Understanding Merkel Cell Carcinoma: Pathogenic Signaling, Extracellular Matrix Dynamics, and Novel Treatment Approaches
Simple Summary
Abstract
1. Introduction
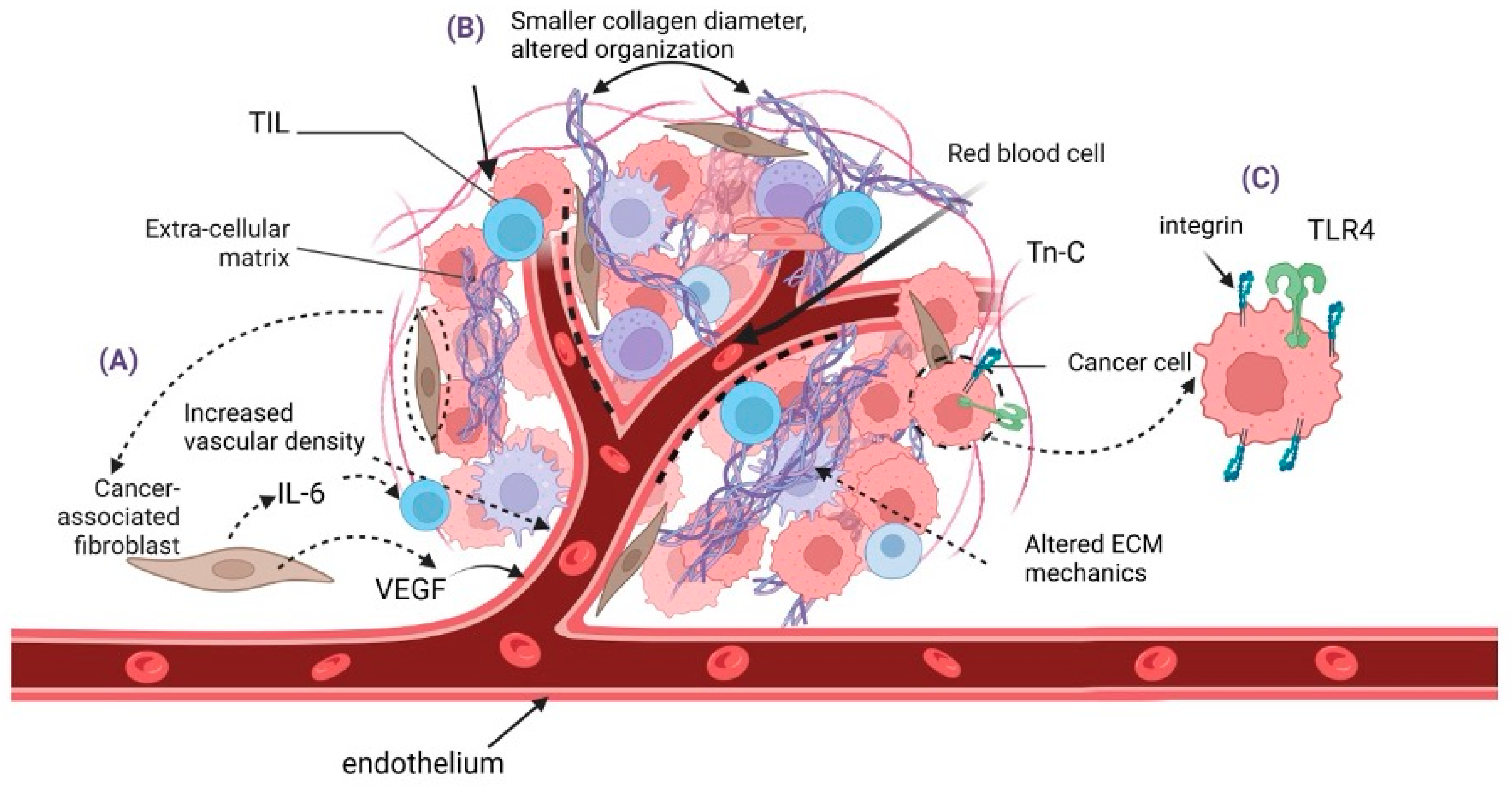
2. Tumor Microenvironment/Tumor Niche
2.1. Role of the Extracellular Matrix in Merkel Cell Carcinoma
2.2. Cancer-Associated Fibroblasts (CAFs)
2.3. Endothelial Cells and Angiogenesis
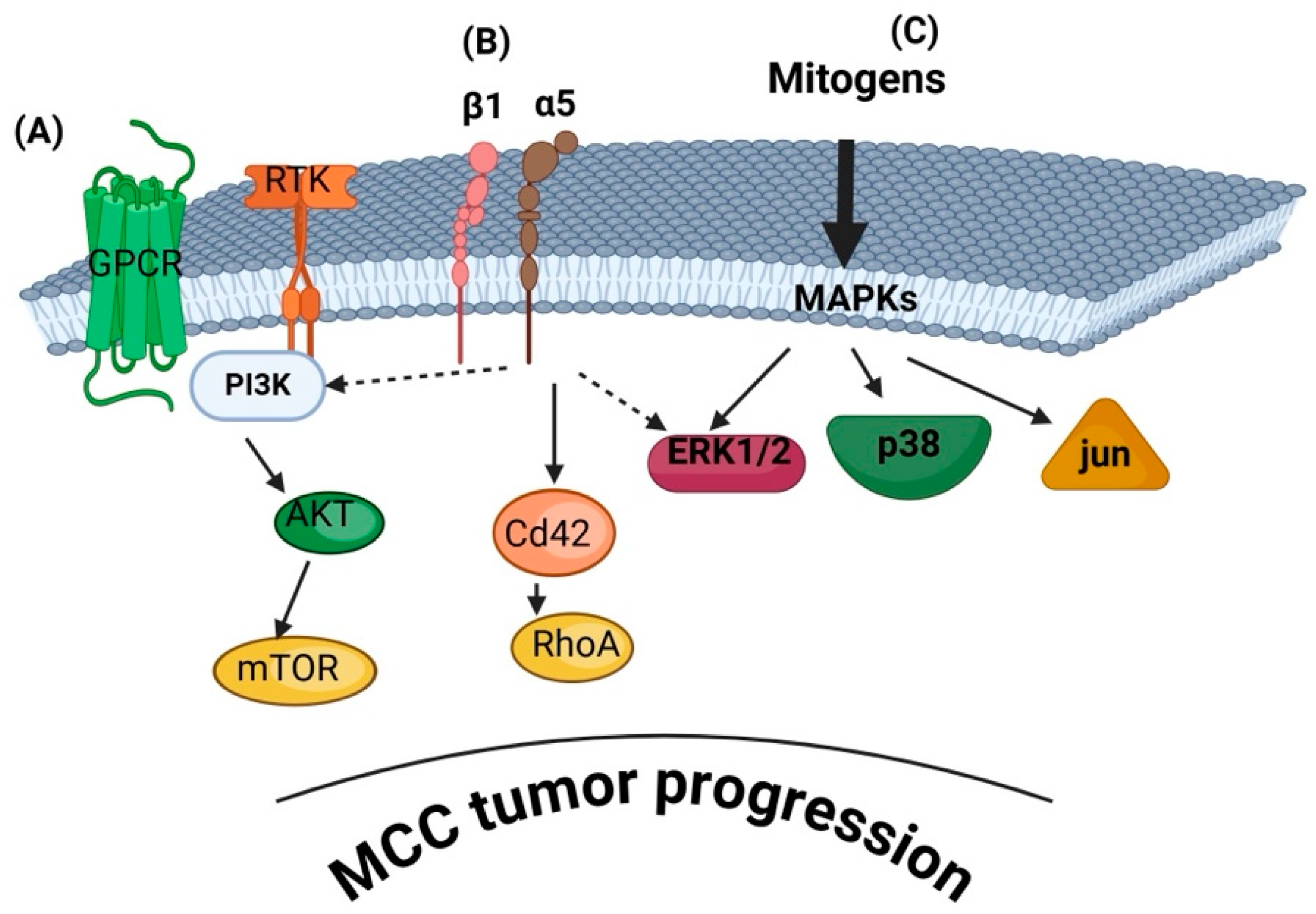
3. Signaling Mechanisms Involved in MCC Progression
3.1. PI3K/AKT/mTOR Pathway
3.2. MAPK/ERK Pathway
3.3. Notch Signaling Pathway
3.4. TP53 and RB Tumor Suppressor Pathways
3.5. Wnt/β-Catenin Pathway
3.6. Extracellular Matrix (ECM)-Derived Signaling in MCC
3.6.1. Integrin Signaling
3.6.2. ECM Remodeling Enzymes
3.6.3. Mechanotransduction
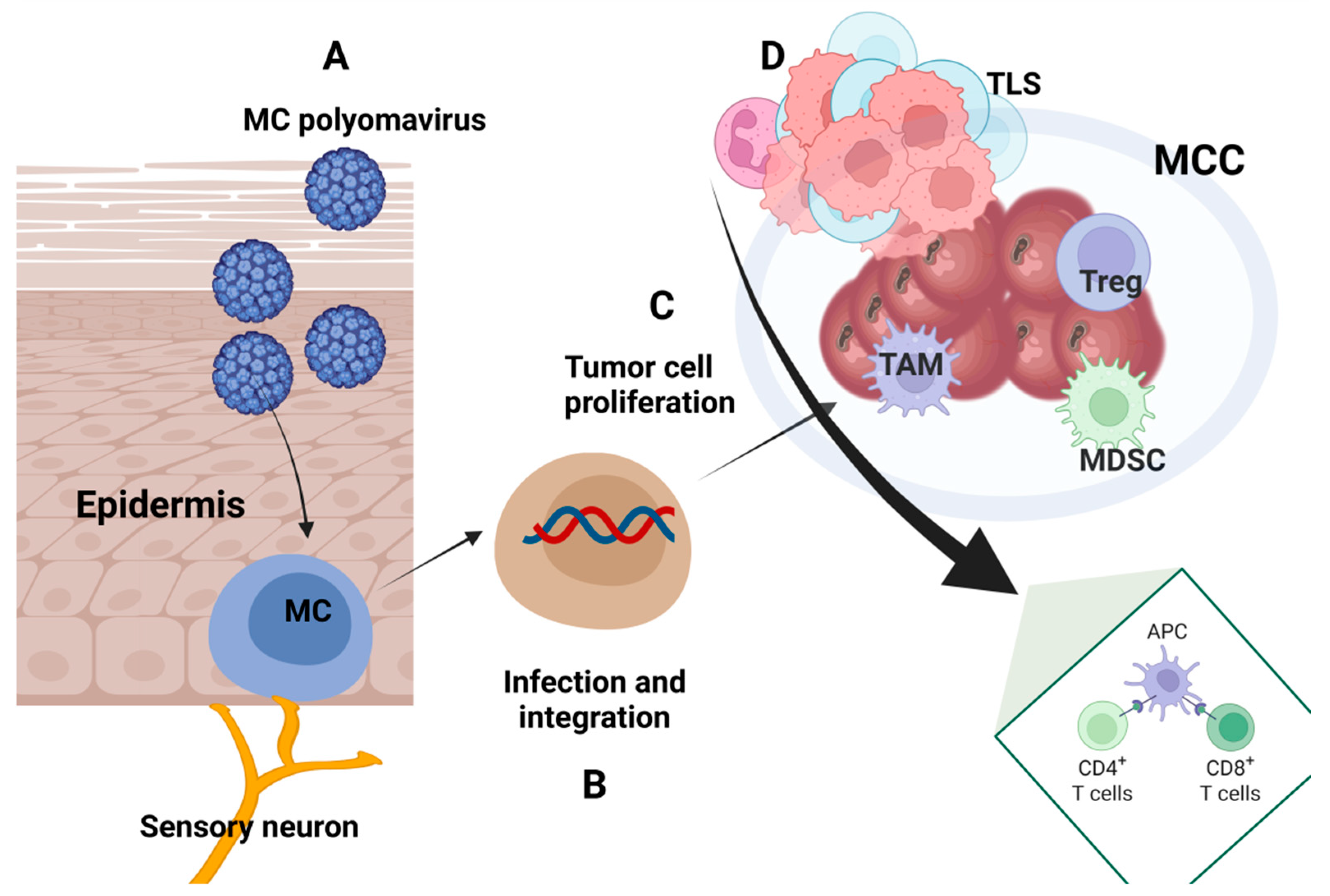
4. Immune Traits in Merkel Cell Carcinoma
4.1. Immune Characteristics of Tumor Tissue
4.2. Circulatory Immune Parameters
4.3. Immune Patterns Induced by MCPyV
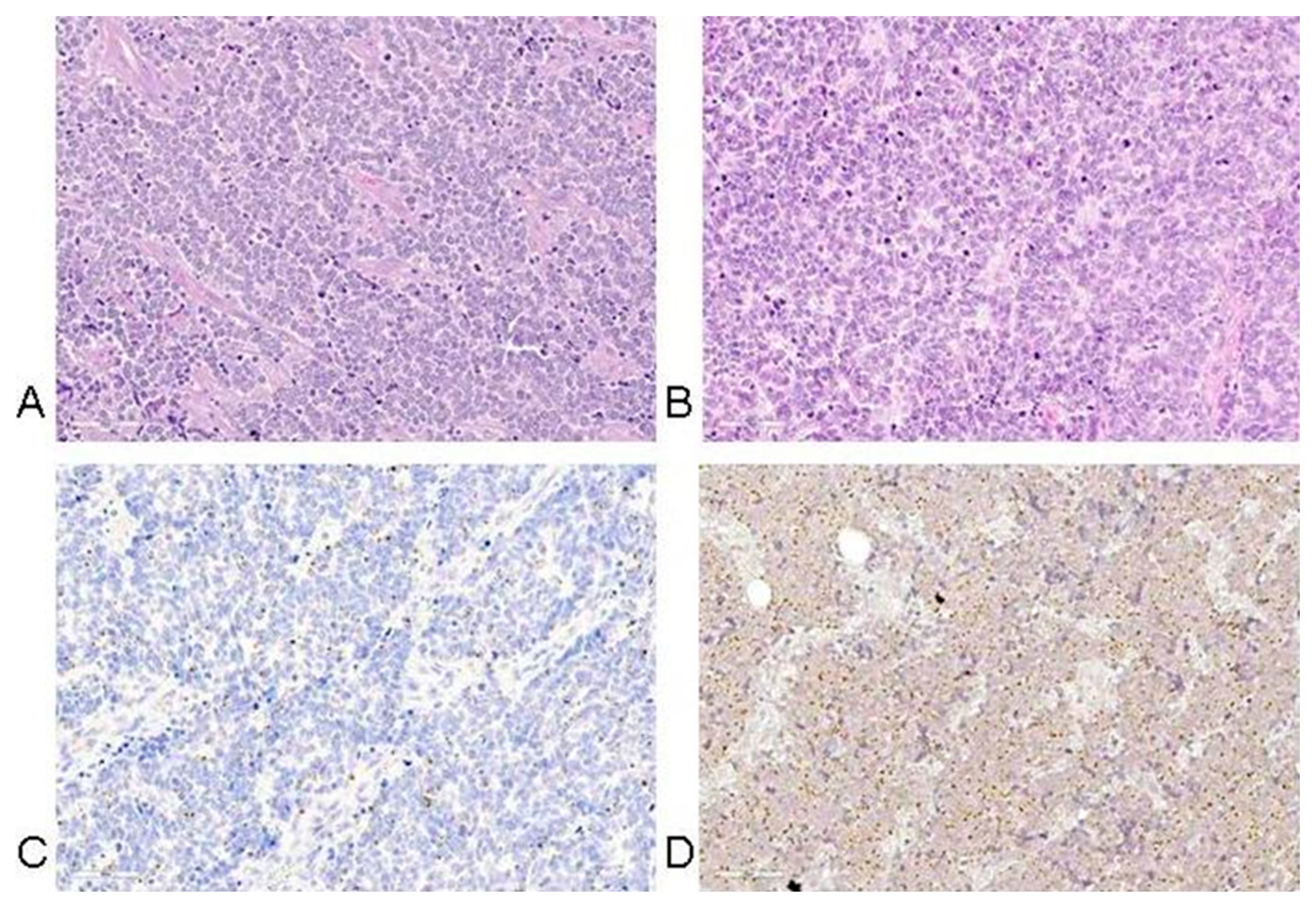
5. Histopathology of MCC
6. Therapy
6.1. Immune Therapy in Merkel Cell Carcinoma
6.2. Other Immune-Mediated Therapies
6.3. Novel Therapy Approaches
6.3.1. VEGF Inhibitors
6.3.2. SSTs (Somatostatin Analogs)
6.3.3. Antivirals
6.3.4. PI3K Inhibitors
6.3.5. P53 Targeting Therapeutics
6.3.6. NOTCH Signaling Targeting Approaches
6.3.7. MAPK/ERK-Targeting Drugs
6.3.8. Integrins as MCC Therapy Targets
6.3.9. MMPs and LOX Inhibitors
6.3.10. CAF-Targeting Approaches
7. Future Developments
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Acronym | Abbreviation |
| MCC | Merkel Cell Carcinoma |
| MCPyV | Merkel Cell Polyomavirus |
| UV exposure | Ultraviolet exposure |
| pRb | Retinoblastoma protein |
| P53 | Tumor protein 53 |
| PI3K | Phosphatidylinositol-3-kinase |
| AKT | Protein kinase B |
| mTOR | Mammalian target of rapamycin |
| MAPK | Mitogen-activated protein kinase |
| TME | Tumor Microenvironment |
| PD-1 | Programmed cell death 1 |
| PD-L1 | Programmed death - ligand 1 |
| ECM | Extracellular matrix |
| MMPs | Matrix metalloproteinases |
| AJCC | American Joint Cancer Committee |
| CAFs | Cancer associated fibroblasts |
| GAGs | Glycosaminoglycans |
| PGs | Proteoglycans |
| LOX | Lysyl oxidase |
| Tn-C | Tenascin-C |
| TLR4 | Toll-like receptor 4 |
| FAK | Focal adhesion kinase |
| YAP | Yes-associated protein |
| TGFβ | Transforming growth facto beta |
| PDGF | Platelet-derived growth factor |
| FGF | Fibroblast growth factor |
| HGF | Hepatocyte growth factor |
| VEGF | Vascular endothelial growth factor |
| TNF | Tumor necrosis factor |
| IFN | Interferon |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| IL-6 | Interleukin-6 |
| CTGFβ | Connective tissue growth factor |
| EGF | Epidermal growth factor |
| GAS6 | Growth arrest-specific protein 6 |
| SFRP1 | Galectin-1, secreted frizzled-related protein 1 |
| SHH | Sonic hedgehog protein |
| BMP | Bone morphogenetic protein |
| scRNAseq | Single-cell RNA sequencing |
| miR-375 | MicroRNA-375 |
| RBPJ | Recombination signal binding protein for immunoglobulin kappa J region |
| CD8 | Cluster of differentiation 8 |
| CD4 | Cluster of differentiation 4 |
| TILs | Tumor-infiltrating Lymphocytes |
| HIFs | Hypoxia-inducible factors |
| αSMA-1+ cells | α Smooth muscle actin positive stromal cells |
| CD31 | Cluster of differentiation 31 |
| B7-H3 | B7 homolog 3 protein |
| VEGFR | Vascular endothelial growth factor receptor |
| RTKs | Receptor tyrosine kinases |
| GPCRs | G-protein-coupled receptors |
| PIP2 | Phosphatidylinositol-4,5-bisphosphate |
| PIP3 | Phosphatidylinositol-3,4,5-trisphosphate |
| FOXO | Forkhead box transcription factors |
| PTEN | Phosphatase and tensin homolog |
| TMB | Tumor mutation burden |
| BRCA | Breast Cancer gene |
| HRAS | Harvey rat sarcoma viral oncogene homolog |
| TSC | Tuberous sclerosis proteins |
| ARID1A | AT-Rich Interaction Domain 1A |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A |
| NOTCH1 | Neurogenic locus notch homolog protein 1 |
| MEK-ERK | Mitogen-activated protein kinase/extracellular-signal-regulated kinase |
| JAK-STAT | Janus kinase/signal transducer and activator of transcription |
| SCF | stem cell factor |
