Oncological Efficacy and Safety of Minimally Invasive Focal and Whole-Gland Interventions in the Treatment of Low- and Intermediate-Risk Prostate Cancer: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Search
2.2. Eligibility Criteria
2.3. Study Selection Process
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
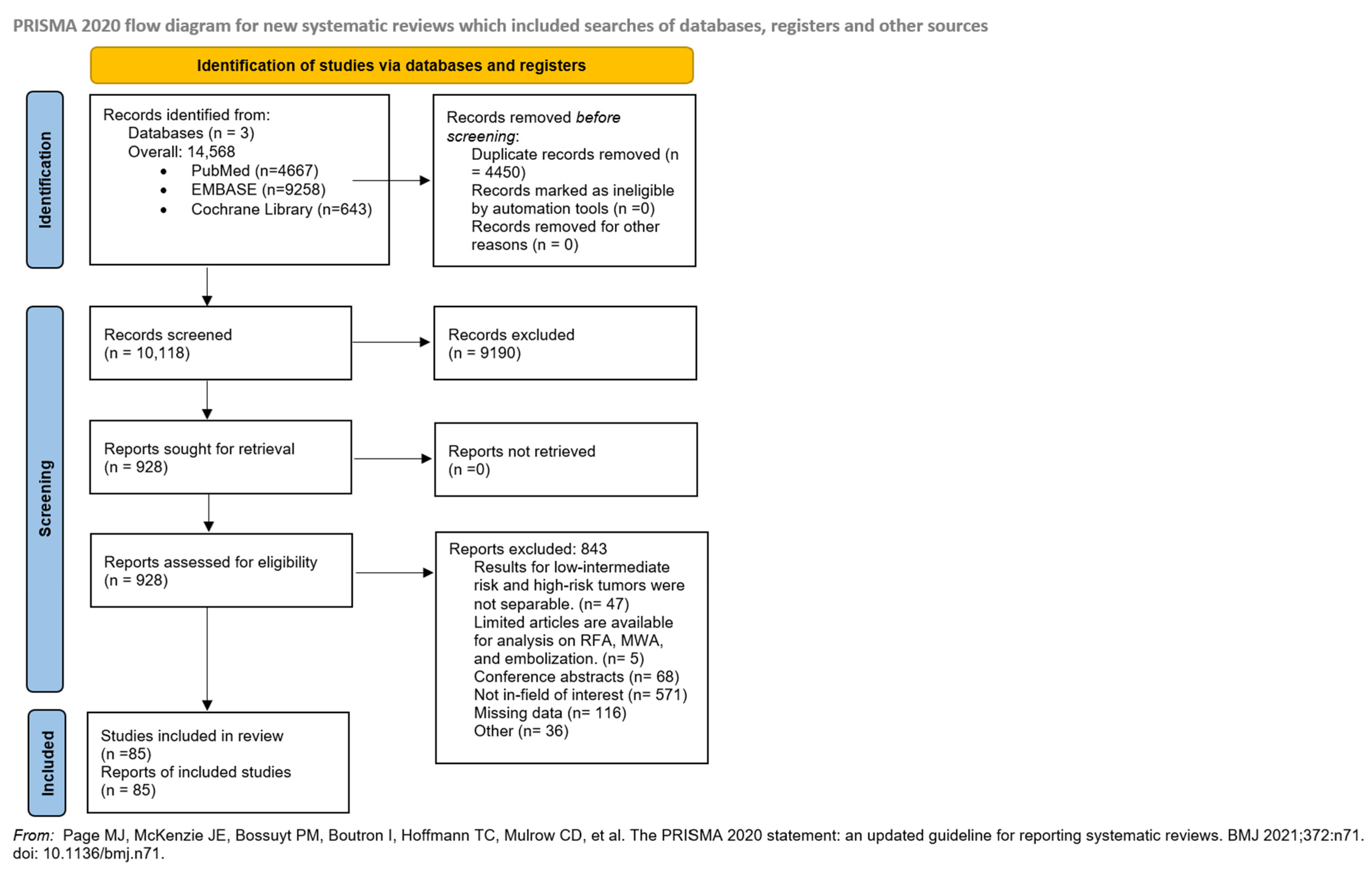
3.1. Search and Selection
3.2. Basic Characteristics of Included Studies
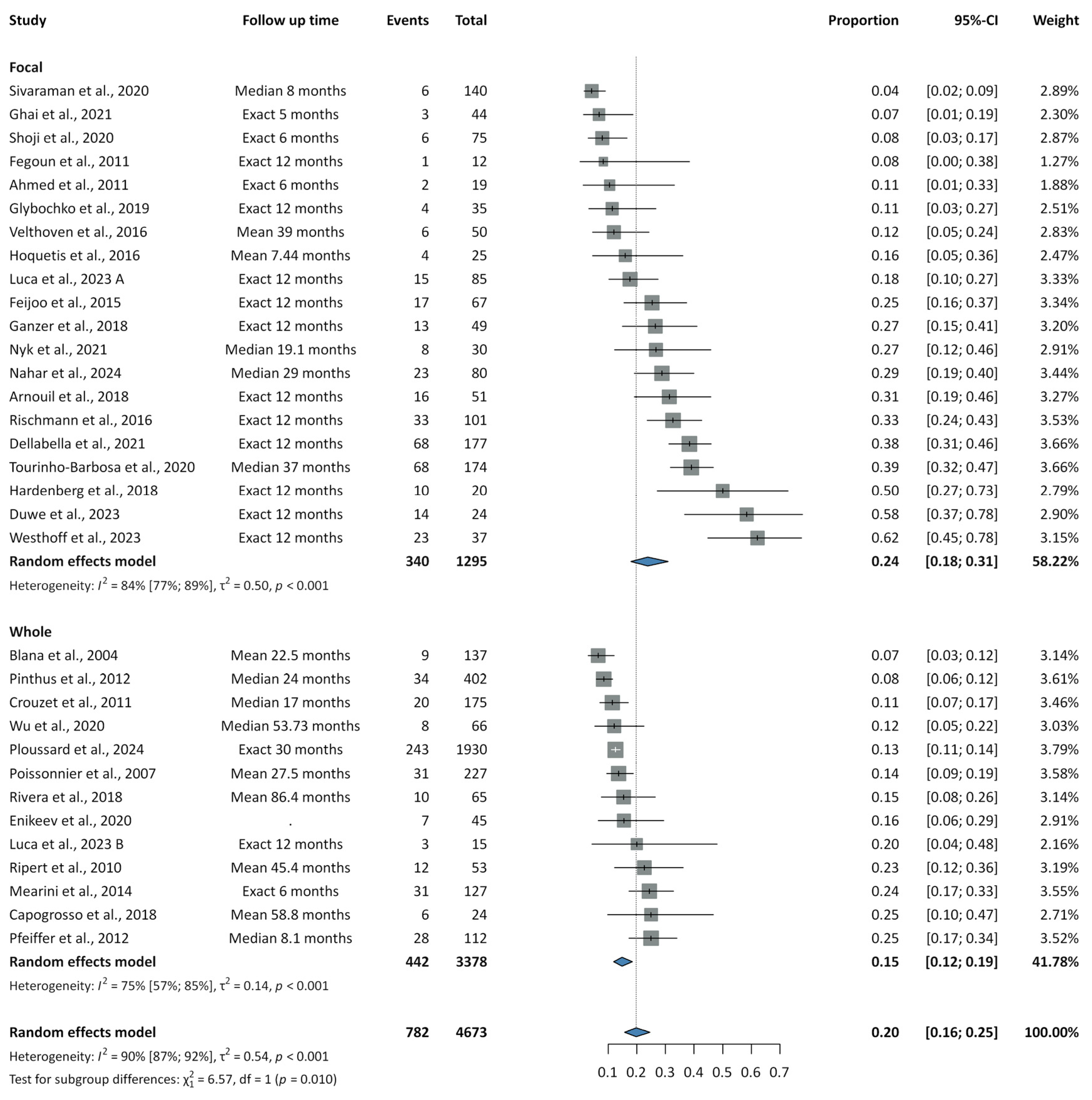
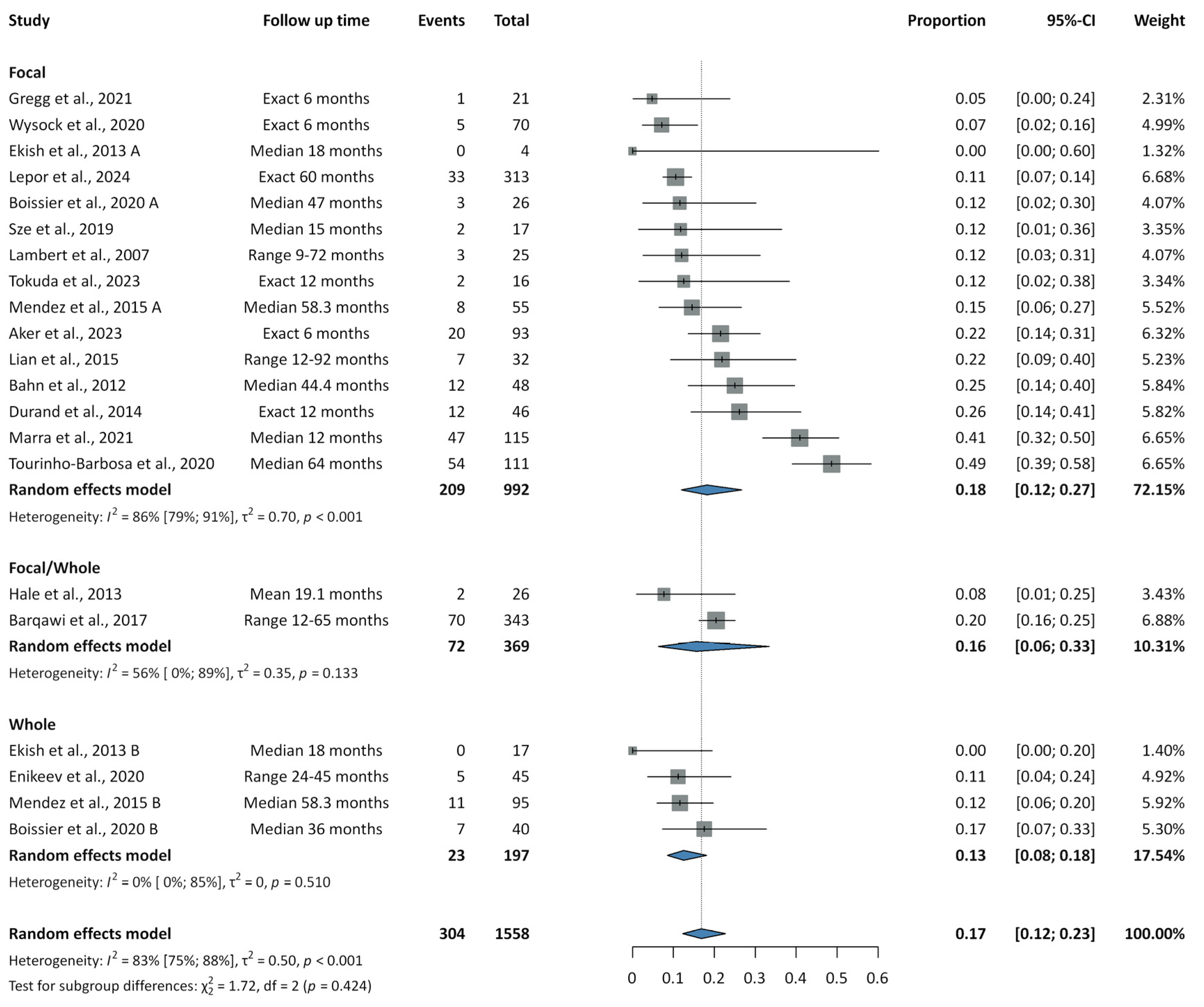
3.3. Recurrence Rates
3.4. Complications
3.5. Survival Outcomes: OS, CSS, and MFS
3.6. Biochemical Outcomes
3.7. Functional Outcomes
3.8. Retreatment
3.9. Risk of Bias Assessment
3.10. Publication Bias and Heterogeneity
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Practice and Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AS | Active surveillance |
| BFRS | Biochemical recurrence-free survival |
| CI | Confidence interval |
| CSS | Cancer-specific survival |
| FFS | Failure-free survival |
| FT | Focal therapy |
| HIFU | High-intensity focused ultrasound |
| IRE | Irreversible electroporation |
| ISUP | International Society of Urological Pathology |
| MFS | Metastasis-free survival |
| MRI | Magnetic resonance imaging |
| NA | Not available |
| NCCN | National Comprehensive Cancer Network |
| OR | Odds ratio |
| OS | Overall survival |
| PCa | Prostate cancer |
| PICO | Population, Intervention, Comparison, Outcome |
| PI | Prediction interval |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PSA | Prostate-specific antigen |
| RCT | Randomized controlled trials |
| ROBINS-I | Risk of Bias In Non-randomized Studies of Interventions |
| RoB 2 | Risk of Bias 2 |
| RP | Radical prostatectomy |
| RT | Radiotherapy |
| TNM | Tumor Node Metastasis |
Appendix A. Risk of Bias Assessment Methodology Regarding Prospective and Retrospective Non-Randomized Cohort and Registry Studies (ROBINS-I Tool)
Appendix B. Risk of Bias Assessment Methodology Regarding Randomized Controlled Trials of Interventions (RoB2 Tool)
References
- Bell, K.J.; Del Mar, C.; Wright, G.; Dickinson, J.; Glasziou, P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer 2015, 137, 1749–1757. [Google Scholar] [CrossRef]
- Bruinsma, S.M.; Roobol, M.J.; Carroll, P.R.; Klotz, L.; Pickles, T.; Moore, C.M.; Gnanapragasam, V.J.; Villers, A.; Rannikko, A.; Valdagni, R.; et al. Expert consensus document: Semantics in active surveillance for men with localized prostate cancer—Results of a modified Delphi consensus procedure. Nat. Rev. Urol. 2017, 14, 312–322. [Google Scholar] [CrossRef]
- Remmers, S.; Bangma, C.H.; Godtman, R.A.; Carlsson, S.V.; Auvinen, A.; Tammela, T.L.J.; Denis, L.J.; Nelen, V.; Villers, A.; Rebillard, X.; et al. Relationship Between Baseline Prostate-specific Antigen on Cancer Detection and Prostate Cancer Death: Long-term Follow-up from the European Randomized Study of Screening for Prostate Cancer. Eur. Urol. 2023, 84, 503–509. [Google Scholar] [CrossRef]
- Hugosson, J.; Roobol, M.J.; Månsson, M.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Tosoian, J.J.; Mamawala, M.; Epstein, J.I.; Landis, P.; Wolf, S.; Trock, B.J.; Carter, H.B. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J. Clin. Oncol. 2015, 33, 3379–3385. [Google Scholar] [CrossRef]
- Loeb, S.; Bruinsma, S.M.; Nicholson, J.; Briganti, A.; Pickles, T.; Kakehi, Y.; Carlsson, S.V.; Roobol, M.J. Active surveillance for prostate cancer: A systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur. Urol. 2015, 67, 619–626. [Google Scholar] [CrossRef]
- Thomsen, F.B.; Brasso, K.; Klotz, L.H.; Røder, M.A.; Berg, K.D.; Iversen, P. Active surveillance for clinically localized prostate cancer—A systematic review. J. Surg. Oncol. 2014, 109, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L. Overdiagnosis in urologic cancer: For World Journal of Urology Symposium on active surveillance in prostate and renal cancer. World J. Urol. 2022, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fanshawe, J.B.; Wai-Shun Chan, V.; Asif, A.; Ng, A.; Van Hemelrijck, M.; Cathcart, P.; Challacombe, B.; Brown, C.; Popert, R.; Elhage, O.; et al. Decision Regret in Patients with Localised Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2023, 6, 456–466. [Google Scholar] [CrossRef] [PubMed]
