The Role of Gamma Knife Surgery in the Treatment of Rare Sellar Neoplasms: A Report of Nine Cases
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
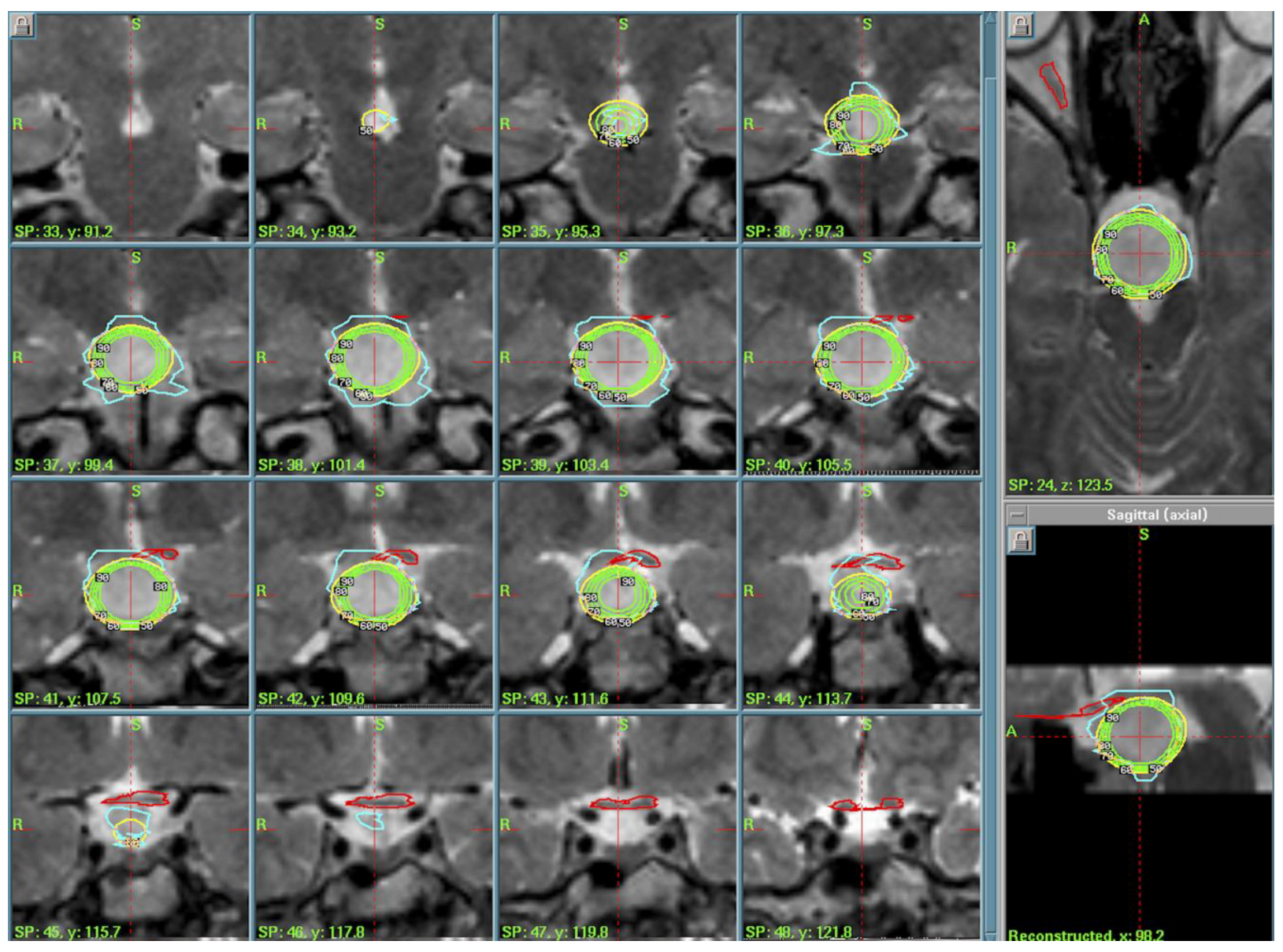
3.1. Hypothalamic Hamartoma (n = 3)
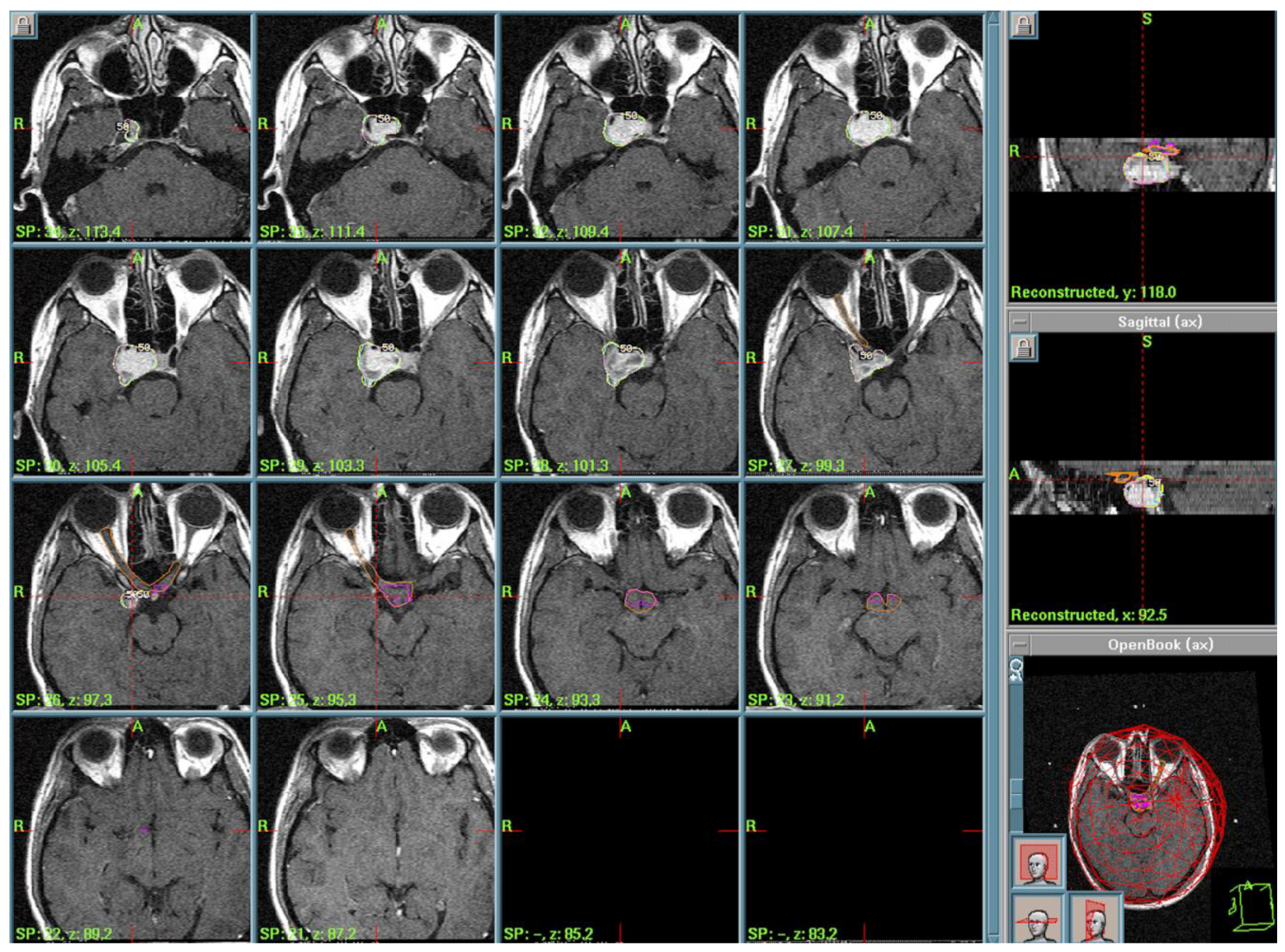
3.2. Pituitary Stalk Langerhans Cell Histiocytosis (LCH)
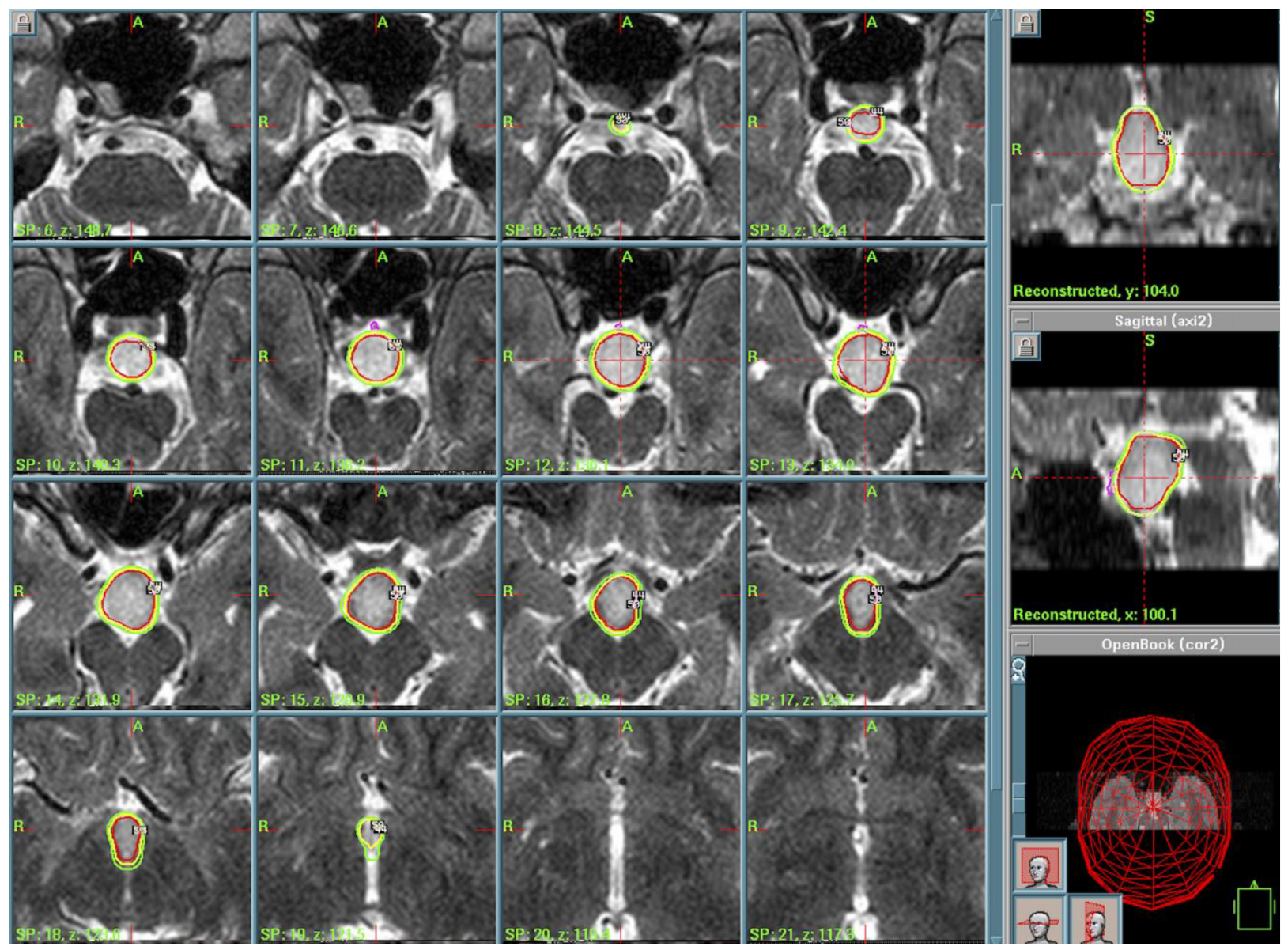
3.3. Rathke’s Cleft Cist (RCC)
3.4. Ossifying Fibroma (OF) of the Ethmoid Sinus
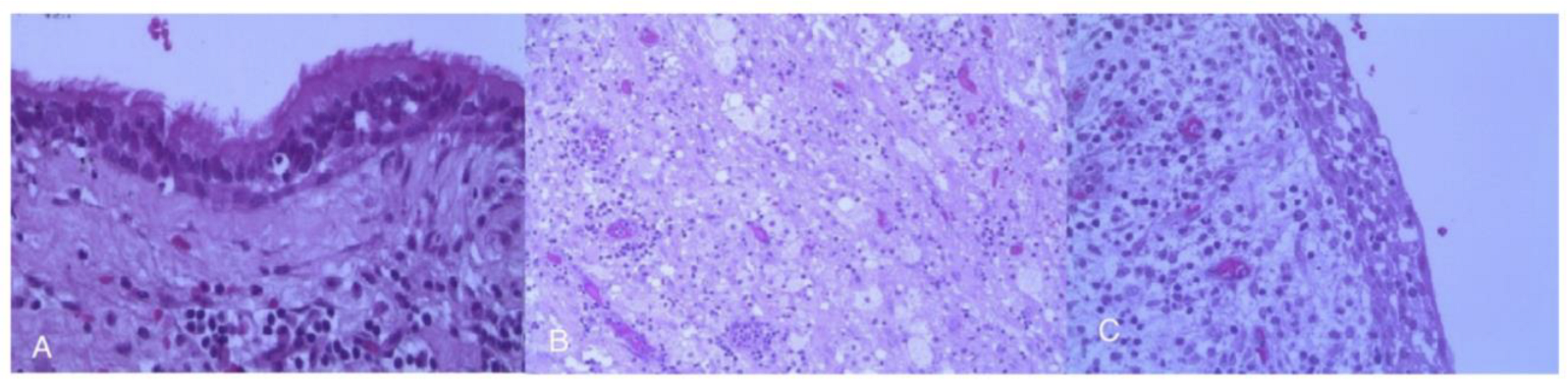
3.5. Spindle Cell Oncocytoma (SCO)
3.6. Choroid Plexus Papilloma (CPP)
4. Discussion
4.1. Pituitary Adenomas and Nonadenomatous Sellar Lesions
4.2. Hypothalamic Hamartomas
4.3. Langerhans Cell Histiocytosis (LCH) and Other Histiocytosis
4.4. Rathke’s Cleft Cysts
4.5. Other Forms
4.6. Radiotherapy as Salvage Therapy
4.7. Emerging Insights and Research Needs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Petrakakis, I.; Pirayesh, A.; Krauss, J.K.; Raab, P.; Hartmann, C.; Nakamura, M. The sellar and suprasellar region: A “hideaway” of rare lesions. Clinical aspects, imaging findings, surgical outcome and comparative analysis. Clin. Neurol. Neurosurg. 2016, 149, 154–165. [Google Scholar] [CrossRef]
- Freda, P.U.; Post, K.D. Differential diagnosis of sellar masses. Endocrinol. Metab. Clin. N. Am. 1999, 28, 81–117. [Google Scholar] [CrossRef]