| MKK4 | Mitogen-activated protein kinase (MAPK) kinase 4 |
| NICD | Notch intracellular domain |
| TA | Tumor antigens |
| HDM-2 | Human double minute 2 |
| BCL-2 | B-cell lymphoma-2 protein |
| FLIP | Fas-associated death domain-like interleukin-1-β-converting enzyme-inhibitory protein |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| ST | Small tumor antigen |
| LT | Large tumor antigen |
| PP4C | Phosphatase catalytic subunit |
| PIEZO | Mechanosensitive ion channels located in the cell membrane and function as key cellular mechanotransducers |
| GPCR | G protein-coupled receptor |
| IHC | Immunohistochemistry |
| HLA | Human leukocyte antigens |
| aAPC | Artificial antigen-presenting |
| ISGF3 | Interferon-stimulated gene factor 3 |
| TAM | Tumor-associated macrophages |
| APC | Antigen-presenting cells |
| NFAT | Nuclear factor of activated T cells |
| NFP | Neurofilament |
| TTF-1 | Thyroid transcription factor 1 |
| SLNB | Sentinel Lymph Node Biopsy |
| TCM | Central memory T cells |
| ROS | Reactive oxygen species |
| STING | Stimulator of interferon genes |
| TCR | T-cell receptor |
| DFs | Dermal fibroblasts |
| ACT | Adoptive T cell transfer |
| PET | Positron Emission Tomography-Computed Tomography |
| HATs | Histone acetyltransferases |
| HDACs | Histone deacetylases |
| BET | Bromodomain and extra-terminal |
References
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel Cell Carcinoma: Current US Incidence and Projected Increases Based on Changing Demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463.e2. [Google Scholar] [CrossRef] [PubMed]
- Silling, S.; Kreuter, A.; Gambichler, T.; Meyer, T.; Stockfleth, E.; Wieland, U. Epidemiology of Merkel Cell Polyomavirus Infection and Merkel Cell Carcinoma. Cancers 2022, 14, 6176. [Google Scholar] [CrossRef]
- Siqueira, S.O.M.; Campos-do-Carmo, G.; dos Santos, A.L.S.; Martins, C.; de Melo, A.C. Merkel Cell Carcinoma: Epidemiology, Clinical Features, Diagnosis and Treatment of a Rare Disease. An. Bras. Dermatol. 2023, 98, 277. [Google Scholar] [CrossRef]
- Paulson, K.G.; Iyer, J.G.; Tegeder, A.R.; Thibodeau, R.; Schelter, J.; Koba, S.; Schrama, D.; Simonson, W.T.; Lemos, B.D.; Byrd, D.R.; et al. Transcriptome-Wide Studies of Merkel Cell Carcinoma and Validation of Intratumoral CD8+ Lymphocyte Invasion as an Independent Predictor of Survival. J. Clin. Oncol. 2011, 29, 1539–1546. [Google Scholar] [CrossRef]
- Nwogu, N.; Boyne, J.R.; Dobson, S.J.; Poterlowicz, K.; Blair, G.E.; Macdonald, A.; Mankouri, J.; Whitehouse, A. Cellular Sheddases Are Induced by Merkel Cell Polyomavirus Small Tumour Antigen to Mediate Cell Dissociation and Invasiveness. PLoS Pathog. 2018, 14, e1007276. [Google Scholar] [CrossRef]
- Laikova, K.V.; Oberemok, V.V.; Krasnodubets, A.M.; Gal’chinsky, N.V.; Useinov, R.Z.; Novikov, I.A.; Temirova, Z.Z.; Gorlov, M.V.; Shved, N.A.; Kumeiko, V.V.; et al. Advances in the Understanding of Skin Cancer: Ultraviolet Radiation, Mutations, and Antisense Oligonucleotides as Anticancer Drugs. Molecules 2019, 24, 1516. [Google Scholar] [CrossRef]
- Zaggana, E.; Konstantinou, M.P.; Krasagakis, G.H.; de Bree, E.; Kalpakis, K.; Mavroudis, D.; Krasagakis, K. Merkel Cell Carcinoma—Update on Diagnosis, Management and Future Perspectives. Cancers 2023, 15, 103. [Google Scholar] [CrossRef]
- Horny, K.; Gerhardt, P.; Hebel-Cherouny, A.; Wülbeck, C.; Utikal, J.; Becker, J.C. Mutational Landscape of Virus- and UV-Associated Merkel Cell Carcinoma Cell Lines Is Comparable to Tumor Tissue. Cancers 2021, 13, 649. [Google Scholar] [CrossRef]
- Moshiri, A.S.; Doumani, R.; Yelistratova, L.; Blom, A.; Lachance, K.; Shinohara, M.M.; Delaney, M.; Chang, O.; McArdle, S.; Thomas, H.; et al. Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J. Investig. Dermatol. 2016, 137, 819. [Google Scholar] [CrossRef]
- Yang, A.; Wijaya, W.A.; Yang, L.; He, Y.; Cen, Y.; Chen, J. The Impact of Merkel Cell Polyomavirus Positivity on Prognosis of Merkel Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 1020805. [Google Scholar] [CrossRef]
- Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Böhling, T.; Joensuu, H. Clinical Factors Associated With Merkel Cell Polyomavirus Infection in Merkel Cell Carcinoma. JNCI J. Natl. Cancer Inst. 2009, 101, 938–945. [Google Scholar] [CrossRef]
- Stachyra, K.; Dudzisz-śledź, M.; Bylina, E.; Szumera-ciećkiewicz, A.; Spałek, M.J.; Bartnik, E.; Rutkowski, P.; Czarnecka, A.M. Merkel Cell Carcinoma from Molecular Pathology to Novel Therapies. Int. J. Mol. Sci. 2021, 22, 6305. [Google Scholar] [CrossRef]
- Brazel, D.; Kumar, P.; Doan, H.; Pan, T.; Shen, W.; Gao, L.; Moyers, J.T. Genomic Alterations and Tumor Mutation Burden in Merkel Cell Carcinoma. JAMA Netw. Open 2023, 6, e2249674. [Google Scholar] [CrossRef]
- Smith, V.A.; Camp, E.R.; Lentsch, E.J. Merkel Cell Carcinoma: Identification of Prognostic Factors Unique to Tumors Located in the Head and Neck Based on Analysis of SEER Data. Laryngoscope 2012, 122, 1283–1290. [Google Scholar] [CrossRef]
- Hussain, S.K.; Sundquist, J.; Hemminki, K. Incidence Trends of Squamous Cell and Rare Skin Cancers in the Swedish National Cancer Registry Point to Calendar Year and Age-Dependent Increases. J. Investig. Dermatol. 2010, 130, 1323–1328. [Google Scholar] [CrossRef]
- Ouyang, K.; Zheng, D.X.; Agak, G.W. T-Cell Mediated Immunity in Merkel Cell Carcinoma. Cancers 2022, 14, 6058. [Google Scholar] [CrossRef]
- Fennig, S.; Landman, Y.; Brenner, R.; Billan, S.; Fenig, E. Merkel Cell Carcinoma in Lymph Nodes with and without Primary Origin. Cancer Med. 2022, 11, 1484. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Andrew, T.W.; Erdmann, S.; Alrawi, M.; Plummer, R.; Shalhout, S.Z.; Sondak, V.; Brownell, I.; Lovat, P.E.; Rose, A. A Multivariable Disease Specific Model Enhances Prognostication beyond Current Merkel Cell Carcinoma Staging: An International Cohort Study of 10,958 Patients. J. Am. Acad. Dermatol. 2024, 92, 520–527. [Google Scholar] [CrossRef]
- Sunshine, J.C.; Jahchan, N.S.; Sage, J.; Choi, J. Are There Multiple Cells of Origin of Merkel Cell Carcinoma? Oncogene 2018, 37, 1409. [Google Scholar] [CrossRef]
- Albertini, S.; Martuscelli, L.; Borgogna, C.; Virdi, S.; Indenbirken, D.; Lo Cigno, I.; Griffante, G.; Calati, F.; Boldorini, R.; Fischer, N.; et al. Cancer-Associated Fibroblasts Exert Proangiogenic Activity in Merkel Cell Carcinoma. J. Investig. Dermatol. 2023, 143, 965–976.e15. [Google Scholar] [CrossRef]
- Zheng, Z.; Yoo, D.S.; Li, S.; Pei, M.; Lee, S.G.; Kim, J.Y.; Chung, K.Y.; Roh, M.R. Implication of IL6-Positive Cancer-Associated Fibroblasts in Merkel Cell Carcinoma Pathogenesis: A Possible Modulator of Immune Microenvironment. Anticancer. Res. 2022, 42, 4359–4369. [Google Scholar] [CrossRef]
- Laurito, T.L.; França, F.T.; Vieira-Damiani, G.; Pelegati, V.B.; Baratti, M.O.; de Carvalho, H.F.; Cesar, C.L.; de Moraes, A.M.; Cintra, M.L.; Teixeira, F. The Texture of Collagen in the Microenvironments of Merkel Cell Carcinoma. Medicine 2021, 100, e27925. [Google Scholar] [CrossRef]
- Fan, K.; Spassova, I.; Gravemeyer, J.; Ritter, C.; Horny, K.; Lange, A.; Gambichler, T.; Ødum, N.; Schrama, D.; Schadendorf, D.; et al. Merkel Cell Carcinoma-Derived Exosome-Shuttle MiR-375 Induces Fibroblast Polarization by Inhibition of RBPJ and P53. Oncogene 2020, 40, 980–996. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of Extracellular Matrix Viscoelasticity on Cellular Behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Berdiaki, A.; Giatagana, E.M.; Tzanakakis, G.; Nikitovic, D. The Landscape of Small Leucine-Rich Proteoglycan Impact on Cancer Pathogenesis with a Focus on Biglycan and Lumican. Cancers 2023, 15, 3549. [Google Scholar] [CrossRef]
- Kavasi, R.M.; Neagu, M.; Constantin, C.; Munteanu, A.; Surcel, M.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Matrix Effectors in the Pathogenesis of Keratinocyte-Derived Carcinomas. Front. Med. 2022, 9, 879500. [Google Scholar] [CrossRef]
- Fromme, J.E.; Zigrino, P. The Role of Extracellular Matrix Remodeling in Skin Tumor Progression and Therapeutic Resistance. Front. Mol. Biosci. 2022, 9, 864302. [Google Scholar] [CrossRef]
