Robot-Assisted Radical Nephroureterectomy: A Safe and Effective Option for Upper Tract Urothelial Carcinoma, Especially for Novice Surgeons
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
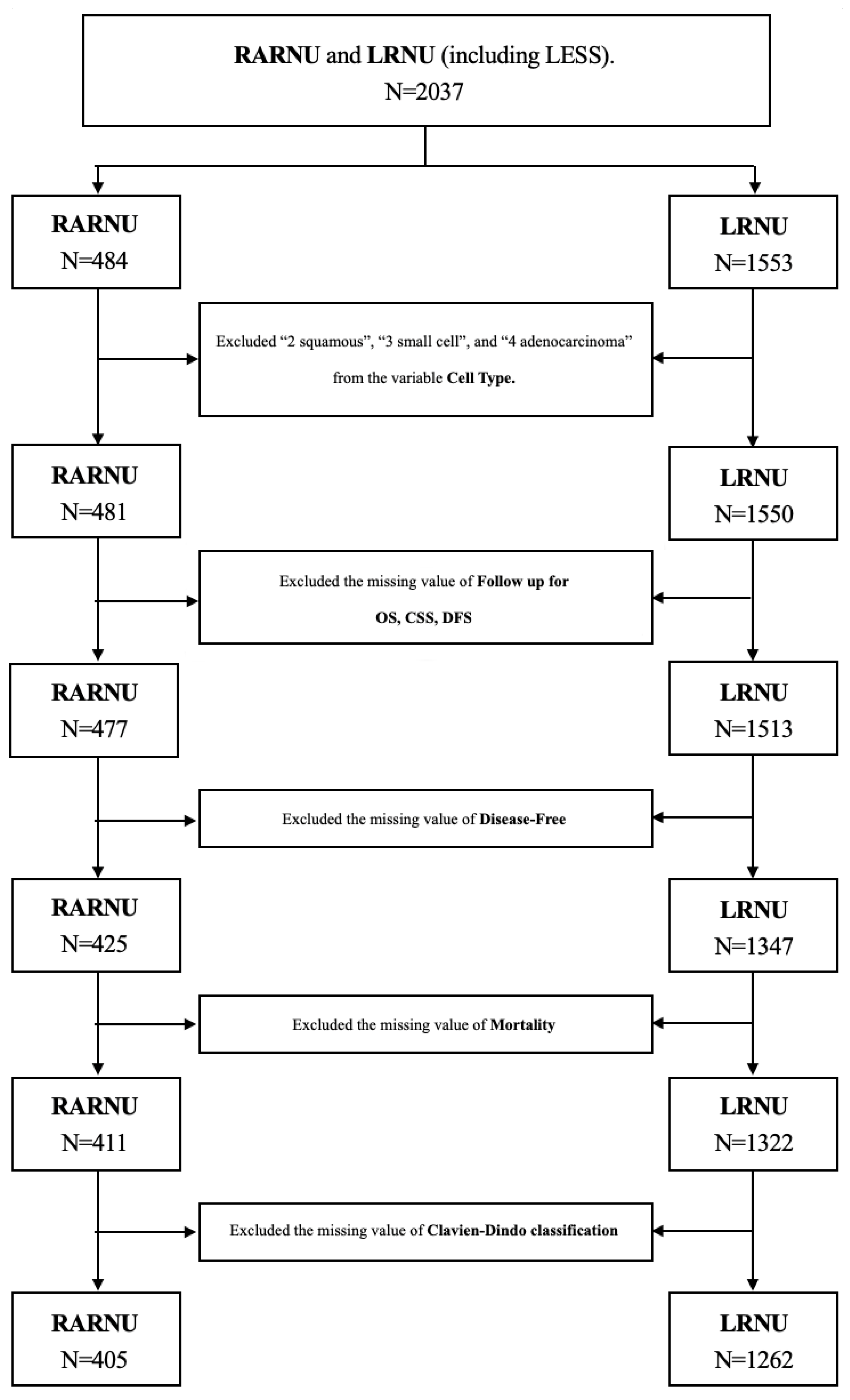
2.1. Study Population
2.2. Definitions and Endpoints
2.3. Follow-Up Protocols
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Surgical Outcomes
3.3. Survival Prediction Model
3.4. Surgeon Experience
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouprêt, M.; Babjuk, M.; Compérat, E.; Zigeuner, R.; Sylvester, R.J.; Burger, M.; Cowan, N.C.; Gontero, P.; Van Rhijn, B.W.; Mostafid, A.H. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur. Urol. 2018, 73, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Tatsumi, Y.; Fujimoto, K.; Nagao, K.; Sakano, S.; Matsuyama, H.; Inamoto, T.; Azuma, H.; Yasumoto, H.; Shiina, H. Changes in oncological outcomes after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma treated in the last two decades: A retrospective analysis based on a multicenter collaborative study. Jpn. J. Clin. Oncol. 2016, 46, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.J.; Ellison, L.M. Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J. Urol. 2000, 164, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Chen, K.-K.; Yen, C.-C.; Wang, W.-S.; Chang, Y.-H.; Huang, W.J.-S.; Fan, F.S.; Chiou, T.-J.; Liu, J.-H.; Chen, P.-M. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology 2002, 59, 681–687. [Google Scholar] [CrossRef]
- Chou, Y.H.; Huang, C.H. Unusual clinical presentation of upper urothelial carcinoma in Taiwan. Cancer 1999, 85, 1342–1344. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Lo, W.-C.; Yang, Y.-W.; You, S.-L.; Chen, C.-J.; Lai, M.-S. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J. Formos. Med. Assoc. 2016, 115, 1076–1088. [Google Scholar] [CrossRef]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef]
- Reddy, K.; Gharde, P.; Tayade, H.; Patil, M.; Reddy, L.S.; Surya, D. Advancements in robotic surgery: A comprehensive overview of current utilizations and upcoming frontiers. Cureus 2023, 15, e50415. [Google Scholar] [CrossRef]
- Tinay, I.; Gelpi-Hammerschmidt, F.; Leow, J.J.; Allard, C.B.; Rodriguez, D.; Wang, Y.; Chung, B.I.; Chang, S.L. Trends in utilisation, perioperative outcomes, and costs of nephroureterectomies in the management of upper tract urothelial carcinoma: A 10-year population-based analysis. BJU Int. 2016, 117, 954–960. [Google Scholar] [CrossRef]