- van der Poel, H.G.; van den Bergh, R.C.N.; Briers, E.; Cornford, P.; Govorov, A.; Henry, A.M.; Lam, T.B.; Mason, M.D.; Rouvière, O.; De Santis, M.; et al. Focal Therapy in Primary Localised Prostate Cancer: The European Association of Urology Position in 2018. Eur. Urol. 2018, 74, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Lin-Brande, M.; Isharwal, S. Primary Focal Therapy for Localized Prostate Cancer: A Review of the Literature. Oncology 2021, 35, 261–268. [Google Scholar] [CrossRef]
- Hopstaken, J.S.; Bomers, J.G.R.; Sedelaar, M.J.P.; Valerio, M.; Fütterer, J.J.; Rovers, M.M. An Updated Systematic Review on Focal Therapy in Localized Prostate Cancer: What Has Changed over the Past 5 Years? Eur. Urol. 2022, 81, 5–33. [Google Scholar] [CrossRef]
- Deivasigamani, S.; Kotamarti, S.; Rastinehad, A.R.; Salas, R.S.; de la Rosette, J.; Lepor, H.; Pinto, P.; Ahmed, H.U.; Gill, I.; Klotz, L.; et al. Primary Whole-gland Ablation for the Treatment of Clinically Localized Prostate Cancer: A Focal Therapy Society Best Practice Statement. Eur. Urol. 2023, 84, 547–560. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hartung, J.; Knapp, G. On tests of the overall treatment effect in meta-analysis with normally distributed responses. Stat. Med. 2001, 20, 1771–1782. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D. Doing Meta-Analysis with R: A Hands-On Guide; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Shi, J.; Luo, D.; Weng, H.; Zeng, X.T.; Lin, L.; Chu, H.; Tong, T. Optimally estimating the sample standard deviation from the five-number summary. Res. Synth. Methods 2020, 11, 641–654. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- de la Rosette, J.; Dominguez-Escrig, J.; Zhang, K.; Teoh, J.; Barret, E.; Ramon-Borja, J.C.; Muir, G.; Bohr, J.; de Reijke, T.; Ng, C.F.; et al. A Multicenter, Randomized, Single-blind, 2-Arm Intervention Study Evaluating the Adverse Events and Quality of Life After Irreversible Electroporation for the Ablation of Localized Low-intermediate Risk Prostate Cancer. J. Urol. 2023, 209, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Collettini, F.; Enders, J.; Stephan, C.; Fischer, T.; Baur, A.D.J.; Penzkofer, T.; Busch, J.; Hamm, B.; Gebauer, B. Image-guided Irreversible Electroporation of Localized Prostate Cancer: Functional and Oncologic Outcomes. Radiology 2019, 292, 250–257. [Google Scholar] [CrossRef]
- Wang, H.; Xue, W.; Yan, W.; Yin, L.; Dong, B.; He, B.; Yu, Y.; Shi, W.; Zhou, Z.; Lin, H.; et al. Extended Focal Ablation of Localized Prostate Cancer With High-Frequency Irreversible Electroporation: A Nonrandomized Controlled Trial. JAMA Surg. 2022, 157, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Yoon, C.E.; Kwon, H.J.; Moon, H.W.; Park, Y.H.; Cho, H.J.; Ha, U.S.; Hong, S.H.; Park, S.Y.; Ha, S.; et al. Irreversible electroporation for prostate cancer using PSMA PET-CT. Prostate Int. 2023, 11, 40–45. [Google Scholar] [CrossRef]
- Giganti, F.; Stabile, A.; Giona, S.; Marenco, J.; Orczyk, C.; Moore, C.M.; Allen, C.; Kirkham, A.; Emberton, M.; Punwani, S. Prostate cancer treated with irreversible electroporation: MRI-based volumetric analysis and oncological outcome. Magn. Reson. Imaging 2019, 58, 143–147. [Google Scholar] [CrossRef]
- Blazevski, A.; Scheltema, M.J.; Yuen, B.; Masand, N.; Nguyen, T.V.; Delprado, W.; Shnier, R.; Haynes, A.M.; Cusick, T.; Thompson, J.; et al. Oncological and Quality-of-life Outcomes Following Focal Irreversible Electroporation as Primary Treatment for Localised Prostate Cancer: A Biopsy-monitored Prospective Cohort. Eur. Urol. Oncol. 2020, 3, 283–290. [Google Scholar] [CrossRef]
- Popeneciu, I.V.; Mohr, M.N.; Strauß, A.; Leitsmann, C.; Trojan, L.; Reichert, M. Personalized Treatment Strategy in "Low-Risk Prostate Cancer Active Surveillance Candidates" Using Irreversible Electroporation: Prospective Evaluation of Feasibility, Morbidity, Functional and Oncological Outcomes. World J. Mens. Health 2024, 42, 821–829. [Google Scholar] [CrossRef]
- Yaxley, W.J.; Gianduzzo, T.; Kua, B.; Oxford, R.; Yaxley, J.W. Focal therapy for prostate cancer with irreversible electroporation: Oncological and functional results of a single institution study. Investig. Clin. Urol. 2022, 63, 285–293. [Google Scholar] [CrossRef]