- Zeltz, C.; Primac, I.; Erusappan, P.; Alam, J.; Noel, A.; Gullberg, D. Cancer-Associated Fibroblasts in Desmoplastic Tumors: Emerging Role of Integrins. Semin. Cancer Biol. 2020, 62, 166–181. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Tzanakakis, P.; Spyridaki, I.; Pérez, S.; Nikitovic, D. Extracellular Matrix Components and Mechanosensing Pathways in Health and Disease. Biomolecules 2024, 14, 1186. [Google Scholar] [CrossRef]
- Kagan, H.M.; Li, W. Lysyl Oxidase: Properties, Specificity, and Biological Roles inside and Outside of the Cell. J. Cell Biochem. 2003, 88, 660–672. [Google Scholar] [CrossRef]
- Becker, J.C.; Kauczok, C.S.; Ugurel, S.; Eib, S.; Bröcker, E.B.; Houben, R. Merkel Cell Carcinoma: Molecular Pathogenesis, Clinical Features and Therapy. J. Dtsch. Dermatol. Ges. 2008, 6, 709–719. [Google Scholar] [CrossRef]
- Koljonen, V.; Jahkola, T.; Tukiainen, E.; Granroth, G.; Haglund, C.; Böhling, T. Tenascin-C in Primary Merkel Cell Carcinoma. J. Clin. Pathol. 2005, 58, 297–300. [Google Scholar] [CrossRef]
- Pilch, H.; Schäffer, U.; Schlenger, K.; Lautz, A.; Tanner, B.; Höckel, M.; Knapstein, P.G. Expression of Tenascin in Human Cervical Cancer--Association of Tenascin Expression with Clinicopathological Parameters. Gynecol. Oncol. 1999, 73, 415–421. [Google Scholar] [CrossRef]
- Stakaitytė, G.; Nwogu, N.; Dobson, S.J.; Knight, L.M.; Wasson, C.W.; Salguero, F.J.; Blackbourn, D.J.; Blair, G.E.; Mankouri, J.; Macdonald, A.; et al. Merkel Cell Polyomavirus Small T Antigen Drives Cell Motility via Rho-GTPase-Induced Filopodium Formation. J. Virol. 2018, 92, e00940-17. [Google Scholar] [CrossRef]
- Konstantinell, A.; Bruun, J.A.; Olsen, R.; Aspar, A.; Škalko-Basnet, N.; Sveinbjørnsson, B.; Moens, U. Secretomic Analysis of Extracellular Vesicles Originating from Polyomavirus-Negative and Polyomavirus-Positive Merkel Cell Carcinoma Cell Lines. Proteomics 2016, 16, 2587–2591. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; van Tienderen, G.S.; Schenke-Layland, K.; van der Laan, L.J.W.; Khalil, A.A.; Verstegen, M.M.A. The Extracellular Matrix as Hallmark of Cancer and Metastasis: From Biomechanics to Therapeutic Targets. Sci. Transl. Med. 2024, 16, eadg3840. [Google Scholar] [CrossRef]
- Liu, W.; Yang, R.; Payne, A.S.; Schowalter, R.M.; Spurgeon, M.E.; Lambert, P.F.; Xu, X.; Buck, C.B.; You, J. Identifying the Target Cells and Mechanisms of Merkel Cell Polyomavirus Infection. Cell Host Microbe 2016, 19, 775–787. [Google Scholar] [CrossRef]
- Suomela, S.; Koljonen, V.; Skoog, T.; Kukko, H.; Böhling, T.; Saarialho-Kere, U. Expression of MMP-10, MMP-21, MMP-26, and MMP-28 in Merkel Cell Carcinoma. Virchows Arch. 2009, 455, 495–503. [Google Scholar] [CrossRef]
- Fernández-Figueras, M.T.; Puig, L.; Musulén, E.; Gilaberte, M.; Lerma, E.; Serrano, S.; Ferrándiz, C.; Ariza, A. Expression Profiles Associated with Aggressive Behavior in Merkel Cell Carcinoma. Mod. Pathol. 2007, 20, 90–101. [Google Scholar] [CrossRef]
- Massi, D.; Franchi, A.; Ketabchi, S.; Paglierani, M.; Pimpinelli, N.; Santucci, M. Expression and Prognostic Significance of Matrix Metalloproteinases and Their Tissue Inhibitors in Primary Neuroendocrine Carcinoma of the Skin. Hum. Pathol. 2003, 34, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Komal, M.; Kedar, M.; Chikhale, S.; Kotkar, P.; Khemnar, P.; More, M. Merkel Cell Carcinoma: Pathogenesis, Clinical Features, Mechanotransduction, and Emerging Therapeutic Perspectives. Int. J. Pharm. Res. Appl. 2025, 10, 1200. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling Pathways in Cancer-Associated Fibroblasts and Targeted Therapy for Cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Glabman, R.A.; Choyke, P.L.; Sato, N. Cancer-Associated Fibroblasts: Tumorigenicity and Targeting for Cancer Therapy. Cancers 2022, 14, 3906. [Google Scholar] [CrossRef]
- Aung, P.P.; Parra, E.R.; Barua, S.; Sui, D.; Ning, J.; Mino, B.; Ledesma, D.A.; Curry, J.L.; Nagarajan, P.; Torres-Cabala, C.A.; et al. B7-H3 Expression in Merkel Cell Carcinoma–Associated Endothelial Cells Correlates with Locally Aggressive Primary Tumor Features and Increased Vascular Density. Clin. Cancer Res. 2019, 25, 3455. [Google Scholar] [CrossRef]
- Expression of Vascular Endothelial Growth Factor Receptor-2 in Merkel Cell Carcinoma—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/17695419/ (accessed on 15 February 2025).