- Glezer, A.; Paraiba, D.B.; Bronstein, M.D. Rare sellar lesions. Endocrinol. Metab. Clin. N. Am. 2008, 37, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Rubin, M.A.; McKeever, P.E. Differential expression of cytokeratins 8 and 20 distinguishes craniopharyngioma from rathke cleft cyst. Arch. Pathol. Lab. Med. 2002, 126, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Bernstein, K.; Lee, C.-C.; Yang, H.-C.; Liscak, R.; May, J.; Martínez-Álvarez, R.; Martínez-Moreno, N.; Bunevicius, A.; Sheehan, J.P. Stereotactic radiosurgery for Rathke’s cleft cysts: An international multicenter study. J. Neurosurg. 2022, 137, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Strickler, S.; Hitchcock, K.E.; Dziegielewski, P.T.; Mendenhall, W.M. Radiotherapy for juvenile ossifying fibroma of the maxillary sinus: Case report and literature review. Head Neck 2017, 39, E81–E84. [Google Scholar] [CrossRef]
- Abushamat, L.A.; Kerr, J.M.; Lopes, M.B.S.; Kleinschmidt-DeMasters, B.K. Very Unusual Sellar/Suprasellar Region Masses: A Review. J. Neuropathol. Exp. Neurol. 2019, 78, 673–684. [Google Scholar] [CrossRef]
- Khade, S.; Shenoy, A. Ectopic Choroid Plexus Papilloma. Asian J. Neurosurg. 2018, 13, 191–194. [Google Scholar] [CrossRef]
- Giantini Larsen, A.M.; Cote, D.J.; Zaidi, H.A.; Bi, W.L.; Schmitt, P.J.; Iorgulescu, J.B.; Miller, M.B.; Smith, T.R.; Lopes, M.B.; Jane, J.A.; et al. Spindle cell oncocytoma of the pituitary gland. J. Neurosurg. 2019, 131, 517–525. [Google Scholar] [CrossRef]
- Huang, B.Y.; Castillo, M. Nonadenomatous tumors of the pituitary and sella turcica. Top. Magn. Reson. Imaging TMRI 2005, 16, 289–299. [Google Scholar] [CrossRef]