- Altan, Ş.A.; Güleryüz Kızıl, P.; Tarhan, N.; Adsan, O. One-year Follow-up Results of Transperineal Biopsy For Patients Undergoing Irreversible Electroporation Treatment in Localized Prostate Cancer. Urol. Res. Pract. 2023, 49, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.S.; Ehdaie, B.; Musser, J.; Mashni, J.; Srimathveeravalli, G.; Durack, J.C.; Solomon, S.B.; Coleman, J.A. Pilot Study to Assess Safety and Clinical Outcomes of Irreversible Electroporation for Partial Gland Ablation in Men with Prostate Cancer. J. Urol. 2016, 196, 883–890. [Google Scholar] [CrossRef]
- Ting, F.; Tran, M.; Böhm, M.; Siriwardana, A.; Van Leeuwen, P.J.; Haynes, A.M.; Delprado, W.; Shnier, R.; Stricker, P.D. Focal irreversible electroporation for prostate cancer: Functional outcomes and short-term oncological control. Prostate Cancer Prostatic Dis. 2016, 19, 46–52. [Google Scholar] [CrossRef]
- Valerio, M.; Dickinson, L.; Ali, A.; Ramachadran, N.; Donaldson, I.; McCartan, N.; Freeman, A.; Ahmed, H.U.; Emberton, M. Nanoknife Electroporation Ablation Trial: A Prospective Development Study Investigating Focal Irreversible Electroporation for Localized Prostate Cancer. J. Urol. 2017, 197, 647–654. [Google Scholar] [CrossRef]
- van den Bos, W.; Scheltema, M.J.; Siriwardana, A.R.; Kalsbeek, A.M.F.; Thompson, J.E.; Ting, F.; Böhm, M.; Haynes, A.M.; Shnier, R.; Delprado, W.; et al. Focal irreversible electroporation as primary treatment for localized prostate cancer. BJU Int. 2018, 121, 716–724. [Google Scholar] [CrossRef] [PubMed]
- López, B.M.; Boville, G.A.; Bernardos, G.B.; Marckert, X.A.; Roca, M.T.; Huerta, L.L.; Aubá, F.V.; Chillón, F.R.d.F.; Ortega, J.S.; Muela, M.A.; et al. Focal Therapy of Prostate Cancer Index Lesion With Irreversible Electroporation. A Prospective Study With a Median Follow-up of 3 Years. J. Urol. 2023, 209, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, B.; Yamada, K.; Takahata, A.; Fujihara, A.; Iwata, T.; Ukimura, O.; Yamada, K. Time-course changes in multiparametric magnetic resonance imaging following focal cryotherapy for localized prostate cancer: Initial experience. Eur. J. Radiol. 2023, 160, 110714. [Google Scholar] [CrossRef]
- Wysock, J.S.; Becher, E.; Gogaj, R.; Velazquez, N.; Lepor, H. Early oncological control following partial gland cryo-ablation: A prospective experience specifying reflex MRI guided biopsy of the ablation zone. Prostate Cancer Prostatic Dis. 2021, 24, 114–119. [Google Scholar] [CrossRef]
- Sze, C.; Tsivian, E.; Tay, K.J.; Schulman, A.A.; Davis, L.G.; Gupta, R.T.; Polascik, T.J. Anterior gland focal cryoablation: Proof-of-concept primary prostate cancer treatment in select men with localized anterior cancers detected by multi-parametric magnetic resonance imaging. BMC Urol. 2019, 19, 127. [Google Scholar] [CrossRef]
- Kim, F.J.; Cerqueira, M.A.; Almeida, J.C.; Pompeo, A.; Sehrt, D.; Calheiros, J.M.; Martins, F.A.; Molina, W.R. Initial brazilian experience in the treatment of localized prostate cancer using a new generation cryotechnology: Feasibility study. Int. Braz. J. Urol. 2012, 38, 620–626. [Google Scholar] [CrossRef][Green Version]
- Bahn, D.; de Castro Abreu, A.L.; Gill, I.S.; Hung, A.J.; Silverman, P.; Gross, M.E.; Lieskovsky, G.; Ukimura, O. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur. Urol. 2012, 62, 55–63. [Google Scholar] [CrossRef]
- Barqawi, A.B.; Huebner, E.; Krughoff, K.; O’Donnell, C.I. Prospective Outcome Analysis of the Safety and Efficacy of Partial and Complete Cryoablation in Organ-confined Prostate Cancer. Urology 2018, 112, 126–131. [Google Scholar] [CrossRef]
- El Hayek, O.R.; Alfer, W., Jr.; Reggio, E.; Pompeo, A.C.; Arap, S.; Lucon, A.M.; Srougi, M. Prostate cryoablation: Prospective analysis comparing high- and low-risk prostate cancer outcomes. Urol. Int. 2008, 81, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Gregg, J.R.; Borregales, L.D.; Choi, H.; Lozano, M.; McRae, S.E.; Venkatesan, A.M.; Davis, J.W.; Nogueras-Gonzalez, G.M.; Pisters, L.L.; Ward, J.F. Prospective trial of regional (hockey-stick) prostate cryoablation: Oncologic and quality of life outcomes. World J. Urol. 2021, 39, 3259–3264. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.H.; Bolte, K.; Masson, P.; Katz, A.E. Focal cryosurgery: Encouraging health outcomes for unifocal prostate cancer. Urology 2007, 69, 1117–1120. [Google Scholar] [CrossRef]
- Lian, H.; Zhuang, J.; Yang, R.; Qu, F.; Wang, W.; Lin, T.; Guo, H. Focal cryoablation for unilateral low-intermediate-risk prostate cancer: 63-month mean follow-up results of 41 patients. Int. Urol. Nephrol. 2016, 48, 85–90. [Google Scholar] [CrossRef]
