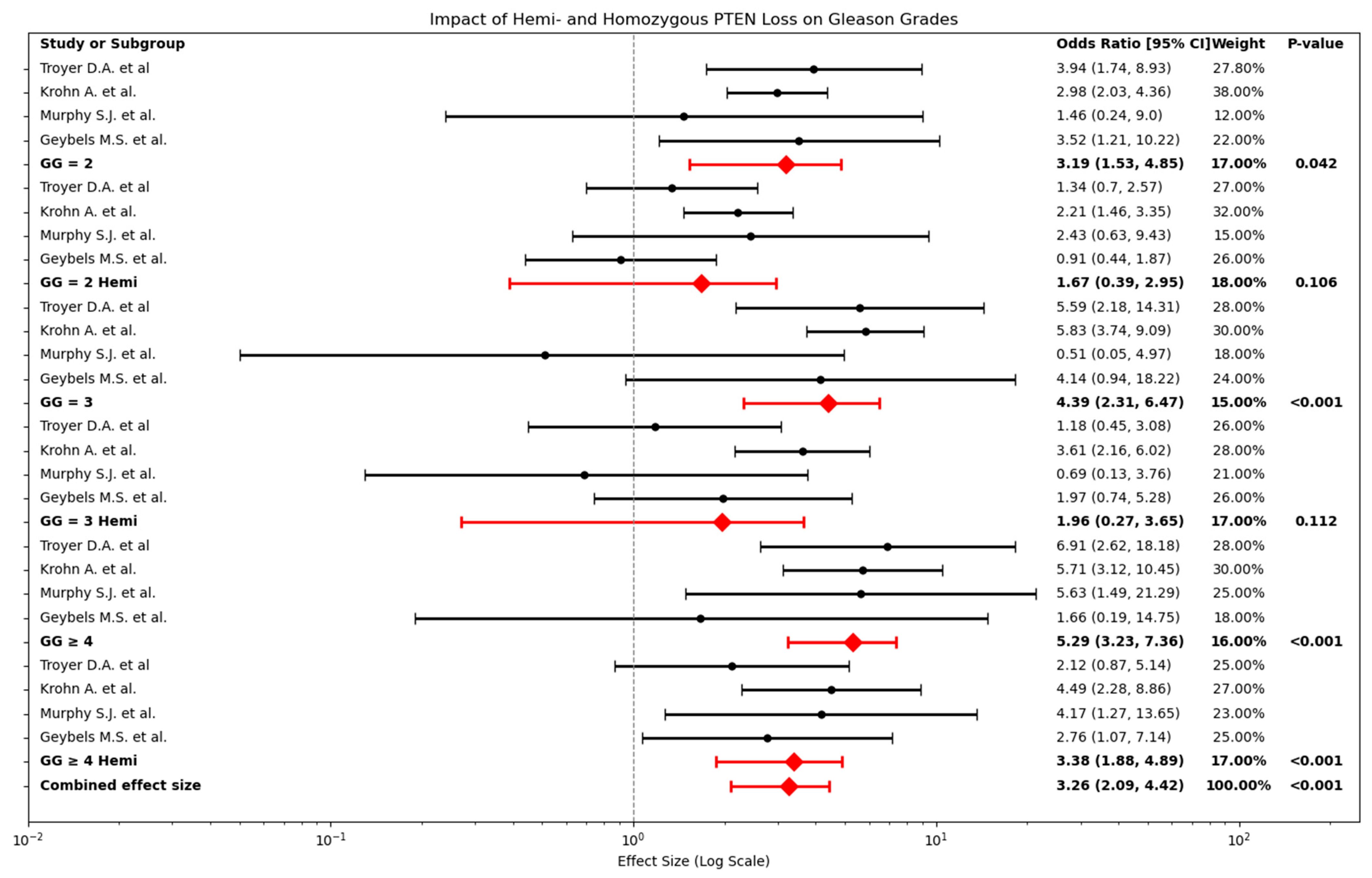
2.3. Relationship Between PTEN Loss and Gleason Grade
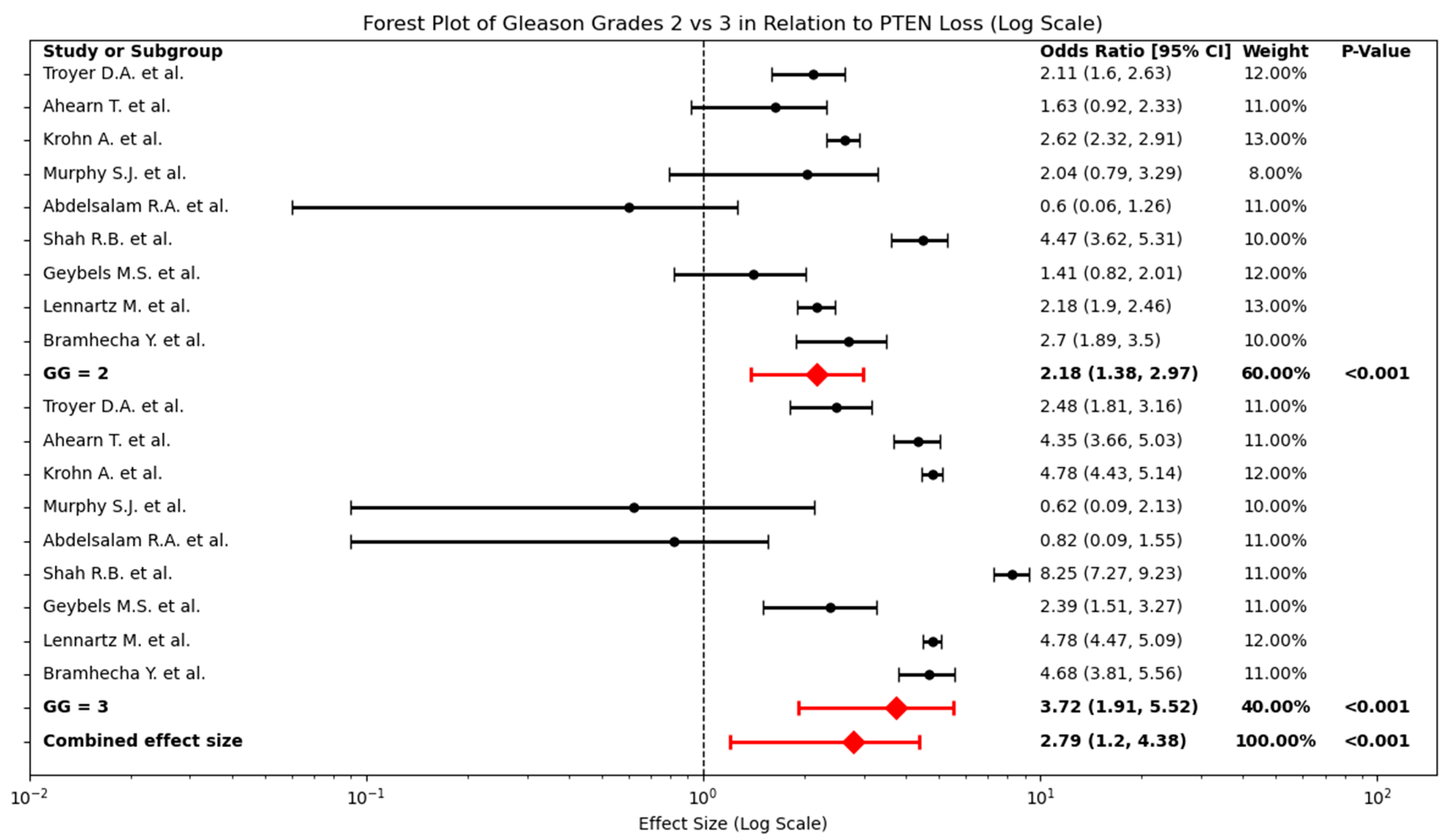
2.4. Relationship Between PTEN Loss and Intermediate Gleason Grades (2 and 3)
2.5. Relationship Between Hemizygous and Homozygous PTEN Loss in Relation to Gleason Grades
2.6. Clinical Outcomes and PTEN Loss: Results
3. Discussion
3.1. PTEN Loss and Gleason Grade
3.2. Homozygous vs. Hemizygous PTEN Loss
3.3. Clinical Outcomes and PTEN Loss: Discussion
3.4. PTEN as a Biomarker to Guide Focal Therapy and Adjuvant Radiation
3.5. Commercial Assays and PTEN Loss Assessment in Practice
3.6. Limitations
3.7. Future Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Underlying Features of Prostate Cancer—Statistics, Risk Factors, and Emerging Methods for Its Diagnosis [Internet]. Available online: https://www.mdpi.com/1718-7729/30/2/178 (accessed on 20 November 2024).
- Cancer Research UK [Internet]. Cancer Research UK. Available online: https://www.cancerresearchuk.org/home (accessed on 29 September 2024).
- Prostate Cancer Statistics|World Cancer Research Fund International [Internet]. WCRF International. Available online: https://www.wcrf.org/cancer-trends/prostate-cancer-statistics/ (accessed on 29 September 2024).