- Conde Blanco, E.; Anciones Martín, C.; Manzanares, I.; Gil López, F.; Roldán, P.; Donaire, A.; Rumiá, J.; Carreño, M. Hypothalamic hamartomas in adulthood: Clinical spectrum and treatment outcome-A unicenter experience. Brain Behav. 2019, 9, e01412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Régis, J.; Scavarda, D.; Tamura, M.; Nagayi, M.; Villeneuve, N.; Bartolomei, F.; Brue, T.; Dafonseca, D.; Chauvel, P. Epilepsy related to hypothalamic hamartomas: Surgical management with special reference to gamma knife surgery. Child’s Nerv. Syst. 2006, 22, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Régis, J.; Scavarda, D.; Tamura, M.; Villeneuve, N.; Bartolomei, F.; Brue, T.; Morange, I.; Dafonseca, D.; Chauvel, P. Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Semin. Pediatr. Neurol. 2007, 14, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.T.; Cross, J.H.; Arzimanoglou, A.; Berkovic, S.F.; Kerrigan, J.F.; Miller, I.P.; Webster, E.; Soeby, L.; Cukiert, A.; Hesdorffer, D.K.; et al. Hypothalamic Hamartomas: Evolving Understanding and Management. Neurology 2021, 97, 864–873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamdi, H.; Ferrante, P.; Spatola, G.; Clawson, W.; McGonigal, A.; Daquin, G.; Villeneuve, N.; Laguitton, V.; Bartolomei, F.; Regis, J. Epileptic hypothalamic hamartomas impact of topography on clinical presentation and radiosurgical outcome. Epilepsy Res. 2021, 173, 106624. [Google Scholar] [CrossRef]
- Hamdi, H.; Albader, F.; Spatola, G.; Laguitton, V.; Trebuchon, A.; Bartolomei, F.; Regis, J. Long-term cognitive outcome after radiosurgery in epileptic hypothalamic hamartomas and review of the literature. Epilepsia 2021, 62, 1369–1381. [Google Scholar] [CrossRef]