- Tothill, R.; Estall, V.; Rischin, D. Merkel Cell Carcinoma: Emerging Biology, Current Approaches, and Future Directions. Am. Soc. Clin. Oncol. Educ. Book. 2015, 35, e519–e526. [Google Scholar] [CrossRef]
- Jacot, W.; Mollevi, C.; Fina, F.; Lopez-Crapez, E.; Martin, P.M.; Colombo, P.E.; Bibeau, F.; Romieu, G.; Lamy, P.J. High EGFR Protein Expression and Exon 9 PIK3CA Mutations Are Independent Prognostic Factors in Triple Negative Breast Cancers. BMC Cancer 2015, 15, 986. [Google Scholar] [CrossRef]
- Hafner, C.; Houben, R.; Baeurle, A.; Ritter, C.; Schrama, D.; Landthaler, M.; Becker, J.C. Activation of the PI3K/AKT Pathway in Merkel Cell Carcinoma. PLoS ONE 2012, 7, e31255. [Google Scholar] [CrossRef]
- Nardi, V.; Song, Y.; Santamaria-Barria, J.A.; Cosper, A.K.; Lam, Q.; Faber, A.C.; Boland, G.M.; Yeap, B.Y.; Bergethon, K.; Scialabba, V.L.; et al. Activation of PI3K Signaling in Merkel Cell Carcinoma. Clin. Cancer Res. 2012, 18, 1227–1236. [Google Scholar] [CrossRef]
- Iwasaki, T.; Matsushita, M.; Nonaka, D.; Kuwamoto, S.; Kato, M.; Murakami, I.; Nagata, K.; Nakajima, H.; Sano, S.; Hayashi, K. Comparison of Akt/MTOR/4E-BP1 Pathway Signal Activation and Mutations of PIK3CA in Merkel Cell Polyomavirus-Positive and Merkel Cell Polyomavirus-Negative Carcinomas. Hum. Pathol. 2015, 46, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, B.; Kilpinen, S.; Böhling, T.; Koljonen, V.; Sihto, H. Activation of Oncogenic and Immune-Response Pathways Is Linked to Disease-Specific Survival in Merkel Cell Carcinoma. Cancers 2022, 14, 3591. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Limmer, A.L.; Narayanan, D.; Doan, H.Q.; Simonette, R.A.; Rady, P.L.; Tyring, S.K. The Novel AKT Inhibitor Afuresertib Suppresses Human Merkel Cell Carcinoma MKL-1 Cell Growth. Clin. Exp. Dermatol. 2021, 46, 1551–1554. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Fabbrocini, G.; Costa, C.; Carmela Annunziata, M.; Scalvenzi, M. Merkel Cell Carcinoma: Therapeutic Update and Emerging Therapies. Dermatol. Ther. 2019, 9, 209–222. [Google Scholar] [CrossRef]
- Saddozai, U.A.K.; Wang, F.; Cheng, Y.; Lu, Z.; Akbar, M.U.; Zhu, W.; Li, Y.; Ji, X.; Guo, X. Gene Expression Profile Identifies Distinct Molecular Subtypes and Potential Therapeutic Genes in Merkel Cell Carcinoma. Transl. Oncol. 2020, 13, 100816. [Google Scholar] [CrossRef]
- Temblador, A.; Topalis, D.; Andrei, G.; Snoeck, R. Synergistic Targeting of the PI3K/MTOR and MAPK/ERK Pathways in Merkel Cell Carcinoma. Tumour Virus Res. 2022, 14, 200244. [Google Scholar] [CrossRef]
- Panelos, J.; Batistatou, A.; Paglierani, M.; Zioga, A.; Maio, V.; Santi, R.; Pimpinelli, N.; De Giorgi, V.; Santucci, M.; Massi, D. Expression of Notch-1 and Alteration of the E-Cadherin/Beta-Catenin Cell Adhesion Complex Are Observed in Primary Cutaneous Neuroendocrine Carcinoma (Merkel Cell Carcinoma). Mod. Pathol. 2009, 22, 959–968. [Google Scholar] [CrossRef]
- Wardhani, L.O.; Matsushita, M.; Kuwamoto, S.; Nonaka, D.; Nagata, K.; Kato, M.; Kitamura, Y.; Hayashi, K. Expression of Notch 3 and Jagged 1 Is Associated With Merkel Cell Polyomavirus Status and Prognosis in Merkel Cell Carcinoma. Anticancer. Res. 2019, 39, 319–329. [Google Scholar] [CrossRef]
- Gambichler, T.; Majchrzak-Stiller, B.; Peters, I.; Becker, J.C.; Strotmann, J.; Abu Rached, N.; Müller, T.; Uhl, W.; Buchholz, M.; Braumann, C. The Effect of GP-2250 on Cultured Virus-Negative Merkel Cell Carcinoma Cells: Preliminary Results. J. Cancer Res. Clin. Oncol. 2023, 149, 10831–10840. [Google Scholar] [CrossRef]
- Karpinski, P.; Rosales, I.; Laczmanski, L.; Kowalik, A.; Wenson, S.; Hoang, M.P. Expression of Genes Associated With Epithelial-Mesenchymal Transition in Merkel Cell Polyomavirus-Negative Merkel Cell Carcinoma. Lab. Investig. 2023, 103, 100177. [Google Scholar] [CrossRef]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Amini, A.; Bibee, K.; Bolotin, D.; Bordeaux, J.; Chen, P.L.; Contreras, C.M.; et al. NCCN Guidelines® Insights: Merkel Cell Carcinoma, Version 1.2024: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2024, 22, e240002. [Google Scholar] [CrossRef]