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Abdelsalam, R.A.; Khalifeh, I.; Box, A.; Kalantarian, M.; Ghosh, S.; Abou-Ouf, H.; Lotfi, T.; Shahait, M.; Palanisamy, N.; Bismar, T.A. Molecular characterization of prostate cancer in Middle Eastern population highlights differences with Western populations with prognostic implication. J. Cancer Res. Clin. Oncol. 2020, 146, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Imada, E.L.; Sanchez, D.F.; Dinalankara, W.; Vidotto, T.; Ebot, E.M.; Tyekucheva, S.; Franco, G.R.; Mucci, L.A.; Loda, M.; Schaeffer, E.M.; et al. Transcriptional landscape of PTEN loss in primary prostate cancer. BMC Cancer 2021, 21, 856. [Google Scholar] [CrossRef] [PubMed]
- Cyll, K.; Haug, E.S.; Pradhan, M.; Vlatkovic, L.; Carlsen, B.; Löffeler, S.; Kildal, W.; Skogstad, K.; Torkelsen, F.H.; Syvertsen, R.A.; et al. DNA ploidy and PTEN as biomarkers for predicting aggressive disease in prostate cancer patients under active surveillance. Br. J. Cancer 2024, 131, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Ullman, D.; Dorn, D.; Rais-Bahrami, S.; Gordetsky, J. Clinical Utility and Biologic Implications of Phosphatase and Tensin Homolog (PTEN) and ETS-related Gene (ERG) in Prostate Cancer. Urology 2018, 113, 59–70. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, H. Interplay Among PI3K/AKT, PTEN/FOXO and AR Signaling in Prostate Cancer. In Prostate Cancer: Cellular and Genetic Mechanisms of Disease Development and Progression; Dehm, S.M., Tindall, D.J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 319–331. [Google Scholar] [CrossRef]
- Correia, N.C.; Gírio, A.; Antunes, I.; Martins, L.R.; Barata, J.T. The multiple layers of non-genetic regulation of PTEN tumour suppressor activity. Eur. J. Cancer 2014, 50, 216–225. [Google Scholar] [CrossRef]
- Gong, L.; Govan, J.M.; Evans, E.B.; Dai, H.; Wang, E.; Lee, S.-W.; Lin, H.-K.; Lazar, A.J.; Mills, G.B.; Lin, S.-Y. Nuclear PTEN tumor-suppressor functions through maintaining heterochromatin structure. Cell Cycle 2015, 14, 2323–2332. [Google Scholar] [CrossRef]
- Uzoh, C.C.; Perks, C.M.; Bahl, A.; Holly, J.M.P.; Sugiono, M.; Persad, R.A. PTEN-mediated pathways and their association with treatment-resistant prostate cancer. BJU Int. 2009, 104, 556–561. [Google Scholar] [CrossRef]
- Ming, M.; He, Y.-Y. PTEN in DNA damage repair. Cancer Lett. 2012, 319, 125–129. [Google Scholar] [CrossRef]
- Armstrong, C.W.; Maxwell, P.J.; Ong, C.W.; Redmond, K.M.; McCann, C.; Neisen, J.; Ward, G.A.; Chessari, G.; Johnson, C.; Crawford, N.T.; et al. PTEN deficiency promotes macrophage infiltration and hypersensitivity of prostate cancer to IAP antagonist/radiation combination therapy. Oncotarget 2016, 7, 7885–7898. [Google Scholar] [CrossRef]
- Sachdeva, A.; Hart, C.A.; Kim, K.; Tawadros, T.; Oliveira, P.; Shanks, J.; Brown, M.; Clarke, N. Non-canonical EphA2 activation underpins PTEN-mediated metastatic migration and poor clinical outcome in prostate cancer. Br. J. Cancer 2022, 127, 1254–1262. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Lee, S.H.; Johnson, D.; Luong, R.; Sun, Z. Crosstalking between Androgen and PI3K/AKT Signaling Pathways in Prostate Cancer Cells. J. Biol. Chem. 2015, 290, 2759–2768. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Cunha, I.W.; Coudry, R.A.; Fonseca, F.P.; Torres, C.H.; Soares, F.A.; Squire, J.A. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br. J. Cancer 2007, 97, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Noh, B.-J.; Sung, J.-Y.; Kim, Y.W.; Chang, S.-G.; Park, Y.-K. Prognostic value of ERG, PTEN, CRISP3 and SPINK1 in predicting biochemical recurrence in prostate cancer. Oncol. Lett. 2016, 11, 3621–3630. [Google Scholar] [CrossRef] [PubMed]
- McCall, P.; Witton, C.J.; Grimsley, S.; Nielsen, K.V.; Edwards, J. Is PTEN loss associated with clinical outcome measures in human prostate cancer? Br. J. Cancer 2008, 99, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Mei, Y.; Sun, H.; Nie, Z.; Liu, X.; Wang, S. The association of Phosphatase and tensin homolog (PTEN) deletion and prostate cancer risk: A meta-analysis. Biomed. Pharmacother. 2016, 83, 114–121. [Google Scholar] [CrossRef]
- Guedes, L.B.; Tosoian, J.J.; Hicks, J.; Ross, A.E.; Lotan, T.L. PTEN Loss in Gleason Score 3 + 4 = 7 Prostate Biopsies is Associated with Nonorgan Confined Disease at Radical Prostatectomy. J. Urol. 2017, 197, 1054–1059. [Google Scholar] [CrossRef]
- Troyer, D.A.; Jamaspishvili, T.; Wei, W.; Feng, Z.; Good, J.; Hawley, S.; Fazli, L.; McKenney, J.K.; Simko, J.; Hurtado-Coll, A.; et al. A multicenter study shows PTEN deletion is strongly associated with seminal vesicle involvement and extracapsular extension in localized prostate cancer. Prostate 2015, 75, 1206–1215. [Google Scholar] [CrossRef]
- A Prospective Investigation of PTEN Loss and ERG Expression in Lethal Prostate Cancer—PubMed [Internet]. Available online: https://pubmed.ncbi.nlm.nih.gov/26615022/ (accessed on 17 November 2024).