- Mendez, M.H.; Passoni, N.M.; Pow-Sang, J.; Jones, J.S.; Polascik, T.J. Comparison of Outcomes Between Preoperatively Potent Men Treated with Focal Versus Whole Gland Cryotherapy in a Matched Population. J. Endourol. 2015, 29, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Marra, G.; Soeterik, T.; Oreggia, D.; Tourinho-Barbosa, R.; Moschini, M.; Filippini, C.; van Melick, H.H.E.; van den Bergh, R.C.N.; Gontero, P.; Cathala, N.; et al. Long-term Outcomes of Focal Cryotherapy for Low- to Intermediate-risk Prostate Cancer: Results and Matched Pair Analysis with Active Surveillance. Eur. Urol. Focus 2022, 8, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Barret, E.; Galiano, M.; Rozet, F.; Sanchez-Salas, R.; Ahallal, Y.; Macek, P.; Gaya, J.M.; Cerruti, J.; Devilliers, H.; et al. Focal cryoablation: A treatment option for unilateral low-risk prostate cancer. BJU Int. 2014, 113, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hale, Z.; Miyake, M.; Palacios, D.A.; Rosser, C.J. Focal cryosurgical ablation of the prostate: A single institute’s perspective. BMC Urol. 2013, 13, 2. [Google Scholar] [CrossRef]
- Bossier, R.; Sanguedolce, F.; Territo, A.; Vanacore, D.; Martínez, C.; Regis, F.; Gallioli, A.; Mercade, A.; Mosquera, L.; Aumatell, J.; et al. Whole and hemi-gland cryoablation for primary localized prostate cancer: Short and medium-term oncological and functional outcomes. Actas Urol. Esp. 2020, 44, 172–178. [Google Scholar] [CrossRef]
- Rodríguez, S.A.; Arias Fúnez, F.; Bueno Bravo, C.; Rodríguez-Patrón Rodríguez, R.; Sanz Mayayo, E.; Palacios, V.H.; Burgos Revilla, F.J. Cryotherapy for primary treatment of prostate cancer: Intermediate term results of a prospective study from a single institution. Prostate Cancer 2014, 2014, 571576. [Google Scholar] [CrossRef]
- Elkjær, M.C.; Borre, M. Oncological outcome after primary prostate cryoablation compared with radical prostatectomy: A single-centre experience. Scand. J. Urol. 2014, 48, 27–33. [Google Scholar] [CrossRef]
- Guo, X.X.; Liu, S.J.; Wang, M.; Hou, H.M.; Wang, X.; Zhang, Z.P.; Liu, M.; Wang, J.Y. Comparing the Oncological Outcomes of Cryoablation vs. Radical Prostatectomy in Low-Intermediate Risk Localized Prostate Cancer. Front. Oncol. 2020, 10, 1489. [Google Scholar] [CrossRef]
- Aker, M.N.; Brisbane, W.G.; Kwan, L.; Gonzalez, S.; Priester, A.M.; Kinnaird, A.; Delfin, M.K.; Felker, E.; Sisk, A.E.; Kuppermann, D.; et al. Cryotherapy for partial gland ablation of prostate cancer: Oncologic and safety outcomes. Cancer Med. 2023, 12, 9351–9362. [Google Scholar] [CrossRef]
- Al Ekish, S.; Nayeemuddin, M.; Maddox, M.; Pareek, G. The role of cryosurgery of the prostate for nonsurgical candidates. JSLS 2013, 17, 423–428. [Google Scholar] [CrossRef]
- Bjerklund Johansen, T.E. Cryosurgical ablation as primary treatment in prostate cancer patients. Actas Urol. Esp. 2007, 31, 651–659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cohen, J.K.; Miller, R.J., Jr.; Ahmed, S.; Lotz, M.J.; Baust, J. Ten-year biochemical disease control for patients with prostate cancer treated with cryosurgery as primary therapy. Urology 2008, 71, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Dhar, N.; Ward, J.F.; Cher, M.L.; Jones, J.S. Primary full-gland prostate cryoablation in older men (> age of 75 years): Results from 860 patients tracked with the COLD Registry. BJU Int. 2011, 108, 508–512. [Google Scholar] [CrossRef]
- Grossgold, E.; Given, R.; Ruckle, H.; Jones, J.S. Does neoadjuvant androgen deprivation therapy before primary whole gland cryoablation of the prostate affect the outcome? Urology 2014, 83, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Chiang, P.H.; Chuang, Y.C.; Lee, W.C.; Cheng, Y.T.; Wang, H.J. Predictors of prostate-specific antigen biochemical recurrence in patients undergoing primary whole-gland prostate cryoablation. Ann. Surg. Oncol. 2015, 22, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Mercader, C.; Musquera, M.; Franco, A.; Alcaraz, A.; Ribal, M.J. Primary cryotherapy for localized prostate cancer treatment. Aging Male 2020, 23, 1460–1466. [Google Scholar] [CrossRef]
- Oishi, M.; Gill, I.S.; Ashrafi, A.N.; Lin-Brande, M.; Nassiri, N.; Shin, T.; Bove, A.; Cacciamani, G.E.; Ukimura, O.; Bahn, D.K.; et al. Primary Whole-gland Cryoablation for Prostate Cancer: Biochemical Failure and Clinical Recurrence at 5.6 Years of Follow-up. Eur. Urol. 2019, 75, 208–214. [Google Scholar] [CrossRef]