- Grossmann, N.C.; Soria, F.; Juvet, T.; Potretzke, A.M.; Djaladat, H.; Ghoreifi, A.; Kikuchi, E.; Mari, A.; Khene, Z.-E.; Fujita, K. Comparing oncological and perioperative outcomes of open versus laparoscopic versus robotic radical nephroureterectomy for the treatment of upper tract urothelial carcinoma: A multicenter, multinational, propensity score-matched analysis. Cancers 2023, 15, 1409. [Google Scholar] [CrossRef] [PubMed]
- Mourmouris, P.; ARGUN, Ö.; Tzelves, L.; Tuna, M.; Gourtzelidou, M.; Tziotis, A.; Kural, A.; Skolarikos, A. Is robotic radical nephroureterectomy a safe alternative to open approach: The first prospective analysis. Arch. Ital. Urol. Androl. 2021, 93, 4. [Google Scholar] [CrossRef] [PubMed]
- Zeuschner, P.; Vollmer, S.G.; Linxweiler, J.; Wagenpfeil, G.; Wagenpfeil, S.; Saar, M.; Siemer, S.; Stöckle, M.; Heinzelbecker, J. Robot-assisted versus open radical nephroureterectomy for urothelial carcinoma of the upper urinary tract: A retrospective cohort study across ten years. Surg. Oncol. 2021, 38, 101607. [Google Scholar] [CrossRef]
- Ambani, S.N.; Weizer, A.Z.; Wolf, J.S., Jr.; He, C.; Miller, D.C.; Montgomery, J.S. Matched comparison of robotic vs laparoscopic nephroureterectomy: An initial experience. Urology 2014, 83, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-Y.; Yang, C.-K.; Huang, C.-Y.; Ou, Y.-C.; Hung, S.-F.; Chung, S.-D.; Pu, Y.-S. Robot-assisted laparoscopic nephroureterectomy versus hand-assisted laparoscopic nephroureterectomy for upper urinary tract urothelial carcinoma: A matched comparison study. BioMed Res. Int. 2015, 2015, 918486. [Google Scholar] [CrossRef]
- Melquist, J.J.; Redrow, G.; Delacroix, S.; Park, A.; Faria, E.E.; Karam, J.A.; Matin, S.F. Comparison of single-docking robotic-assisted and traditional laparoscopy for retroperitoneal lymph node dissection during nephroureterectomy with bladder cuff excision for upper-tract urothelial carcinoma. Urology 2016, 87, 216–223. [Google Scholar] [CrossRef]
- Li, C.-C.; Chang, C.-H.; Huang, C.-P.; Hong, J.-H.; Huang, C.-Y.; Chen, I.-H.A.; Lin, J.-T.; Lo, C.-W.; Yu, C.-C.; Tseng, J.-S. Comparing oncological outcomes and surgical complications of hand-assisted, laparoscopic and robotic nephroureterectomy for upper tract urothelial carcinoma. Front. Oncol. 2021, 11, 731460. [Google Scholar] [CrossRef]
- Pearce, S.M.; Pariser, J.J.; Patel, S.G.; Steinberg, G.D.; Shalhav, A.L.; Smith, N.D. The effect of surgical approach on performance of lymphadenectomy and perioperative morbidity for radical nephroureterectomy. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 121.e115–121.e121. [Google Scholar] [CrossRef]
- Lenis, A.T.; Donin, N.M.; Faiena, I.; Salmasi, A.; Johnson, D.C.; Drakaki, A.; Gollapudi, K.; Blumberg, J.; Belldegrun, A.; Pantuck, A. Role of surgical approach on lymph node dissection yield and survival in patients with upper tract urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 9.e1–9.e9. [Google Scholar] [CrossRef]
- Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2009. Available online: https://hastie.su.domains/ElemStatLearn/ (accessed on 19 April 2025).
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap weighting: A propensity score method that mimics attributes of a randomized clinical trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Cheng, C.; Li, F.; Thomas, L.E.; Li, F. Addressing extreme propensity scores in estimating counterfactual survival functions via the overlap weights. Am. J. Epidemiol. 2022, 191, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Clancy, O.; Grover, V.; Darzi, A. The evolution of robotic surgery: Surgical and anaesthetic aspects. BJA Br. J. Anaesth. 2017, 119, i72–i84. [Google Scholar] [CrossRef] [PubMed]
- Castilho, T.M.L.; Lemos, G.C.; Cha, J.D.; Colombo, J.R.; Claros, O.R.; Lemos, M.B.; Carneiro, A. Transition from open partial nephrectomy directly to robotic surgery: Experience of a single surgeon to achieve “TRIFECTA”. Int. Braz. J. Urol. 2020, 46, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Ghani, K.R.; Sukumar, S.; Sammon, J.D.; Rogers, C.G.; Trinh, Q.-D.; Menon, M. Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: Results from the nationwide inpatient sample. J. Urol. 2014, 191, 907–913. [Google Scholar] [CrossRef]
- Patel, M.N.; Bhandari, M.; Menon, M.; Rogers, C.G. Robotic-assisted partial nephrectomy. BJU Int. 2009, 103, 1296–1311. [Google Scholar] [CrossRef]
- Chatterjee, S.; Das, S.; Ganguly, K.; Mandal, D. Advancements in robotic surgery: Innovations, challenges and future prospects. J. Robot. Surg. 2024, 18, 28. [Google Scholar] [CrossRef]
- Tyson II, M.D.; Andrews, P.E.; Ferrigni, R.F.; Humphreys, M.R.; Parker, A.S.; Castle, E.P. Radical prostatectomy trends in the United States: 1998 to 2011. Mayo Clin. Proc. 2016, 91, 10–16. [Google Scholar] [CrossRef]
- Morriss, S.; Zargar, H.; Dias, B.H. Management of the distal ureter during nephroureterectomy for upper tract urothelial carcinoma: A comprehensive review of literature. Urol. J. 2021, 18, 585–599. [Google Scholar]
- Xylinas, E.; Colin, P.; Audenet, F.; Phe, V.; Cormier, L.; Cussenot, O.; Houlgatte, A.; Karsenty, G.; Bruyère, F.; Polguer, T. Intravesical recurrence after radical nephroureterectomy for upper tract urothelial carcinomas: Predictors and impact on subsequent oncological outcomes from a national multicenter study. World J. Urol. 2013, 31, 61–68. [Google Scholar] [CrossRef]