- Bismar, T.A.; Hegazy, S.; Feng, Z.; Yu, D.; Donnelly, B.; Palanisamy, N.; Trock, B.J. Clinical utility of assessing PTEN and ERG protein expression in prostate cancer patients: A proposed method for risk stratification. J. Cancer Res. Clin. Oncol. 2018, 144, 2117–2125. [Google Scholar] [CrossRef]
- Krohn, A.; Diedler, T.; Burkhardt, L.; Mayer, P.-S.; De Silva, C.; Meyer-Kornblum, M.; Kötschau, D.; Tennstedt, P.; Huang, J.; Gerhäuser, C.; et al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am. J. Pathol. 2012, 181, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Jeldres, C.; Glaskova, L.; Friedman, C.; Schroeder, S.; Nelson, P.S.; Porter, C.; Fang, M. Identification of Combinatorial Genomic Abnormalities Associated with Prostate Cancer Early Recurrence. J. Mol. Diagn. 2016, 18, 215–224. [Google Scholar] [CrossRef][Green Version]
- Murphy, S.J.; Karnes, R.J.; Kosari, F.; Castellar, B.E.R.P.; Kipp, B.R.; Johnson, S.H.; Terra, S.; Harris, F.R.; Halling, G.C.; Klein, J.L.S.; et al. Integrated analysis of the genomic instability of PTEN in clinically insignificant and significant prostate cancer. Mod. Pathol. 2016, 29, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.A.; Gray, K.P.; Huang, Y.; Bowden, M.; Pomerantz, M.; Loda, M.; Sweeney, C.J. Loss of PTEN Expression Detected by Fluorescence Immunohistochemistry Predicts Lethal Prostate Cancer in Men Treated with Prostatectomy. Eur. Urol. Oncol. 2019, 2, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Lahdensuo, K.; Erickson, A.; Saarinen, I.; Seikkula, H.; Lundin, J.; Lundin, M.; Nordling, S.; Bützow, A.; Vasarainen, H.; Boström, P.J.; et al. Loss of PTEN expression in ERG-negative prostate cancer predicts secondary therapies and leads to shorter disease-specific survival time after radical prostatectomy. Mod. Pathol. 2016, 29, 1565–1574. [Google Scholar] [CrossRef]
- p16INK4a, PTEN, E-cadherin, Bcl-2 and Ki-67 Expression in Prostate Cancer: Its Relationship with the Metastatic Potential and Known Prognostic Factors. [Internet]. Available online: https://www.jpatholtm.org/journal/view.php?number=2885 (accessed on 17 November 2024).