- Lepor, H.; Rapoport, E.; Tafa, M.; Gogaj, R.; Wysock, J.S. Five-year Oncologic Outcomes Following Primary Partial Gland Cryo-ablation Prospective Cohort Study of Men With Intermediate-risk Prostate Cancer. Urology 2025, 196, 189–195. [Google Scholar] [CrossRef]
- Westhoff, N.; Ernst, R.; Kowalewski, K.F.; Derigs, F.; Neuberger, M.; Nörenberg, D.; Popovic, Z.V.; Ritter, M.; Stephan Michel, M.; von Hardenberg, J. Medium-term Oncological Efficacy and Patient-reported Outcomes After Focal High-intensity Focused Ultrasound: The FOXPRO Trial. Eur. Urol. Focus. 2023, 9, 283–290. [Google Scholar] [CrossRef]
- Glybochko, P.V.; Amosov, A.V.; Krupinov, G.E.; Petrovskii, N.V.; Lumpov, I.S. Hemiablation of Localized Prostate Cancer by High-Intensity Focused Ultrasound: A Series of 35 Cases. Oncology 2019, 97, 44–48. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Freeman, A.; Kirkham, A.; Sahu, M.; Scott, R.; Allen, C.; Van der Meulen, J.; Emberton, M. Focal therapy for localized prostate cancer: A phase I/II trial. J. Urol. 2011, 185, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Aoun, F.; Limani, K.; Peltier, A.; Marcelis, Q.; Zanaty, M.; Chamoun, A.; Vanden Bossche, M.; Roumeguère, T.; van Velthoven, R. High Intensity Focused Ultrasound versus Brachytherapy for the Treatment of Localized Prostate Cancer: A Matched-Pair Analysis. Adv. Urol. 2015, 2015, 350324. [Google Scholar] [CrossRef] [PubMed]
- Blana, A.; Walter, B.; Rogenhofer, S.; Wieland, W.F. High-intensity focused ultrasound for the treatment of localized prostate cancer: 5-year experience. Urology 2004, 63, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, P.; Barret, E.; Sanchez-Salas, R.; Nunes-Silva, I.; Rozet, F.; Galiano, M.; Ventimiglia, E.; Briganti, A.; Salonia, A.; Montorsi, F.; et al. Oncological and functional outcomes of elderly men treated with HIFU vs. minimally invasive radical prostatectomy: A propensity score analysis. Eur. J. Surg. Oncol. 2018, 44, 185–191. [Google Scholar] [CrossRef]
- Dellabella, M.; Branchi, A.; Di Rosa, M.; Pucci, M.; Gasparri, L.; Claudini, R.; Carnevali, F.; Cecchini, S.; Castellani, D. Oncological and functional outcome after partial prostate HIFU ablation with Focal-One(®): A prospective single-center study. Prostate Cancer Prostatic Dis. 2021, 24, 1189–1197. [Google Scholar] [CrossRef]
- Duwe, G.; Boehm, K.; Haack, M.; Sparwasser, P.; Brandt, M.P.; Mager, R.; Tsaur, I.; Haferkamp, A.; Höfner, T. Single-center, prospective phase 2 trial of high-intensity focused ultrasound (HIFU) in patients with unilateral localized prostate cancer: Good functional results but oncologically not as safe as expected. World J. Urol. 2023, 41, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- El Fegoun, A.B.; Barret, E.; Prapotnich, D.; Soon, S.; Cathelineau, X.; Rozet, F.; Galiano, M.; Sanchez-Salas, R.; Vallancien, G. Focal therapy with high-intensity focused ultrasound for prostate cancer in the elderly. A feasibility study with 10 years follow-up. Int. Braz. J. Urol. 2011, 37, 213–219, discussion 220–212. [Google Scholar] [CrossRef]
- Feijoo, E.R.; Sivaraman, A.; Barret, E.; Sanchez-Salas, R.; Galiano, M.; Rozet, F.; Prapotnich, D.; Cathala, N.; Mombet, A.; Cathelineau, X. Focal High-intensity Focused Ultrasound Targeted Hemiablation for Unilateral Prostate Cancer: A Prospective Evaluation of Oncologic and Functional Outcomes. Eur. Urol. 2016, 69, 214–220. [Google Scholar] [CrossRef]
- Ganzer, R.; Hadaschik, B.; Pahernik, S.; Koch, D.; Baumunk, D.; Kuru, T.; Heidenreich, A.; Stolzenburg, J.U.; Schostak, M.; Blana, A. Prospective Multicenter Phase II Study on Focal Therapy (Hemiablation) of the Prostate with High Intensity Focused Ultrasound. J. Urol. 2018, 199, 983–989. [Google Scholar] [CrossRef]
- Hoquetis, L.; Malavaud, B.; Game, X.; Beauval, J.B.; Portalez, D.; Soulie, M.; Rischmann, P. MRI evaluation following partial HIFU therapy for localized prostate cancer: A single-center study. Prog. Urol. 2016, 26, 517–523. [Google Scholar] [CrossRef]
- Nyk, Ł.; Michalak, W.; Szempliński, S.; Woźniak, R.; Zagożdżon, B.; Krajewski, W.; Kryst, P.; Kamecki, H.; Poletajew, S. High-Intensity Focused-Ultrasound Focal Therapy Versus Laparoscopic Radical Prostatectomy: A Comparison of Oncological and Functional Outcomes in Low- and Intermediate-Risk Prostate Cancer Patients. J. Pers. Med. 2022, 12, 251. [Google Scholar] [CrossRef]