- Popp, S.; Waltering, S.; Herbst, C.; Moll, I.; Boukamp, P. UV-B-Type Mutations and Chromosomal Imbalances Indicate Common Pathways for the Development of Merkel and Skin Squamous Cell Carcinomas. Int. J. Cancer 2002, 99, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Böhling, T.; Joensuu, H. Merkel Cell Polyomavirus Infection, Large T Antigen, Retinoblastoma Protein and Outcome in Merkel Cell Carcinoma. Clin. Cancer Res. 2011, 17, 4806–4813. [Google Scholar] [CrossRef]
- Park, D.E.; Cheng, J.; Berrios, C.; Montero, J.; Cortés-Cros, M.; Ferretti, S.; Arora, R.; Tillgren, M.L.; Gokhale, P.C.; DeCaprio, J.A. Dual Inhibition of MDM2 and MDM4 in Virus-Positive Merkel Cell Carcinoma Enhances the P53 Response. Proc. Natl. Acad. Sci. USA 2019, 116, 1027–1032. [Google Scholar] [CrossRef]
- Lassacher, A.; Heitzer, E.; Kerl, H.; Wolf, P. P14ARF Hypermethylation Is Common but INK4a-ARF Locus or P53 Mutations Are Rare in Merkel Cell Carcinoma. J. Investig. Dermatol. 2008, 128, 1788–1796. [Google Scholar] [CrossRef]
- Houben, R.; Dreher, C.; Angermeyer, S.; Borst, A.; Utikal, J.; Haferkamp, S.; Peitsch, W.K.; Schrama, D.; Hesbacher, S. Mechanisms of P53 Restriction in Merkel Cell Carcinoma Cells Are Independent of the Merkel Cell Polyoma Virus T Antigens. J. Investig. Dermatol. 2013, 133, 2453–2460. [Google Scholar] [CrossRef]
- Hesbacher, S.; Pfitzer, L.; Wiedorfer, K.; Angermeyer, S.; Borst, A.; Haferkamp, S.; Scholz, C.J.; Wobser, M.; Schrama, D.; Houben, R. RB1 Is the Crucial Target of the Merkel Cell Polyomavirus Large T Antigen in Merkel Cell Carcinoma Cells. Oncotarget 2016, 7, 32956–32968. [Google Scholar] [CrossRef]
- Harms, P.W.; Vats, P.; Verhaegen, M.E.; Robinson, D.R.; Wu, Y.M.; Dhanasekaran, S.M.; Palanisamy, N.; Siddiqui, J.; Cao, X.; Su, F.; et al. The Distinctive Mutational Spectra of Polyomavirus-Negative Merkel Cell Carcinoma. Cancer Res. 2015, 75, 3720. [Google Scholar] [CrossRef]
- Sahi, H.; Savola, S.; Sihto, H.; Koljonen, V.; Bohling, T.; Knuutila, S. RB1 Gene in Merkel Cell Carcinoma: Hypermethylation in All Tumors and Concurrent Heterozygous Deletions in the Polyomavirus-Negative Subgroup. APMIS 2014, 122, 1157–1166. [Google Scholar] [CrossRef]
- Aster, J.C.; Pear, W.S.; Blacklow, S.C. The Varied Roles of Notch in Cancer. Annu. Rev. Pathol. 2016, 12, 245. [Google Scholar] [CrossRef]
- Becker, J.C.; Houben, R. Pathogenesis of Merkel Cell Carcinoma. In Skin Cancer—World-Wide Perspective; Springer: Cham, Switzerland, 2010; pp. 81–86. [Google Scholar] [CrossRef]
- Akgül, B.; Zigrino, P.; Hufbauer, M.; Liu, X.; Moore, P.S.; Mauch, C.; Pfister, H. Lack of Integrin Β5 in Merkel Cell Carcinomas and Derived Cell Lines Is Frequently Associated with Merkel Cell Polyomavirus Positivity. J. Dermatol. Sci. 2012, 67, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Nwogu, N.; Ortiz, L.E.; Whitehouse, A.; Kwun, H.J. Merkel Cell Polyomavirus Small Tumor Antigen Activates Matrix Metallopeptidase-9 Gene Expression for Cell Migration and Invasion. J. Virol. 2020, 94, e00786-20. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The Evolution of Phosphatidylinositol 3-Kinases as Regulators of Growth and Metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Broussard, L.; Howland, A.; Ryu, S.; Song, K.; Norris, D.; Armstrong, C.A.; Song, P.I. Melanoma Cell Death Mechanisms. Chonnam Med. J. 2018, 54, 135. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-Kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Leo, M.S.; Sivamani, R.K. Phytochemical Modulation of the Akt/MTOR Pathway and Its Potential Use in Cutaneous Disease. Arch. Dermatol. Res. 2014, 306, 861–871. [Google Scholar] [CrossRef]
- Wu, H.; Goel, V.; Haluska, F.G. PTEN Signaling Pathways in Melanoma. Oncogene 2003, 22, 3113–3122. [Google Scholar] [CrossRef]
- Dai, D.L.; Martinka, M.; Li, G. Prognostic Significance of Activated Akt Expression in Melanoma: A Clinicopathologic Study of 292 Cases. J. Clin. Oncol. 2005, 23, 1473–1482. [Google Scholar] [CrossRef]
- Constitutive Activation of Akt/Protein Kinase B in Melanoma Leads to Up-Regulation of Nuclear Factor-KappaB and Tumor Progression—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12499277/ (accessed on 24 January 2025).