- Cuzick, J.; Yang, Z.H.; Fisher, G.; Tikishvili, E.; Stone, S.; Lanchbury, J.S.; Camacho, N.; Merson, S.; Brewer, D.; Cooper, C.S.; et al. Prognostic value of PTEN loss in men with conservatively managed localised prostate cancer. Br. J. Cancer 2013, 108, 2582–2589. [Google Scholar] [CrossRef]
- Shah, R.B.; Shore, K.T.; Yoon, J.; Mendrinos, S.; McKenney, J.K.; Tian, W. PTEN loss in prostatic adenocarcinoma correlates with specific adverse histologic features (intraductal carcinoma, cribriform Gleason pattern 4 and stromogenic carcinoma). Prostate 2019, 79, 1267–1273. [Google Scholar] [CrossRef]
- Geybels, M.S.; Fang, M.; Wright, J.L.; Qu, X.; Bibikova, M.; Klotzle, B.; Fan, J.-B.; Feng, Z.; Ostrander, E.A.; Nelson, P.S.; et al. PTEN loss is associated with prostate cancer recurrence and alterations in tumor DNA methylation profiles. Oncotarget 2017, 8, 84338–84348. [Google Scholar] [CrossRef]
- Lennartz, M.; Minner, S.; Brasch, S.; Wittmann, H.; Paterna, L.; Angermeier, K.; Öztürk, E.; Shihada, R.; Ruge, M.; Kluth, M.; et al. The Combination of DNA Ploidy Status and PTEN/6q15 Deletions Provides Strong and Independent Prognostic Information in Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2802–2811. [Google Scholar] [CrossRef]
- Bramhecha, Y.M.; Rouzbeh, S.; Guérard, K.-P.; Scarlata, E.; Brimo, F.; Chevalier, S.; Hamel, L.; Aprikian, A.G.; Lapointe, J. The combination of PTEN deletion and 16p13.3 gain in prostate cancer provides additional prognostic information in patients treated with radical prostatectomy. Mod. Pathol. 2019, 32, 128–138. [Google Scholar] [CrossRef]
- Risk of Bias Tools—Robvis (Visualization Tool) [Internet]. Available online: https://www.riskofbias.info/welcome/robvis-visualization-tool (accessed on 21 August 2025).
- Reid, A.H.M.; Attard, G.; Ambroisine, L.; Fisher, G.; Kovacs, G.; Brewer, D.; Clark, J.; Flohr, P.; Edwards, S.; Berney, D.M.; et al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br. J. Cancer 2010, 102, 678–684. [Google Scholar] [CrossRef]
- Sircar, K.; Yoshimoto, M.; Monzon, F.A.; Koumakpayi, I.H.; Katz, R.L.; Khanna, A.; Alvarez, K.; Chen, G.; Darnel, A.D.; Aprikian, A.G.; et al. PTEN genomic deletion is associated with p-Akt and AR signalling in poorer outcome, hormone refractory prostate cancer. J. Pathol. 2009, 218, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Lotan, T.L.; Wei, W.; Ludkovski, O.; Morais, C.L.; Guedes, L.B.; Jamaspishvili, T.; Lopez, K.; Hawley, S.T.; Feng, Z.; Fazli, L.; et al. Analytic validation of a clinical-grade PTEN immunohistochemistry assay in prostate cancer by comparison with PTEN FISH. Mod. Pathol. 2016, 29, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, J.; Klevebring, D.; Liu, W.; Neiman, M.; Xu, J.; Wiklund, P.; Wiklund, F.; Mills, I.G.; Egevad, L.; Grönberg, H. Exome sequencing of prostate cancer supports the hypothesis of independent tumour origins. Eur. Urol. 2013, 63, 347–353. [Google Scholar] [CrossRef]
- Ferraldeschi, R.; Welti, J.; Powers, M.V.; Yuan, W.; Smyth, T.; Seed, G.; Riisnaes, R.; Hedayat, S.; Wang, H.; Crespo, M.; et al. Second-Generation HSP90 Inhibitor Onalespib Blocks mRNA Splicing of Androgen Receptor Variant 7 in Prostate Cancer Cells. Cancer Res. 2016, 76, 2731–2742. [Google Scholar] [CrossRef]
- Tosoian, J.J.; Guedes, L.B.; Morais, C.L.; Mamawala, M.; Ross, A.E.; De Marzo, A.M.; Trock, B.J.; Han, M.; Carter, H.B.; Lotan, T.L. PTEN Status Assessment in the Johns Hopkins Active Surveillance Cohort. Prostate Cancer Prostatic Dis. Prostate Cancer Prostatic Dis. 2019, 22, 176–181. [Google Scholar] [CrossRef]
- E Ross, A.; D’AMico, A.V.; Freedland, S.J. Which, when and why? Rational use of tissue-based molecular testing in localized prostate cancer. Prostate Cancer Prostatic Dis. 2016, 19, 1–6. [Google Scholar] [CrossRef]
- Lee, E.; Oliveira, L.D.; Dairo, O.; Abadchi, S.N.; Cha, E.; Mendes, A.A.; Wang, J.H.; Song, D.Y.; Lotan, T.L. PTEN Loss Is Associated with Adverse Outcomes in the Setting of Salvage Radiation Therapy. Eur. Urol. Oncol. 2024, 7, 1513–1519. [Google Scholar] [CrossRef]
- Ong, C.W.; Maxwell, P.; A Alvi, M.; McQuaid, S.; Waugh, D.; Mills, I.; Salto-Tellez, M.; Alvi, A. A gene signature associated with PTEN activation defines good prognosis intermediate risk prostate cancer cases. J. Pathol. Clin. Res. 2018, 4, 103–113. [Google Scholar] [CrossRef]
- Den, R.B.; Santiago-Jimenez, M.; Alter, J.; Schliekelman, M.; Wagner, J.R.; Renzulli, J.F., II; Lee, D.I.; Brito, C.G.; Monahan, K.; Gburek, B.; et al. Decipher correlation patterns post prostatectomy: Initial experience from 2 342 prospective patients. Prostate Cancer Prostatic Dis. 2016, 19, 374–379. [Google Scholar] [CrossRef]
- Anu, R.I.; Shamsudeen, S.; Leslie, S.W. Prostate Cancer Tissue-Based Biomarkers. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK587345/ (accessed on 25 August 2025).