- Ricciardi, G.K.; Paio, F.; Zivelonghi, C.; Longhi, M.; Bulgarelli, G.; Tagliamonte, M.; Polloniato, P.M.; Mantovani, E.; Ferlisi, M.; Nicolato, A.; et al. MRgFUS disconnection surgery for hypothalamic hamartoma-related epilepsy: Case report and literature review. Neurol. Sci. 2025, 46, 1399–1404. [Google Scholar] [CrossRef]
- Castinetti, F.; Brue, T.; Morange, I.; Carron, R.; Régis, J. Gamma Knife radiosurgery for hypothalamic hamartoma preserves endocrine functions. Epilepsia 2017, 58 (Suppl. S2), 72–76. [Google Scholar] [CrossRef]
- Butragueño Laiseca, L.; Oikonomopoulou, N.; Miranda Herrero, M.C.; Barredo Valderrama, E.; Vázquez López, M.; Jiménez de Domingo, A.; Aguado Del Hoyo, A.; García-Leal, R.; Meiriño, R.M. Neurological complications after gamma-knife radiosurgery for hypothalamic hamartoma. Eur. J. Paediatr. Neurol. 2016, 20, 745–749. [Google Scholar] [CrossRef]
- Romanelli, P. CyberKnife® Radiosurgery as First-line Treatment for Catastrophic Epilepsy Caused by Hypothalamic Hamartoma. Cureus 2018, 10, e2968. [Google Scholar] [CrossRef]
- Tripathi, M.; Maskara, P.; Deora, H.; Bansal, D.; Mohindra, S.; Tripathi, S.; Kaur, R.; Sheehan, J.P.; Rana, R.; Kumar, N. Role of Stereotactic Radiosurgery in Intracranial Histiocytosis: A Systematic Review of Literature of an Emerging Modality for Localized Disease. World Neurosurg. 2021, 150, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.-C.; Murovic, J.A.; Gibbs, I.; Vogel, H.; Chang, S.D. Pituitary stalk Langerhans cell histiocytosis treated with CyberKnife radiosurgery. Clin. Neurol. Neurosurg. 2013, 115, 573–577. [Google Scholar] [CrossRef]