- Stahl, J.M.; Sharma, A.; Cheung, M.; Zimmerman, M.; Cheng, J.Q.; Bosenberg, M.W.; Kester, M.; Sandirasegarane, L.; Robertson, G.P. Deregulated Akt3 Activity Promotes Development of Malignant Melanoma. Cancer Res. 2004, 64, 7002–7010. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, H.T. MAPK Signal Pathways in the Regulation of Cell Proliferation in Mammalian Cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Pathological Roles of MAPK Signaling Pathways in Human Diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP Kinase Signalling Pathways in Cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef]
- Sebolt-Leopold, J.S.; Herrera, R. Targeting the Mitogen-Activated Protein Kinase Cascade to Treat Cancer. Nat. Rev. Cancer 2004, 4, 937–947. [Google Scholar] [CrossRef]
- Bollag, G.; Hirth, P.; Tsai, J.; Zhang, J.; Ibrahim, P.N.; Cho, H.; Spevak, W.; Zhang, C.; Zhang, Y.; Habets, G.; et al. Clinical Efficacy of a RAF Inhibitor Needs Broad Target Blockade in BRAF-Mutant Melanoma. Nature 2010, 467, 596–599. [Google Scholar] [CrossRef]
- Krasagakis, K.; Fragiadaki, I.; Metaxari, M.; Krüger-Krasagakis, S.; Tzanakakis, G.N.; Stathopoulos, E.N.; Eberle, J.; Tavernarakis, N.; Tosca, A.D. KIT Receptor Activation by Autocrine and Paracrine Stem Cell Factor Stimulates Growth of Merkel Cell Carcinoma in Vitro. J. Cell Physiol. 2011, 226, 1099–1109. [Google Scholar] [CrossRef]
- Dobson, S.J.; Anene, A.; Boyne, J.R.; Mankouri, J.; Macdonald, A.; Whitehouse, A. Merkel Cell Polyomavirus Small Tumour Antigen Activates the P38 MAPK Pathway to Enhance Cellular Motility. Biochem. J. 2020, 477, 2721–2733. [Google Scholar] [CrossRef]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef]
- Ranganathan, P.; Weaver, K.L.; Capobianco, A.J. Notch Signalling in Solid Tumours: A Little Bit of Everything but Not All the Time. Nat. Rev. Cancer 2011, 11, 338–351. [Google Scholar] [CrossRef]
- Weng, A.P.; Ferrando, A.A.; Lee, W.; Morris IV, J.P.; Silverman, L.B.; Sanchez-Irizarry, C.; Blacklow, S.C.; Look, A.T.; Aster, J.C. Activating Mutations of NOTCH1 in Human T Cell Acute Lymphoblastic Leukemia. Science 2004, 306, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Nowell, C.; Radtke, F. Cutaneous Notch Signaling in Health and Disease. Cold Spring Harb. Perspect. Med. 2013, 3, a017772. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; McCormick, F. The RB and P53 Pathways in Cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell Cycle Regulation: P53-P21-RB Signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Zhou, Y.; Nakajima, R.; Shirasawa, M.; Fikriyanti, M.; Zhao, L.; Iwanaga, R.; Bradford, A.P.; Kurayoshi, K.; Araki, K.; Ohtani, K. Expanding Roles of the E2F-RB-P53 Pathway in Tumor Suppression. Biology 2023, 12, 1511. [Google Scholar] [CrossRef]
- Cheng, J.; Rozenblatt-Rosen, O.; Paulson, K.G.; Nghiem, P.; DeCaprio, J.A. Merkel Cell Polyomavirus Large T Antigen Has Growth-Promoting and Inhibitory Activities. J. Virol. 2013, 87, 6118. [Google Scholar] [CrossRef]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/Beta-Catenin Pathway: Modulating Anticancer Immune Response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Zent, R.; Pozzi, A. Cell-Extracellular Matrix Interactions in Cancer; Springer: Cham, Switzerland, 2010; pp. 1–314. [Google Scholar] [CrossRef]
- Ronan, S.G.; Green, A.D.; Shilkaitis, A.; Huang, T.S.W.; Das Gupta, T.K. Merkel Cell Carcinoma: In Vitro and in Vivo Characteristics of a New Cell Line. J. Am. Acad. Dermatol. 1993, 29, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.H.; Dash, P.; Holland, P.; Kearsley, J.H.; Bell, J.R. Characterisation of Four Merkel Cell Carcinoma Adherent Cell Lines. Int. J. Cancer 1995, 60, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Van Gele, M.; Leonard, J.H.; Van Roy, N.; Van Limbergen, H.; Van Belle, S.; Cocquyt, V.; Salwen, H.; De Paepe, A.; Speleman, F. Combined Karyotyping, CGH and M-FISH Analysis Allows Detailed Characterization of Unidentified Chromosomal Rearrangements in Merkel Cell Carcinoma. Int. J. Cancer 2002, 101, 137–145. [Google Scholar] [CrossRef]
- Houben, R.; Shuda, M.; Weinkam, R.; Schrama, D.; Feng, H.; Chang, Y.; Moore, P.S.; Becker, J.C. Merkel Cell Polyomavirus-Infected Merkel Cell Carcinoma Cells Require Expression of Viral T Antigens. J. Virol. 2010, 84, 7064–7072. [Google Scholar] [CrossRef]
- Shuda, M.; Feng, H.; Kwun, H.J.; Rosen, S.T.; Gjoerup, O.; Moore, P.S.; Chang, Y. T Antigen Mutations Are a Human Tumor-Specific Signature for Merkel Cell Polyomavirus. Proc. Natl. Acad. Sci. USA 2008, 105, 16272–16277. [Google Scholar] [CrossRef]
- Brown, G.T.; Murray, G.I. Current Mechanistic Insights into the Roles of Matrix Metalloproteinases in Tumour Invasion and Metastasis. J. Pathol. 2015, 237, 273–281. [Google Scholar] [CrossRef]
- Richards, K.F.; Guastafierro, A.; Shuda, M.; Toptan, T.; Moore, P.S.; Chang, Y. Merkel Cell Polyomavirus T Antigens Promote Cell Proliferation and Inflammatory Cytokine Gene Expression. J. General. Virol. 2015, 96, 3532–3544. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Overall, C.M.; López-Otín, C. Strategies for MMP Inhibition in Cancer: Innovations for the Post-Trial Era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Frost, T.C.; Gartin, A.K.; Liu, M.; Cheng, J.; Dharaneeswaran, H.; Keskin, D.B.; Wu, C.J.; Giobbie-Hurder, A.; Thakuria, M.; DeCaprio, J.A. YAP1 and WWTR1 Expression Inversely Correlates with Neuroendocrine Markers in Merkel Cell Carcinoma. J. Clin. Investig. 2023, 133, e157171. [Google Scholar] [CrossRef]
- García-Mesa, Y.; Martín-Sanz, R.; García-Piqueras, J.; Cobo, R.; Muñoz-Bravo, S.; García-Suárez, O.; Martín-Biedma, B.; Vega, J.A.; Feito, J. Merkel Cell Carcinoma Display PIEZO2 Immunoreactivity. J. Pers. Med. 2022, 12, 894. [Google Scholar] [CrossRef]
- Jani, S.; Church, C.D.; Nghiem, P. Insights into Anti-Tumor Immunity via the Polyomavirus Shared across Human Merkel Cell Carcinomas. Front. Immunol. 2023, 14, 1172913. [Google Scholar] [CrossRef]
- Yusuf, M.; Gaskins, J.; Mandish, S.; May, M.E.; Wall, W.; Fisher, W.; Tennant, P.; Jorgensen, J.; Bumpous, J.; Dunlap, N. Tumor Infiltrating Lymphocyte Grade in Merkel Cell Carcinoma: Relationships with Clinical Factors and Independent Prognostic Value. Acta Oncol. 2020, 59, 1409–1415. [Google Scholar] [CrossRef]
- Acikalin, A.; Bagir, E.; Paydas, S. Prognostic and Predictive Value of EZH2 Expression and the Tumor Immune Microenvironment in Merkel Cell Carcinoma. Pol. J. Pathol. 2021, 72, 140–147. [Google Scholar] [CrossRef]
- Gherardin, N.A.; Waldeck, K.; Caneborg, A.; Martelotto, L.G.; Balachander, S.; Zethoven, M.; Petrone, P.M.; Pattison, A.; Wilmott, J.S.; Quiñones-Parra, S.M.; et al. Γδ T Cells in Merkel Cell Carcinomas Have a Proinflammatory Profile Prognostic of Patient Survival. Cancer Immunol. Res. 2021, 9, 612–623. [Google Scholar] [CrossRef]