- Pinthus, J.H.; Farrokhyar, F.; Hassouna, M.M.; Woods, E.; Whelan, K.; Shayegan, B.; Orovan, W.L. Single-session primary high-intensity focused ultrasonography treatment for localized prostate cancer: Biochemical outcomes using third generation-based technology. BJU Int. 2012, 110, 1142–1148. [Google Scholar] [CrossRef]
- Poissonnier, L.; Chapelon, J.Y.; Rouvière, O.; Curiel, L.; Bouvier, R.; Martin, X.; Dubernard, J.M.; Gelet, A. Control of prostate cancer by transrectal HIFU in 227 patients. Eur. Urol. 2007, 51, 381–387. [Google Scholar] [CrossRef]
- Ghai, S.; Finelli, A.; Corr, K.; Chan, R.; Jokhu, S.; Li, X.; McCluskey, S.; Konukhova, A.; Hlasny, E.; van der Kwast, T.H.; et al. MRI-guided Focused Ultrasound Ablation for Localized Intermediate-Risk Prostate Cancer: Early Results of a Phase II Trial. Radiology 2021, 298, 695–703. [Google Scholar] [CrossRef]
- Rischmann, P.; Gelet, A.; Riche, B.; Villers, A.; Pasticier, G.; Bondil, P.; Jung, J.L.; Bugel, H.; Petit, J.; Toledano, H.; et al. Focal High Intensity Focused Ultrasound of Unilateral Localized Prostate Cancer: A Prospective Multicentric Hemiablation Study of 111 Patients. Eur. Urol. 2017, 71, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, A.; Marra, G.; Stabile, A.; Mombet, A.; Macek, P.; Lanz, C.; Cathala, N.; Moschini, M.; Carneiro, A.; Sanchez-Salas, R.; et al. Does mpMRI guidance improve HIFU partial gland ablation compared to conventional ultrasound guidance? Early functional outcomes and complications from a single center. Int. Braz. J. Urol. 2020, 46, 984–992. [Google Scholar] [CrossRef]
- van Velthoven, R.; Aoun, F.; Marcelis, Q.; Albisinni, S.; Zanaty, M.; Lemort, M.; Peltier, A.; Limani, K. A prospective clinical trial of HIFU hemiablation for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2016, 19, 79–83. [Google Scholar] [CrossRef]
- von Hardenberg, J.; Westhoff, N.; Baumunk, D.; Hausmann, D.; Martini, T.; Marx, A.; Porubsky, S.; Schostak, M.; Michel, M.S.; Ritter, M. Prostate cancer treatment by the latest focal HIFU device with MRI/TRUS-fusion control biopsies: A prospective evaluation. Urol. Oncol. 2018, 36, 401.e401–401.e409. [Google Scholar] [CrossRef]
- Arnouil, N.; Gelet, A.; Matillon, X.; Rouviere, O.; Colombel, M.; Ruffion, A.; Mège-Lechevallier, F.; Subtil, F.; Badet, L.; Crouzet, S. Focal HIFU vs robot-assisted total prostatectomy: Functionnal and oncologic outcomes at one year. Prog. Urol. 2018, 28, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, S.; Poissonnier, L.; Murat, F.J.; Pasticier, G.; Rouvière, O.; Mège-Lechevallier, F.; Chapelon, J.Y.; Martin, X.; Gelet, A. Outcomes of HIFU for localised prostate cancer using the Ablatherm Integrate Imaging® device. Prog. Urol. 2011, 21, 191–197. [Google Scholar] [CrossRef] [PubMed]
- DE Luca, S.; Checcucci, E.; Piramide, F.; Russo, F.; Alessio, P.; Garrou, D.; Peretti, D.; Sica, M.; Volpi, G.; Piana, A.; et al. MRI/real-time ultrasound image fusion guided high-intensity focused ultrasound: A prospective comparative and functional analysis of different ablative techniques. Minerva Urol. Nephrol. 2023, 75, 172–179. [Google Scholar] [CrossRef]
- Misraï, V.; Rouprêt, M.; Chartier-Kastler, E.; Comperat, E.; Renard-Penna, R.; Haertig, A.; Bitker, M.O.; Richard, F.; Conort, P. Oncologic control provided by HIFU therapy as single treatment in men with clinically localized prostate cancer. World J. Urol. 2008, 26, 481–485. [Google Scholar] [CrossRef]
- Rosenhammer, B.; Ganzer, R.; Zeman, F.; Näger, T.; Fritsche, H.M.; Blana, A.; Burger, M.; Bründl, J. Oncological long-term outcome of whole gland HIFU and open radical prostatectomy: A comparative analysis. World J. Urol. 2019, 37, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Hiraiwa, S.; Uemura, K.; Nitta, M.; Hasegawa, M.; Kawamura, Y.; Hashida, K.; Hasebe, T.; Tajiri, T.; Miyajima, A. Focal therapy with high-intensity focused ultrasound for the localized prostate cancer for Asian based on the localization with MRI-TRUS fusion image-guided transperineal biopsy and 12-cores transperineal systematic biopsy: Prospective analysis of oncological and functional outcomes. Int. J. Clin. Oncol. 2020, 25, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Durán-Rivera, A.; Montoliu García, A.; Juan Escudero, J.; Garrido Abad, P.; Fernández Arjona, M.; López Alcina, E. High-intensity focused ultrasound therapy for the treatment of prostate cancer: Medium-term experience. Actas Urol. Esp. 2018, 42, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Chiang, P.H. Cohort study of high-intensity focused ultrasound in the treatment of localised prostate cancer treatment: Medium-term results from a single centre. PLoS ONE 2020, 15, e0236026. [Google Scholar] [CrossRef]