- Tan, H.; Yu, K.; Yu, Y.; An, Z.; Li, J. Isolated hypothalamic-pituitary langerhans’ cell histiocytosis in female adult: A case report. Medicine 2019, 98, e13853. [Google Scholar] [CrossRef] [PubMed]
- Tritos, N.A.; Biller, B.M.K.; Swearingen, B. Management of Cushing disease. Nat. Rev. Endocrinol. 2011, 7, 279–289. [Google Scholar] [CrossRef]
- Faramand, A.; Niranjan, A.; Flickinger, J.; Monaco, E.; Lunsford, L.D. Salvage Gamma Knife Stereotactic Radiosurgery for Recurrent Intracranial Langerhans Cell Histiocytosis: A 36-Year Saga. World Neurosurg. 2020, 144, 205–208. [Google Scholar] [CrossRef]
- Aydin, S.; Darko, K.; Detchou, D.; Barrie, U. Rathke’s cleft cysts: From pathophysiology to management. Neurosurg. Rev. 2024, 47, 522. [Google Scholar] [CrossRef]
- Agarwalla, P.K.; Koch, M.J.; Royce, T.J.; Redjal, N.; Bussière, M.R.; Loeffler, J.S.; Swearingen, B. Stereotactic Radiation as Salvage Therapy for Recurrent Rathke Cleft Cysts. Neurosurgery 2020, 87, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Mohsenifar, Z.; Nouhi, S.; Abbas, F.M.; Farhadi, S.; Abedin, B. Ossifying fibroma of the ethmoid sinus: Report of a rare case and review of literature. J. Res. Med. Sci. 2011, 16, 841–847. [Google Scholar]
- Nagar, S.R.; Mittal, N.; Rane, S.U.; Bal, M.; Patil, A.; Ankathi, S.K.; Thiagarajan, S. Ossifying Fibromas of the Head and Neck Region: A Clinicopathological Study of 45 Cases. Head Neck Pathol. 2022, 16, 248–256. [Google Scholar] [CrossRef]