- Gene Expression Profiling and Protein Biomarkers for Prostate Cancer—CAM 241HB [Internet]. 2025. Available online: https://www.southcarolinablues.com/web/public/brands/medicalpolicyhb/external-policies/gene-expression-profiling-and-protein-biomarkers-for-prostate-cancer/#:~:text=imaging,NCCN%2C%202023a (accessed on 29 July 2025).
- Lotan, T.L.; Gurel, B.; Sutcliffe, S.; Esopi, D.; Liu, W.; Xu, J.; Hicks, J.L.; Park, B.H.; Humphreys, E.; Partin, A.W.; et al. PTEN Protein Loss by Immunostaining: Analytic Validation and Prognostic Indicator for a High Risk Surgical Cohort of Prostate Cancer Patients. Clin. Cancer Res. 2011, 17, 6563–6573. [Google Scholar] [CrossRef]
- Ferguson, D.; Kroeger-Lui, N.; Dreisbach, D.; Hart, C.A.; Sanchez, D.F.; Oliveira, P.; Brown, M.; Clarke, N.; Sachdeva, A.; Gardner, P. Full fingerprint hyperspectral imaging of prostate cancer tissue microarrays within clinical timeframes using quantum cascade laser microscopy. Anal. 2025, 150, 1741–1753. [Google Scholar] [CrossRef]
- Ferguson, D.; Sachdeva, A.; Hart, C.A.; Sanchez, D.F.; Oliveira, P.; Brown, M.; Clarke, N.; Gardner, P.; Alfano, R.R.; Seddon, A.B.; et al. Identification of at-risk prostate cancer patients using Fourier transform infrared spectroscopy and machine learning. In Optical Biopsy XXIII: Toward Real-Time Spectroscopic Imaging and Diagnosis; SPIE: Bellingham, WA, USA, 2025; pp. 4–11. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/13311/1331103/Identification-of-at-risk-prostate-cancer-patients-using-Fourier-transform/10.1117/12.3048498.full (accessed on 29 July 2025).


| Study ID | PTEN Assessment Method | GG | Undeleted, n | Hemi- Deletion, n | Homo- Deletion, n | Complete PTEN Loss, n |
|---|---|---|---|---|---|---|
| Troyer D.A. et al. (2015) [23] | FISH | <1 | 216 | 19 | 8 | 27 |
| 2 | 178 | 21 | 26 | 47 | ||
| 3 | 58 | 6 | 12 | 18 | ||
| ≥4 | 43 | 8 | 11 | 19 | ||
| Ahearn T. et al. (2015) [24] | IHC | <1 | 173 | 11 | ||
| 2 | 338 | 35 | ||||
| 3 | 199 | 55 | ||||
| ≥4 | 168 | 65 | ||||
| Bismar T.A. et al. (2018) [25] | IHC | <1 | 120 | 15 | ||
| 2 | 50 | 29 | ||||
| 3 | ||||||
| ≥4 | 103 | 123 | ||||
| Krohn A. et al. (2012) [26] | FISH | <1 | 647 | 32 | 36 | 68 |
| 2 | 815 | 89 | 135 | 224 | ||
| 3 | 185 | 33 | 60 | 93 | ||
| ≥4 | 63 | 14 | 20 | 34 | ||
| Qu X. et al. (2016) [27] | FISH | <1 | 63 | 14 | ||
| 2 | 44 | 4 | ||||
| 3 | ||||||
| ≥4 | 11 | 9 | ||||
| Murphy S.J. et al. (2016) [28] | FISH and IHC | <1 | 35 | 6 | 4 | 10 |
| 2 | 12 | 5 | 2 | 7 | ||
| 3 | 17 | 2 | 1 | 3 | ||
| ≥4 | 14 | 10 | 9 | 19 | ||
| Hamid A.A. et al. (2019) [29] | FISH | <1 | 28 | 7 | ||
| 2 | 38 | 10 | ||||
| 3 | ||||||
| ≥4 | 17 | 9 | ||||
| Lahdensuo K. et al. (2016) [30] | IHC | <1 | 243 | 18 | ||
| 2 | 337 | 67 | ||||
| 3 | ||||||
| ≥4 | 100 | 50 | ||||
| Abdelsalam R.A. et al. (2020) [5] | IHC | <1 | 35 | 27 | ||
| 2 | 69 | 32 | ||||
| 3 | 38 | 24 | ||||
| ≥4 | 27 | 7 | ||||
| Park S.J. et al. (2010) [31] | IHC | <1 | 8 | 12 | ||
| 2 | 7 | 18 | ||||
| 3 | ||||||
| ≥4 | 10 | 50 | ||||
| Noh B.-J. et al. (2016) [19] | IHC | <1 | 8 | 11 | ||
| 2 | 7 | 29 | ||||
| 3 | ||||||
| ≥4 | 1 | 12 | ||||
| Cuzick J. et al. (2013) [32] | FISH and IHC | <1 | 317 | 10 | ||
| 2 | 144 | 37 | ||||
| 3 | ||||||
| ≥4 | 95 | 72 | ||||
| Shah R.B. et al. (2019) [33] | IHC | <1 | 63 | 8 | ||
| 2 | 67 | 38 | ||||
| 3 | 21 | 22 | ||||