- Braun, A.E.; Srivastava, A.; Maffucci, F.; Kutikov, A. Controversies in management of the bladder cuff at nephroureterectomy. Transl. Androl. Urol. 2020, 9, 1868. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.J.; Lee, S.E.; Hong, S.K.; Byun, S.-S. Comparison of oncological and perioperative outcomes of open, laparoscopic, and robotic nephroureterectomy approaches in patients with non-metastatic upper-tract urothelial carcinoma. PLoS ONE 2019, 14, e0210401. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-C.; Chen, C.-H.; Hong, J.-H.; Ke, H.-L.; Li, W.-M.; Chung, S.-D.; Wu, W.-C.; Chen, Y.-T.; Jiang, Y.-H.; Lin, Y.-H. Comparison of oncological outcomes for hand-assisted and pure laparoscopic radical nephroureterectomy: Results from the Taiwan Upper Tract Urothelial Cancer Collaboration Group. Surg. Endosc. 2022, 36, 4342–4434. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Maeda, T.; Tanaka, T.; Fukuta, F.; Kobayashi, K.; Nishiyama, N.; Takahashi, S.; Masumori, N. Comparison of laparoscopic, hand-assisted, and open surgical nephroureterectomy. JSLS J. Soc. Laparoendosc. Surg. 2014, 18, 288. [Google Scholar] [CrossRef]
- Ji, R.; He, Z.; Fang, S.; Yang, W.; Wei, M.; Dong, J.; Xu, W.; Ji, Z. Robot-assisted vs. laparoscopic nephroureterectomy for upper urinary tract urothelial carcinoma: A systematic review and meta-analysis based on comparative studies. Front. Oncol. 2022, 12, 964256. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.G.; Khandwala, Y.S.; Kim, J.H.; Han, D.H.; Li, S.; Wang, Y.; Chang, S.L.; Chung, B.I. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA 2017, 318, 1561–1568. [Google Scholar] [CrossRef]
- Peng, L.; Mehmud, I.; Meng, C.; Tang, D.; Li, K.; Gan, L.; Li, J.; Yi, F.; Li, Y. Comparison of perioperative outcomes and complications of laparoscopic and robotic nephroureterectomy approaches in patients with upper-tract urothelial carcinoma. Ann. Surg. Oncol. 2023, 30, 3805–3816. [Google Scholar] [CrossRef]

| RARNU | LRNU | p | SMD | ||
|---|---|---|---|---|---|
| N | 405 | 1262 | |||
| NU | 394 | 1257 | |||
| Segmental | 11 | 5 | |||
| ECOG (%) | 0 | 211 (52.1) | 631 (50.0) | 0.217 | 0.153 |
| 1 | 172 (42.5) | 521 (41.3) | |||
| 2 | 20 (4.9) | 89 (7.1) | |||
| 3 | 2 (0.5) | 16 (1.3) | |||
| 4 | 0 (0.0) | 5 (0.4) | |||
| Sex (%) | Male | 172 (42.5) | 520 (41.2) | 0.695 | 0.026 |
| Female | 233 (57.5) | 742 (58.8) | |||
| Age (mean (SD)) | 69.25 (10.26) | 68.15 (10.51) | 0.066 * | 0.106 | |
| BMI (%) | Normal | 241 (59.5) | 776 (61.5) | 0.513 | 0.041 |
| Overweight | 164 (40.5) | 486 (38.5) | |||
| Cell Type (%) | Urothelial | 346 (85.4) | 1124 (89.1) | 0.126 | 0.112 |
| UC with variants | 55 (13.6) | 131 (10.4) | |||
| Others | 4 (1.0) | 7 (0.6) | |||
| Side (%) | Left | 209 (51.6) | 643 (51.0) | 0.948 | 0.019 |
| Right | 189 (46.7) | 599 (47.5) | |||
| Both | 7 (1.7) | 20 (1.6) | |||
| Location (%) | Non-Visible | 1 (0.2) | 1 (0.1) | 0.645 | 0.086 |