- Abreu, A.L.; Peretsman, S.; Iwata, A.; Shakir, A.; Iwata, T.; Brooks, J.; Tafuri, A.; Ashrafi, A.; Park, D.; Cacciamani, G.E.; et al. High Intensity Focused Ultrasound Hemigland Ablation for Prostate Cancer: Initial Outcomes of a United States Series. J. Urol. 2020, 204, 741–747. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chiang, P.H.; Liu, Y.Y.; Chuang, Y.C.; Cheng, Y.T. Primary whole-gland ablation for localized prostate cancer with high-intensity focused ultrasound: The important predictors of biochemical recurrence. Int. J. Urol. 2018, 25, 615–620. [Google Scholar] [CrossRef]
- Dickinson, L.; Arya, M.; Afzal, N.; Cathcart, P.; Charman, S.C.; Cornaby, A.; Hindley, R.G.; Lewi, H.; McCartan, N.; Moore, C.M.; et al. Medium-term Outcomes after Whole-gland High-intensity Focused Ultrasound for the Treatment of Nonmetastatic Prostate Cancer from a Multicentre Registry Cohort. Eur. Urol. 2016, 70, 668–674. [Google Scholar] [CrossRef]
- Reddy, D.; Peters, M.; Shah, T.T.; van Son, M.; Tanaka, M.B.; Huber, P.M.; Lomas, D.; Rakauskas, A.; Miah, S.; Eldred-Evans, D.; et al. Cancer Control Outcomes Following Focal Therapy Using High-intensity Focused Ultrasound in 1379 Men with Nonmetastatic Prostate Cancer: A Multi-institute 15-year Experience. Eur. Urol. 2022, 81, 407–413. [Google Scholar] [CrossRef]
- Komura, K.; Inamoto, T.; Takai, T.; Uchimoto, T.; Saito, K.; Tanda, N.; Kono, J.; Minami, K.; Uehara, H.; Fujisue, Y.; et al. Single session of high-intensity focused ultrasound for localized prostate cancer: Treatment outcomes and potential effect as a primary therapy. World J. Urol. 2014, 32, 1339–1345. [Google Scholar] [CrossRef]
- Limani, K.; Aoun, F.; Holz, S.; Paesmans, M.; Peltier, A.; van Velthoven, R. Single high intensity focused ultrasound session as a whole gland primary treatment for clinically localized prostate cancer: 10-year outcomes. Prostate Cancer 2014, 2014, 186782. [Google Scholar] [CrossRef]
- Mearini, L.; D’Urso, L.; Collura, D.; Nunzi, E.; Muto, G.; Porena, M. High-intensity focused ultrasound for the treatment of prostate cancer: A prospective trial with long-term follow-up. Scand. J. Urol. 2015, 49, 267–274. [Google Scholar] [CrossRef]
- Pfeiffer, D.; Berger, J.; Gross, A.J. Single application of high-intensity focused ultrasound as a first-line therapy for clinically localized prostate cancer: 5-year outcomes. BJU Int. 2012, 110, 1702–1707. [Google Scholar] [CrossRef]
- Ripert, T.; Azémar, M.D.; Ménard, J.; Barbe, C.; Messaoudi, R.; Bayoud, Y.; Pierrevelcin, J.; Duval, F.; Staerman, F. Six years’ experience with high-intensity focused ultrasonography for prostate cancer: Oncological outcomes using the new ‘Stuttgart’ definition for biochemical failure. BJU Int. 2011, 107, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.Y.; Lin, C.T.; Chiang, P.H.; Chiang, P.H.; Chiang, P.C. High-Intensity Focused Ultrasound (Sonablate(®)) for Prostate Cancer: Preliminary Outcomes in Taiwan. Ann. Surg. Oncol. 2023, 30, 8764–8769. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; Coloby, P.; Chevallier, T.; Occéan, B.V.; Houédé, N.; Villers, A.; Rischmann, P. Whole-gland or Subtotal High-intensity Focused Ultrasound Versus Radical Prostatectomy: The Prospective, Noninferiority, Nonrandomized HIFI Trial. Eur. Urol. 2025, 87, 526–533. [Google Scholar] [CrossRef]
- Nahar, B.; Ajami, T.; Williams, A.; Soodana Prakash, N.; Khandekar, A.; Freitas, P.F.S.; Malpani, A.; Rayan, J.; Sureshkumar, K.; Ritch, C.R.; et al. Survival Outcomes and Recurrence Patterns Following Focal High-intensity Focused Ultrasound Treatment for Localized Prostate Cancer: Insights on Patient Selection and Lessons Learned. Eur. Urol. Focus. 2024. [Google Scholar] [CrossRef]
- Barret, E.; Ahallal, Y.; Sanchez-Salas, R.; Galiano, M.; Cosset, J.M.; Validire, P.; Macek, P.; Durand, M.; Prapotnich, D.; Rozet, F.; et al. Morbidity of focal therapy in the treatment of localized prostate cancer. Eur. Urol. 2013, 63, 618–622. [Google Scholar] [CrossRef]