- Azab, M.A. Ossifying fibroma of the ethmoid and sphenoid sinuses: A report of a rare case and literature review. Surg. Neurol. Int. 2024, 15, 38. [Google Scholar] [CrossRef]
- Hasegawa, H.; Van Gompel, J.J.; Oushy, S.H.; Pollock, B.E.; Link, M.J.; Meyer, F.B.; Bancos, I.; Erickson, D.; Davidge-Pitts, C.J.; Little, J.T.; et al. A Comprehensive Study of Spindle Cell Oncocytoma of the Pituitary Gland: Series of 6 Cases and Meta-Analysis of 85 Cases. World Neurosurg. 2021, 149, e197–e216. [Google Scholar] [CrossRef]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Oushy, S.; Graffeo, C.S.; Perry, A.; Stafford, S.L.; Link, M.J.; Pollock, B.E. Single-fraction stereotactic radiosurgery for spindle cell oncocytoma: Preliminary experience and systematic review of the literature. J. Neurooncol. 2019, 144, 325–332. [Google Scholar] [CrossRef]
- Sethi, D.; Arora, R.; Garg, K.; Tanwar, P. Choroid plexus papilloma. Asian J. Neurosurg. 2017, 12, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-H.; Ye, K.; Zhan, R.-Y.; Wang, L.-J. Primary choroid plexus papilloma of the sellar region. J. Neurooncol. 2008, 88, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Lukins, D.; Villano, J.L.; Sudhakar, P. Not So Benign: A Rare Atypical Ectopic Choroid Plexus Papilloma. J. Ophthalmol. Vis. Sci. 2021, 6, 1050. [Google Scholar] [CrossRef]