| Renal Pelvis | 163 (40.2) | 546 (43.3) | |||
| Ureter | 142 (35.1) | 402 (31.9) | |||
| Bladder Cuff | 1 (0.2) | 2 (0.2) | |||
| Multiple | 98 (24.2) | 311 (24.6) | |||
| Multiple (%) | No | 262 (64.7) | 825 (65.4) | 0.849 | 0.014 |
| Yes | 143 (35.3) | 437 (34.6) | |||
| Size (%) | <2 cm | 131 (32.3) | 416 (33.0) | 0.865 | 0.013 |
| ≥2 cm | 274 (67.7) | 846 (67.0) | |||
| Pathological Stage (%) | Stage 0a/0is | 64 (15.8) | 204 (16.2) | 0.167 | 0.14 |
| Stage I | 104 (25.7) | 359 (28.4) | |||
| Stage II | 70 (17.3) | 238 (18.9) | |||
| Stage III | 125 (30.9) | 375 (29.7) | |||
| Stage IV | 42 (10.4) | 86 (6.8) | |||
| Grade (%) | Low Grade | 43 (10.6) | 122 (9.7) | 0.001 ** | 0.237 |
| High Grade | 351 (86.7) | 1039 (82.3) | |||
| Not Available | 11 (2.7) | 101 (8.0) | |||
| Bladder Cancer (%) | No | 316 (78.0) | 936 (74.2) | 0.268 | 0.094 |
| Previous History of Bladder UC | 24 (5.9) | 96 (7.6) | |||
| Concurrent Bladder UC | 65 (16.0) | 230 (18.2) | |||
| CIS (%) | No | 280 (69.1) | 919 (72.8) | 0.17 | 0.081 |
| Yes | 125 (30.9) | 343 (27.2) | |||
| LVI (%) | No | 326 (80.5) | 1051 (83.3) | 0.226 | 0.072 |
| Yes | 79 (19.5) | 211 (16.7) | |||
| Surgical Margin (%) | Free | 384 (94.8) | 1226 (97.1) | 0.037 * | 0.119 |
| Positive | 21 (5.2) | 36 (2.9) | |||
| Pre-operation Hydronephrosis (%) | No | 230 (56.8) | 660 (52.3) | 0.129 | 0.09 |
| Yes | 175 (43.2) | 602 (47.7) | |||
| Tumor Necrosis (%) | No | 352 (86.9) | 1079 (85.5) | 0.53 | 0.041 |
| Yes | 53 (13.1) | 183 (14.5) | |||
| Chemotherapy Type (%) | No | 275 (67.9) | 907 (71.9) | 0.062 * | 0.128 |
| Peri-OP Adjuvant | 99 (24.4) | 295 (23.4) | |||
| Salvage/Palliative | 31 (7.7) | 60 (4.8) | |||
| Bladder Cuff Resection (%) | Not Perform BCR | 11 (2.7) | 42 (3.3) | <0.001 *** | 2.402 |
| Open Incision | 81 (20.0) | 879 (69.7) | |||
| Residual Bladder Cuff (%) | 14 (17.3) | 166 (18.9) | |||
| Transurethral Incision | 9 (2.2) | 52 (4.1) | |||
| Laparoscopy | 7 (1.7) | 284 (22.5) | |||
| Residual Bladder Cuff (%) | 46 (16.2) | ||||
| Robot-Assisted | 297 (73.3) | 5 (0.4) | |||
| Residual Bladder Cuff (%) | 42 (14.1) | ||||
| Post-Operation Intravesical C/T Instillation (%) | No | 335 (82.7) | 1200 (95.1) | <0.001 *** | 0.402 |
| Intravesical Therapy | 70 (17.3) | 62 (4.9) | |||
| Overlap | Unweighted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RARNU | LRNU | p | SMD | RARNU | LRNU | p | SMD | ||
| Clavien-Dindo Classification (%) | No Complication | 468.6 (56.3) | 541.5 (65.0) | 0.122 | 0.245 | 259 (64.0) | 861 (68.2) | 0.079 * | 0.180 |
| Grade I | 154.7 (18.6) | 105.3 (12.6) | 66 (16.3) | 148 (11.7) | |||||
| Grade II | 194.4 (23.3) | 160.5 (19.3) | 71 (17.5) | 205 (16.2) | |||||