- Enikeev, D.; Taratkin, M.; Amosov, A.; Rivas, J.G.; Podoinitsin, A.; Potoldykova, N.; Karageziyan, M.; Glybochko, P.; Barret, E. Whole-gland ablation therapy versus active surveillance for low-risk prostate cancer: A prospective study. Cent. Eur. J. Urol. 2020, 73, 127–133. [Google Scholar] [CrossRef]
- Tourinho-Barbosa, R.R.; Sanchez-Salas, R.; Claros, O.R.; Collura-Merlier, S.; Bakavicius, A.; Carneiro, A.; Stabile, A.; Moschini, M.; Cathala, N.; Tobias-Machado, M.; et al. Focal Therapy for Localized Prostate Cancer with Either High Intensity Focused Ultrasound or Cryoablation: A Single Institution Experience. J. Urol. 2020, 203, 320–330. [Google Scholar] [CrossRef]
- Tay, K.J.; Fong, K.Y.; Stabile, A.; Dominguez-Escrig, J.L.; Ukimura, O.; Rodriguez-Sanchez, L.; Blana, A.; Becher, E.; Laguna, M.P. Established focal therapy-HIFU, IRE, or cryotherapy-where are we now?-a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Alberti, A.; Castellani, D.; Yee, C.H.; Zhang, K.; Poon, D.M.C.; Chiu, P.K.; Campi, R.; Resta, G.R.; Dibilio, E.; et al. Oncological results and cancer control definition in focal therapy for Prostate Cancer: A systematic review. Prostate Cancer Prostatic Dis. 2024, 27, 623–634. [Google Scholar] [CrossRef]
- Shah, T.T.; Reddy, D.; Peters, M.; Ball, D.; Kim, N.H.; Gomez, E.G.; Miah, S.; Evans, D.E.; Guillaumier, S.; van Rossum, P.S.N.; et al. Focal therapy compared to radical prostatectomy for non-metastatic prostate cancer: A propensity score-matched study. Prostate Cancer Prostatic Dis. 2021, 24, 567–574. [Google Scholar] [CrossRef]
- Bründl, J.; Osberghaus, V.; Zeman, F.; Breyer, J.; Ganzer, R.; Blana, A.; Gierth, M.; Denzinger, S.; Burger, M.; Rosenhammer, B. Oncological Long-term Outcome After Whole-gland High-intensity Focused Ultrasound for Prostate Cancer-21-yr Follow-up. Eur. Urol. Focus 2022, 8, 134–140. [Google Scholar] [CrossRef]
- Tan, W.P.; Kotamarti, S.; Chen, E.; Mahle, R.; Arcot, R.; Chang, A.; Ayala, A.; Michael, Z.; Seguier, D.; Polascik, T.J. Oncological and functional outcomes of men undergoing primary whole gland cryoablation of the prostate: A 20-year experience. Cancer 2022, 128, 3824–3830. [Google Scholar] [CrossRef]
- Ganzer, R.; Fritsche, H.M.; Brandtner, A.; Bründl, J.; Koch, D.; Wieland, W.F.; Blana, A. Fourteen-year oncological and functional outcomes of high-intensity focused ultrasound in localized prostate cancer. BJU Int. 2013, 112, 322–329. [Google Scholar] [CrossRef]
- Hegyi, P.; Petersen, O.H.; Holgate, S.; Erőss, B.; Garami, A.; Szakács, Z.; Dobszai, D.; Balaskó, M.; Kemény, L.; Peng, S.; et al. Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. J. Clin. Med. 2020, 9, 1532. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, P.; Erőss, B.; Izbéki, F.; Párniczky, A.; Szentesi, A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021, 27, 1317–1319. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skribek, B.; Szabó, A.; Ács, J.; Cavalcante, B.G.N.; Sipos, B.D.; Hegyi, P.; Mátrai, P.; Nyirády, P.; Ács, N.; Majoros, A.; et al. Oncological Efficacy and Safety of Minimally Invasive Focal and Whole-Gland Interventions in the Treatment of Low- and Intermediate-Risk Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 2863. https://doi.org/10.3390/cancers17172863
Skribek B, Szabó A, Ács J, Cavalcante BGN, Sipos BD, Hegyi P, Mátrai P, Nyirády P, Ács N, Majoros A, et al. Oncological Efficacy and Safety of Minimally Invasive Focal and Whole-Gland Interventions in the Treatment of Low- and Intermediate-Risk Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(17):2863. https://doi.org/10.3390/cancers17172863
Chicago/Turabian StyleSkribek, Benjamin, Anett Szabó, Júlia Ács, Bianca Golzio Navarro Cavalcante, Boglárka Dorina Sipos, Péter Hegyi, Péter Mátrai, Péter Nyirády, Nándor Ács, Attila Majoros, and et al. 2025. "Oncological Efficacy and Safety of Minimally Invasive Focal and Whole-Gland Interventions in the Treatment of Low- and Intermediate-Risk Prostate Cancer: A Systematic Review and Meta-Analysis" Cancers 17, no. 17: 2863. https://doi.org/10.3390/cancers17172863
APA StyleSkribek, B., Szabó, A., Ács, J., Cavalcante, B. G. N., Sipos, B. D., Hegyi, P., Mátrai, P., Nyirády, P., Ács, N., Majoros, A., & Deák, P. Á. (2025). Oncological Efficacy and Safety of Minimally Invasive Focal and Whole-Gland Interventions in the Treatment of Low- and Intermediate-Risk Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers, 17(17), 2863. https://doi.org/10.3390/cancers17172863