- Vasquez, J.A.; Fonnegra, J.R.; Diez, J.C.; Fonnegra, A. Treatment of epidermoid tumors with gamma knife radiosurgery: Case series. Surg. Neurol. Int. 2016, 7 (Suppl. S4), S116–S120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen-Inbar, O.; Lee, C.C.; Mousavi, S.H.; Kano, H.; Mathieu, D.; Meola, A.; Nakaji, P.; Honea, N.; Johnson, M.; Abbassy, M.; et al. Stereotactic radiosurgery for intracranial hemangiopericytomas: A multicenter study. J. Neurosurg. 2017, 126, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Albano, L.; Losa, M.; Barzaghi, L.R.; Spatola, G.; Panni, P.; Terreni, M.R.; Mortini, P. Primary sellar melanocytoma: Pathological, clinical and treatment review. J. Endocrinol. Investig. 2020, 43, 575–585. [Google Scholar] [CrossRef] [PubMed]

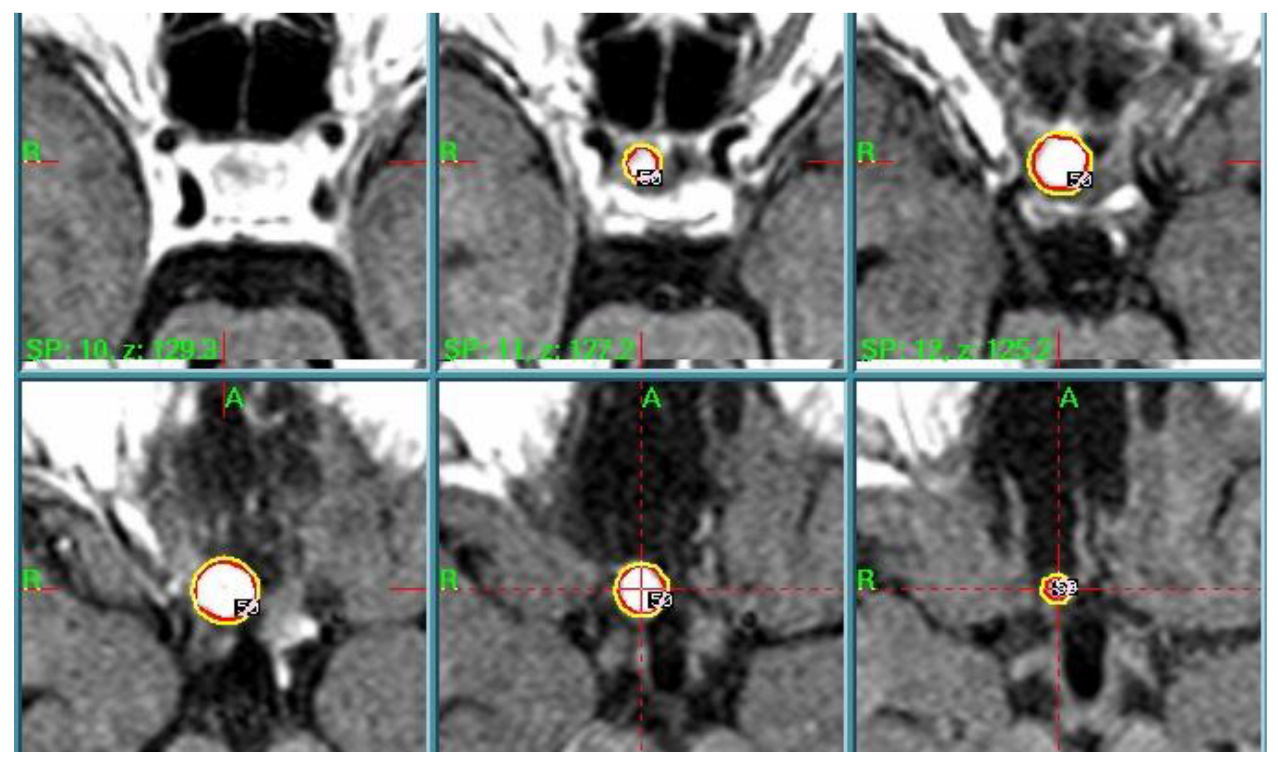

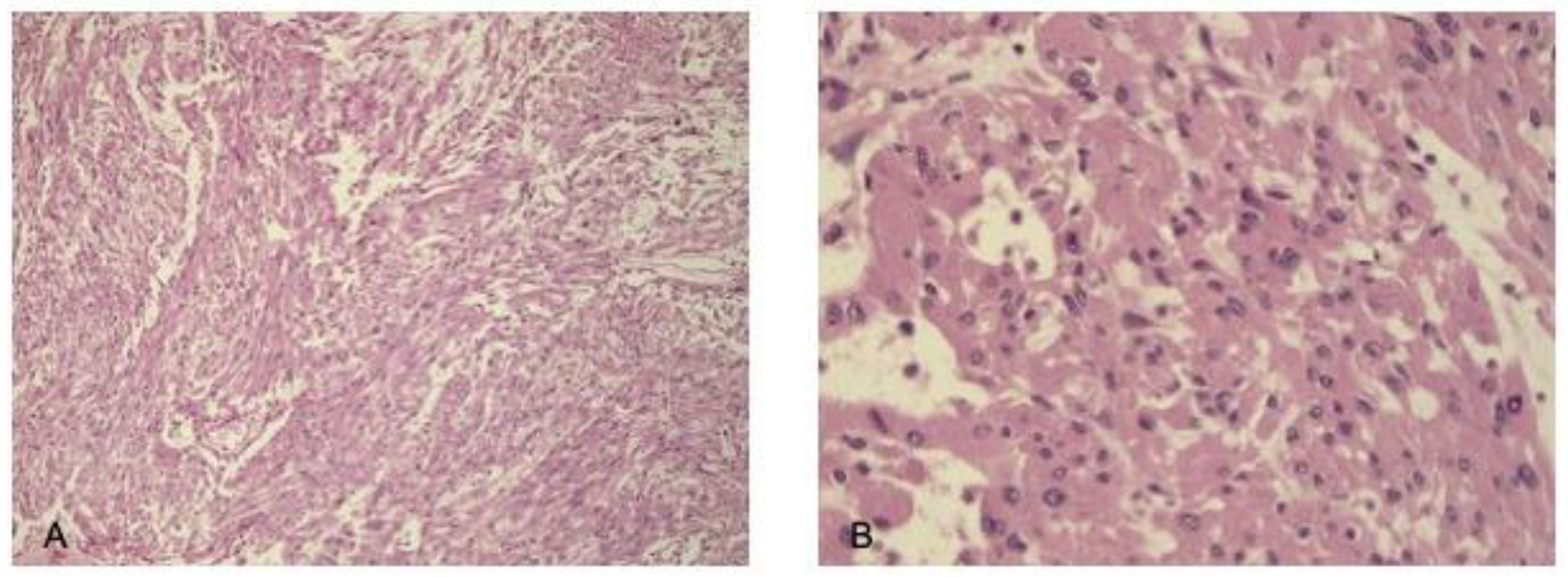
| N | Histology | Treatment Parameters | Treatment Date—Last FU |
|---|---|---|---|
| 1. | Hypothalamic Hamartoma | PI of 50%, PD of 11 Gy, MD of 22 Gy | 2004–2020 |
| 2. | Hypothalamic Hamartoma | PI of 50%, PD of 13.5 Gy, MD of 27 Gy | 2005–2010 |
| 3. | Hypothalamic Hamartoma | PI of 50%, PD of 13 Gy, MD of 26 Gy | 2009–2019 |
| 4. | Langerhans Cell Histiocytosis (LCH) | PI of 50%, PD of 11 Gy, MD of 22 Gy | 2004–2009 |
| 5. | Rathke’s Cleft Cyst | PI of 50%, PD of 12 Gy MD of 24 Gy | 2007–2014 |
| 6. | Rathke’s Cleft Cyst | PI of 50%, PD of 10 Gy, MD of 20 Gy | 2015–2023 |
| 7. | Ossifying Fibroma | PI of 50%, PD of 11 Gy, MD of 22 Gy | 2004–2016 |
| 8. | Spindle Cell Oncocytoma | IP of 50%, Ds of 12 Gy, Dm of 24 Gy | 2013–2019 |