| Grade III | 14.7 (1.8) | 18.2 (2.2) | 6 (1.5) | 31 (2.4) | |||||
| Grade IV | 0.3 (0.0) | 7.3 (0.9) | 2 (0.5) | 16 (1.3) | |||||
| Grade V | 0.2 (0.0) | 0.1 (0.0) | 1 (0.2) | 1 (0.1) | |||||
| Residual Bladder Cuff (%) | No | 656.1 (78.8) | 652.7 (78.4) | 0.921 | 0.010 | 339 (83.7) | 1020 (80.8) | 0.22 | 0.075 |
| Yes | 176.9 (21.2) | 180.3 (21.6) | 66 (16.3) | 242 (19.2) | |||||
| Overall Mortality (%) | No | 575.5 (69.1) | 487.2 (58.5) | 0.034 * | 0.222 | 285 (70.4) | 747 (59.2) | <0.001 *** | 0.236 |
| (Within 5 years) | Yes | 257.5 (30.9) | 345.8 (41.5) | 120 (29.6) | 515 (40.8) | ||||
| UTUC Mortality (%) | No | 687.8 (82.6) | 696.1 (83.6) | 0.793 | 0.027 | 344 (84.9) | 1102 (87.3) | 0.252 | 0.069 |
| (Within 5 years) | Yes | 145.2 (17.4) | 136.9 (16.4) | 61 (15.1) | 160 (12.7) | ||||
| Disease-Free (%) | No | 176.1 (21.1) | 183.4 (22.0) | 0.834 | 0.021 | 98 (24.2) | 244 (19.3) | 0.042 * | 0.118 |
| Yes | 656.9 (78.9) | 649.6 (78.0) | 307 (75.8) | 1018 (80.7) | |||||
| Bladder Recurrence (%) | No | 616.4 (74.0) | 615.5 (73.9) | 0.981 | 0.002 | 308 (76.0) | 921 (73.0) | 0.247 | 0.07 |
| Yes | 216.6 (26.0) | 217.5 (26.1) | 97 (24.0) | 341 (27.0) | |||||
| Follow-Up OS/CSS (Mean(SD)) | 52.03 (33.43) | 51.65 (37.79) | 0.913 | 0.011 | 44.45 (30.64) | 52.51 (38.88) | <0.001 *** | 0.23 | |
| OS | CSS | DFS | |||||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | ||
| Approach | RARNU | 1 | 1 | 1 | |||
| LRNU | 1.21 (0.85, 1.71) | 0.298 | 0.80 (0.49, 1.30) | 0.362 | 0.80 (0.63, 1.02) | 0.071 * | |
| ECOG | 0 | 1 | 1 | ||||
| 1 | 1.22 (1.03, 1.46) | 0.024 * | 1.33 (1.06, 1.68) | 0.015 * | |||
| 2 | 1.82 (1.38, 2.41) | <0.001 *** | 1.29 (0.84, 1.99) | 0.25 | |||
| 3 | 2.02 (1.20, 3.38) | 0.008 ** | 1.74 (0.54, 5.57) | 0.352 | |||
| 4 | 1.53 (0.56, 4.19) | 0.406 | 2.06 (0.28,14.92) | 0.476 | |||
| Sex | Male | 1 | 1 | ||||
| Female | 0.80 (0.68, 0.95) | 0.009 ** | 0.70 (0.53, 0.92) | 0.010 * | |||
| Age | Mean | 1.03 (1.02, 1.04) | <0.001 *** | 1.04 (1.02, 1.05) | <0.001 *** | 1.03 (1.01, 1.04) | <0.001 *** |
| BMI | Normal | 1 | |||||
| Overweight | 0.79 (0.67, 0.94) | 0.007 ** | |||||
| Multiple | No | 1 | 1 | 1 | |||
| Yes | 1.22 (1.04, 1.45) | 0.017 * | 1.65 (1.26, 2.16) | <0.001 *** | 1.26 (1.00, 1.58) | 0.049 * | |
| Size | <2 cm | 1 | 1 | ||||
| ≥2 cm | 1.50 (1.02, 2.19) | 0.038 * | 1.28 (0.96, 1.71) | 0.087 * | |||
| Pathological Stage | Stage 0a/0is | 1 | 1 | 1 | |||
| Stage I | 1.34 (0.98, 1.83) | 0.067 * | 2.32 (0.96, 5.63) | 0.062 * | 1.31 (0.76, 2.25) | 0.327 | |
| Stage II | 1.35 (0.96, 1.90) | 0.088 * | 3.58 (1.48, 8.66) | 0.005 ** | 1.84 (1.06, 3.17) | 0.030 * | |
| Stage III | 2.04 (1.47, 2.84) | <0.001 *** | 5.25 (2.22, 12.42) | <0.001 *** | 3.32 (1.97, 5.60) | <0.001 *** | |