| ≥4 | 21 | 20 | ||||
| Geybels M.S. et al. (2017) [34] | IHC | <1 | 207 | 21 | 5 | 26 |
| 2 | 141 | 13 | 12 | 25 | ||
| 3 | 30 | 6 | 3 | 9 | ||
| ≥4 | 25 | 7 | 1 | 8 | ||
| Lennartz M. et al. (2016) [35] | IHC and FISH | <1 | 716 | 64 | ||
| 2 | 1840 | 359 | ||||
| 3 | 454 | 194 | ||||
| ≥4 | 150 | 68 | ||||
| Bramhecha Y.M. et al. (2019) [36] | IHC and FISH | <1 | 52 | 9 | ||
| 2 | 90 | 42 | ||||
| 3 | 37 | 30 | ||||
| ≥4 | 11 | 16 |
| Study ID | Outcome Type | HR (95% CI) | p-Value | Adjustments | Additional Findings |
|---|---|---|---|---|---|
| Troyer D.A. et al. (2015) [23] | RFS | 1.64 (1.13–2.37) | 0.009 | Preoperative PSA, seminal vesicle invasion | PTEN homozygous deletion strongly linked to shorter RFS |
| RFS | 1.28 (0.84–1.95) | 0.25 | Preoperative PSA, seminal vesicle invasion | Hemizygous deletion not significantly associated with RFS | |
| Ahearn T. et al. (2015) [24] | LP | 1.8 (1.2–2.9) | Not specified | age, BMI, Gleason grade, and TNM stage | Complete PTEN loss associated with lethal progression |
| LP (ERG-negative) | 3.1 (1.7–5.7) | Not specified | Gleason grade and clinical stage | PTEN loss in ERG-negative cases shows strong association | |
| Bismar T.A. et al. (2018) [25] | CSS | 0.27 (0.18–0.42) | <0.0001 | Gleason score, age | PTEN positivity significantly associated with improved CSS, strongest in non-ADT-treated cohort |
| CSS | 0.25 (0.16–0.39) | <0.0001 | Gleason score, age | Reduced CSS risk for weak/moderate PTEN intensity | |
| CSS | 0.43 (0.20–0.92) | 0.029 | Gleason score, age | High PTEN intensity associated with improved survival in multivariable model | |
| Krohn A. et al. (2012) [26] | RFS | 1.3 (1.05–1.60) | 0.0158 | Gleason grade, preoperative PSA level, pT stage | PTEN deletion independently predicts worse RFS |
| Qu X. et al. (2016) [27] | BCR | H3.58 (1.39–9.22) | 0.008 | Gleason grade, tumour stage, PSA | PTEN deletion significantly increases risk of BCR following radical prostatectomy |
| Murphy S.J. et al. (2016) [28] | BCR | HR not reported; recurrence rates reported instead. | Not specified | Not provided | PTEN deletion observed in ~60% of BCR cases, with ~80% recurrence in Gleason 7+ cases with PTEN loss |
| Hamid A.A. et al. (2019) [29] | MFS | 1.49 (1.14–1.92) | <0.003 | age, Gleason grade, and stage | Low PTEN expression strongly associated with metastasis in both continuous and dichotomous models |
| OS | 1.89 (1.37–2.63) | <0.001 | Adjusted for age, Gleason grade, and stage | Lower PTEN expression linked to poorer overall survival outcomes | |
| Lahdensuo K. et al. (2016) [30] | DSS | 2.156 (1.169–3.976) | 0.014 | Univariate analysis | PTEN loss significantly associated with shorter DSS, especially in ERG-negative cancers |
| Secondary therapy-free survival | 2.782 (1.846–4.193) | <0.001 | Adjusted for ERG/PTEN combined status | Higher likelihood of requiring secondary therapy post-radical prostatectomy with PTEN loss | |
| Abdelsalam R.A. et al. (2020) [5] | BCR | OR 2.68 (0.98–7.33) | 0.05 | Adjusted for Gleason grade, path stage, surgical margin | PTEN-negative and ERG-positive cases show increased BCR risk |
| Park S.J. et al. (2010) [31] | PCa progression | HR not reported | 0.019 | None reported | Loss of PTEN expression significantly associated with elevated PSA levels, indicative of progression risk |