| 9. | Ectopic Choroid Plexus Papilloma (CPP) | Dose Staging: PI of 50%, PD of 10 Gy, MD of 20 Gy, PI of 50%; PD of 9 Gy, MD of 18 Gy after 1 month | 2011–2019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longhi, M.; Lavezzo, R.; Barresi, V.; Bulgarelli, G.; D’Amico, A.; Lombardo, A.; Zivelonghi, E.; Polloniato, P.M.; Ricciardi, G.K.; Sala, F.; et al. The Role of Gamma Knife Surgery in the Treatment of Rare Sellar Neoplasms: A Report of Nine Cases. Cancers 2025, 17, 1564. https://doi.org/10.3390/cancers17091564
Longhi M, Lavezzo R, Barresi V, Bulgarelli G, D’Amico A, Lombardo A, Zivelonghi E, Polloniato PM, Ricciardi GK, Sala F, et al. The Role of Gamma Knife Surgery in the Treatment of Rare Sellar Neoplasms: A Report of Nine Cases. Cancers. 2025; 17(9):1564. https://doi.org/10.3390/cancers17091564
Chicago/Turabian StyleLonghi, Michele, Riccardo Lavezzo, Valeria Barresi, Giorgia Bulgarelli, Anna D’Amico, Antonella Lombardo, Emanuele Zivelonghi, Paolo Maria Polloniato, Giuseppe Kenneth Ricciardi, Francesco Sala, and et al. 2025. "The Role of Gamma Knife Surgery in the Treatment of Rare Sellar Neoplasms: A Report of Nine Cases" Cancers 17, no. 9: 1564. https://doi.org/10.3390/cancers17091564
APA StyleLonghi, M., Lavezzo, R., Barresi, V., Bulgarelli, G., D’Amico, A., Lombardo, A., Zivelonghi, E., Polloniato, P. M., Ricciardi, G. K., Sala, F., Musumeci, A., Pinna, G., & Nicolato, A. (2025). The Role of Gamma Knife Surgery in the Treatment of Rare Sellar Neoplasms: A Report of Nine Cases. Cancers, 17(9), 1564. https://doi.org/10.3390/cancers17091564