| Stage IV | 5.06 (3.35, 7.64) | <0.001 *** | 13.81 (5.55, 34.36) | <0.001 *** | 8.31 (4.62,14.93) | <0.001 *** | |
| Grade | Low Grade | 1 | |||||
| High Grade | 1.58 (1.08, 2.32) | 0.019 * | |||||
| Not Available | 3.13 (2.03, 4.83) | <0.001 *** | |||||
| Bladder Cancer | No | 1 | 1 | ||||
| Previous History of Bladder UC | 0.99 (0.72, 1.37) | 0.957 | 1.36 (0.85, 2.17) | 0.204 | |||
| Concurrent Bladder UC | 1.23 (1.01, 1.51) | 0.043 * | 1.36 (1.02, 1.80) | 0.034 * | |||
| CIS | No | 1 | 1 | ||||
| Yes | 0.81 (0.67, 0.98) | 0.026 * | 0.75 (0.59, 0.96) | 0.023 * | |||
| LVI | No | 1 | 1 | 1 | |||
| Yes | 1.19 (0.97, 1.47) | 0.096 * | 1.42 (1.04, 1.93) | 0.025 * | 1.37 (1.06, 1.77) | 0.015 * | |
| Surgical Margin | Free | 1 | 1 | 1 | |||
| Positive | 1.64 (1.14, 2.35) | 0.007 ** | 1.67 (1.05, 2.65) | 0.029 * | 1.70 (1.16, 2.47) | 0.006 ** | |
| Pre-operation Hydronephrosis | No | 1 | |||||
| Yes | 0.78 (0.62, 0.99) | 0.039 * | |||||
| Bladder Cuff Resection | Not Perform BCR | 1 | 1 | ||||
| Open Incision | 0.52 (0.34, 0.79) | 0.002 ** | 0.57 (0.28, 1.19) | 0.137 | |||
| Transurethral Incision | 0.31 (0.17, 0.56) | <0.001 *** | 0.53 (0.21, 1.34) | 0.181 | |||
| Laparoscopy | 0.39 (0.25, 0.61) | <0.001 *** | 0.50 (0.23, 1.07) | 0.075 * | |||
| Robot-Assisted | 0.48 (0.28, 0.83) | 0.009 ** | 0.44 (0.19, 1.03) | 0.058 * | |||
| Residual Bladder Cuff | No | 1 | 1 | 1 | |||
| Yes | 0.54 (0.42, 0.70) | <0.001 *** | 0.55 (0.36, 0.82) | 0.004 ** | 0.76 (0.58, 1.01) | 0.058 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-L.; Tsai, C.-Y.; Cheng, P.-Y.; Wu, W.-J.; Tsai, Y.-C. Robot-Assisted Radical Nephroureterectomy: A Safe and Effective Option for Upper Tract Urothelial Carcinoma, Especially for Novice Surgeons. Cancers 2025, 17, 1394. https://doi.org/10.3390/cancers17091394
Chang C-L, Tsai C-Y, Cheng P-Y, Wu W-J, Tsai Y-C. Robot-Assisted Radical Nephroureterectomy: A Safe and Effective Option for Upper Tract Urothelial Carcinoma, Especially for Novice Surgeons. Cancers. 2025; 17(9):1394. https://doi.org/10.3390/cancers17091394
Chicago/Turabian StyleChang, Chia-Lun, Chung-You Tsai, Pai-Yu Cheng, Wen-Jeng Wu, and Yao-Chou Tsai. 2025. "Robot-Assisted Radical Nephroureterectomy: A Safe and Effective Option for Upper Tract Urothelial Carcinoma, Especially for Novice Surgeons" Cancers 17, no. 9: 1394. https://doi.org/10.3390/cancers17091394
APA StyleChang, C.-L., Tsai, C.-Y., Cheng, P.-Y., Wu, W.-J., & Tsai, Y.-C. (2025). Robot-Assisted Radical Nephroureterectomy: A Safe and Effective Option for Upper Tract Urothelial Carcinoma, Especially for Novice Surgeons. Cancers, 17(9), 1394. https://doi.org/10.3390/cancers17091394