| Noh B.-J. et al. (2016) [19] | High-risk group (Low PTEN, High CRISP3) | 9.979 (1.244–80.031) | 0.03 | Adjusted for subgroup risk (high-risk vs. low-risk). Low risk: Low PTEN and low CRISP3, high PTEN and low CRISP3, and high PTEN and high CRISP3 expression. High risk: Low PTEN and high CRISP3 expression | Low PTEN combined with high CRISP3 strongly associated with increased risk in cancer progression |
| Cuzick J. et al. (2013) [32] | PCa specific mortality | 3.51 (2.60–4.73) | <0.0001 | None specified in univariate; Adjusted in multivariate for Gleason score, PSA, and Ki-67 score | PTEN loss significantly predicts prostate cancer-specific mortality in low-risk patients |
| Shah R.B. et al. (2019) [33] | Intraductal carcinoma (IDC-P) | RR 4.993 (3.451–7.223) | <0.001 | None | PTEN loss significantly associated with IDC-P, which shows the highest relative risk |
| Cribriform Gleason Pattern 4 | RR 2.459 (1.814–3.333) | <0.001 | None | Strong association between PTEN loss and cribriform pattern, indicating poor prognosis | |
| Stromogenic PCa | RR 2.255 (1.634–3.112) | <0.001 | None | PTEN loss linked with stromogenic PCa, a distinct morphological feature associated with worse outcomes | |
| Geybels M.S. et al. (2017) [34] | RFS | 1.39 (0.73–2.64) | <0.05 | None specified | Hemizygous PTEN loss not significantly associated |
| RFS | 2.84 (1.30–6.19) | <0.06 | None specified | Homozygous PTEN loss associated with increased recurrence risk | |
| RFS | 1.74 (1.03–2.93) | <0.07 | None specified | Any PTEN loss (hemi/homozygous) linked to a higher overall recurrence rate compared to PTEN intact cases | |
| Lennartz M. et al. (2016) [35] | RFS | 1.5 (1.3–1.8) for Intermediate vs. Low, 1.4 (1.2–1.7) for High vs. Intermediate | <0.0001 | Multivariate adjustments including Gleason grade, pT stage, resection margin, and PSA levels | 6q15 deletions and PTEN alterations significantly associated with poorer prognosis |
| Bramhecha Y.M. et al. (2019) [36] | BCR | 3.00 (1.81–4.98) for PTEN deletion alone; 4.70 (2.12–10.42) for combined PTEN deletion and 16p13.3 gain | <0.0001 | Adjusted for CAPRA-S score | PTEN deletion and 16p13.3 gain together strongly predict worse BCR, enhancing CAPRA-S-based stratification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisiel, F.; Ferguson, D.; Hart, C.; Brown, M.; Oliveira, P.; Sachdeva, A.; Gardner, P. Prognostic Significance of PTEN Loss in Prostate Cancer: A Meta-Analysis of Gleason Grade and Clinical Outcomes. Cancers 2025, 17, 2862. https://doi.org/10.3390/cancers17172862
Kisiel F, Ferguson D, Hart C, Brown M, Oliveira P, Sachdeva A, Gardner P. Prognostic Significance of PTEN Loss in Prostate Cancer: A Meta-Analysis of Gleason Grade and Clinical Outcomes. Cancers. 2025; 17(17):2862. https://doi.org/10.3390/cancers17172862
Chicago/Turabian StyleKisiel, Filip, Dougal Ferguson, Claire Hart, Mick Brown, Pedro Oliveira, Ashwin Sachdeva, and Peter Gardner. 2025. "Prognostic Significance of PTEN Loss in Prostate Cancer: A Meta-Analysis of Gleason Grade and Clinical Outcomes" Cancers 17, no. 17: 2862. https://doi.org/10.3390/cancers17172862
APA StyleKisiel, F., Ferguson, D., Hart, C., Brown, M., Oliveira, P., Sachdeva, A., & Gardner, P. (2025). Prognostic Significance of PTEN Loss in Prostate Cancer: A Meta-Analysis of Gleason Grade and Clinical Outcomes. Cancers, 17(17), 2862. https://doi.org/10.3390/cancers17172862