- Lai, J.; Madan, V.; Qadri, A.; Danilova, L.; Yuan, L.; Jacobs, V.; Ogurtsova, A.; Engle, L.L.; Sunshine, J.C. Lymphocyte Activation Gene 3 Expression, Γδ T-Cell/Major Histocompatibility Complex Class I Interactions, and Prognosis in Merkel Cell Carcinoma. Lab. Investig. 2025, 105, 102178. [Google Scholar] [CrossRef]
- Donizy, P.; Wu, C.L.; Kopczynski, J.; Pieniazek, M.; Biecek, P.; Ryś, J.; Hoang, M.P. Prognostic Role of Tumoral PD-L1 and IDO1 Expression, and Intratumoral CD8+ and FoxP3+ Lymphocyte Infiltrates in 132 Primary Cutaneous Merkel Cell Carcinomas. Int. J. Mol. Sci. 2021, 22, 5489. [Google Scholar] [CrossRef]
- Ah-Thiane, L.; Samimi, M.; Kervarrec, T.; Khammari, A.; Dréno, B. Complete Spontaneous Regression of Primary Merkel Cell Carcinoma with Tumoural Infiltration: A Systematic Review. Eur. J. Dermatol. 2021, 31, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Sauerer, T.; Lischer, C.; Weich, A.; Berking, C.; Vera, J.; Dörrie, J. Single-Molecule RNA Sequencing Reveals IFNγ-Induced Differential Expression of Immune Escape Genes in Merkel Cell Polyomavirus-Positive MCC Cell Lines. Front. Microbiol. 2021, 12, 785662. [Google Scholar] [CrossRef]
- Lee, P.C.; Klaeger, S.; Le, P.M.; Korthauer, K.; Cheng, J.; Ananthapadmanabhan, V.; Frost, T.C.; Stevens, J.D.; Wong, A.Y.L.; Iorgulescu, J.B.; et al. Reversal of Viral and Epigenetic HLA Class I Repression in Merkel Cell Carcinoma. J. Clin. Investig. 2022, 132, e151666. [Google Scholar] [CrossRef]
- Reinstein, Z.Z.; Zhang, Y.; Ospina, O.E.; Nichols, M.D.; Chu, V.A.; Pulido, A.d.M.; Prieto, K.; Nguyen, J.V.; Yin, R.; Moran Segura, C.; et al. Preexisting Skin-Resident CD8 and Γδ T-Cell Circuits Mediate Immune Response in Merkel Cell Carcinoma and Predict Immunotherapy Efficacy. Cancer Discov. 2024, 14, 1631–1652. [Google Scholar] [CrossRef]
- Simon, S.; Voillet, V.; Vignard, V.; Wu, Z.; Dabrowski, C.; Jouand, N.; Beauvais, T.; Khammari, A.; Braudeau, C.; Josien, R.; et al. PD-1 and TIGIT Coexpression Identifies a Circulating CD8 T Cell Subset Predictive of Response to Anti-PD-1 Therapy. J. Immunother. Cancer 2020, 8, e001631. [Google Scholar] [CrossRef]
- Von Der Grün, J.; Winkelmann, R.; Meissner, M.; Wieland, U.; Silling, S.; Martin, D.; Fokas, E.; Rödel, C.; Rödel, F.; Balermpas, P. Merkel Cell Polyoma Viral Load and Intratumoral CD8+ Lymphocyte Infiltration Predict Overall Survival in Patients with Merkel Cell Carcinoma. Front. Oncol. 2019, 9, 20. [Google Scholar] [CrossRef]
- Jing, L.; Ott, M.; Church, C.D.; Kulikauskas, R.M.; Ibrani, D.; Iyer, J.G.; Afanasiev, O.K.; Colunga, A.; Cook, M.M.; Xie, H.; et al. Prevalent and Diverse Intratumoral Oncoprotein-Specific CD8+ T Cells within Polyomavirus-Driven Merkel Cell Carcinomas. Cancer Immunol. Res. 2020, 8, 648–659. [Google Scholar] [CrossRef]
- Mazziotta, C.; Lanzillotti, C.; Govoni, M.; Pellielo, G.; Mazzoni, E.; Tognon, M.; Martini, F.; Rotondo, J.C. Decreased IgG Antibody Response to Viral Protein Mimotopes of Oncogenic Merkel Cell Polyomavirus in Sera From Healthy Elderly Subjects. Front. Immunol. 2021, 12, 738486. [Google Scholar] [CrossRef]
- Mazziotta, C.; Lanzillotti, C.; Govoni, M.; Falzoni, S.; Tramarin, M.L.; Mazzoni, E.; Tognon, M.; Martini, F.; Rotondo, J.C. Immunological Evidence of an Early Seroconversion to Oncogenic Merkel Cell Polyomavirus in Healthy Children and Young Adults. Immunology 2023, 168, 671–683. [Google Scholar] [CrossRef]
- Krump, N.A.; Wang, R.; Liu, W.; Yang, J.F.; Ma, T.; You, J. Merkel Cell Polyomavirus Infection Induces an Antiviral Innate Immune Response in Human Dermal Fibroblasts. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- Ohnezeit, D.; Huang, J.; Westerkamp, U.; Brinschwitz, V.; Schmidt, C.; Günther, T.; Czech-Sioli, M.; Weißelberg, S.; Schlemeyer, T.; Nakel, J.; et al. Merkel Cell Polyomavirus Small Tumor Antigen Contributes to Immune Evasion by Interfering with Type I Interferon Signaling. PLoS Pathog. 2024, 20, e1012426. [Google Scholar] [CrossRef]
- Ricci, C.; Righi, A.; Ambrosi, F.; Gibertoni, D.; Maletta, F.; Uccella, S.; Sessa, F.; Asioli, S.; Pellilli, M.; Maragliano, R.; et al. Prognostic Impact of MCPyV and TIL Subtyping in Merkel Cell Carcinoma: Evidence from a Large European Cohort of 95 Patients. Endocr. Pathol. 2020, 31, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.M.; Castonguay, M.C.; Carter, M.D.; Pasternak, S.; Ly, T.Y.; Doucette, S.; Hanly, J.G.; Saggini, A.; Cerroni, L. Global PD-L1 Signals and Tumor-Infiltrating Lymphocytes: Markers of Immunogenicity in Different Subsets of Merkel Cell Carcinoma and Potential Therapeutic Implications. Am. J. Dermatopathol. 2019, 41, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Blitzblau, R.; Bowen, G.M.; Contreras, C.M.; Daniels, G.A.; Decker, R.; et al. Merkel Cell Carcinoma, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 742–774. [Google Scholar] [CrossRef]
- Coggshall, K.; Tello, T.L.; North, J.P.; Yu, S.S. Merkel Cell Carcinoma: An Update and Review: Pathogenesis, Diagnosis, and Staging. J. Am. Acad. Dermatol. 2018, 78, 433–442. [Google Scholar] [CrossRef]
- Ball, N.J.; Tanhuanco-Kho, G. Merkel Cell Carcinoma Frequently Shows Histologic Features of Basal Cell Carcinoma: A Study of 30 Cases. J. Cutan. Pathol. 2007, 34, 612–619. [Google Scholar] [CrossRef]
- Samimi, M. Immune Checkpoint Inhibitors and Beyond: An Overview of Immune-Based Therapies in Merkel Cell Carcinoma. Am. J. Clin. Dermatol. 2019, 20, 391–407. [Google Scholar] [CrossRef]
- Andea, A.A.; Coit, D.G.; Amin, B.; Busam, K.J. Merkel Cell Carcinoma: Histologic Features and Prognosis. Cancer 2008, 113, 2549–2558. [Google Scholar] [CrossRef]
- D’Agostino, M.; Cinelli, C.; Willard, R.; Hofmann, J.; Jellinek, N.; Robinson-Bostom, L. Epidermotropic Merkel Cell Carcinoma: A Case Series with Histopathologic Examination. J. Am. Acad. Dermatol. 2010, 62, 463–468. [Google Scholar] [CrossRef]
- Llombart, B.; Monteagudo, C.; López-Guerrero, J.A.; Carda, C.; Jorda, E.; Sanmartín, O.; Almenar, S.; Molina, I.; Martín, J.M.; Llombart-Bosch, A. Clinicopathological and Immunohistochemical Analysis of 20 Cases of Merkel Cell Carcinoma in Search of Prognostic Markers. Histopathology 2005, 46, 622–634. [Google Scholar] [CrossRef]
- Llombart, B.; Requena, C.; Cruz, J. Update on Merkel Cell Carcinoma: Epidemiology, Etiopathogenesis, Clinical Features, Diagnosis, and Staging. Actas Dermosifiliogr. 2017, 108, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kervarrec, T.; Tallet, A.; Miquelestorena-Standley, E.; Houben, R.; Schrama, D.; Gambichler, T.; Berthon, P.; Le Corre, Y.; Hainaut-Wierzbicka, E.; Aubin, F.; et al. Morphologic and Immunophenotypical Features Distinguishing Merkel Cell Polyomavirus-Positive and Negative Merkel Cell Carcinoma. Mod. Pathol. 2019, 32, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, S.; Kervarrec, T.; Weiss, G.J.; Samimi, M. Glypican-3 (GPC3) Is Associated with MCPyV-Negative Status and Impaired Outcome in Merkel Cell Carcinoma. Oncotarget 2022, 13, 960–967. [Google Scholar] [CrossRef] [PubMed]
- González-Vela, M.d.C.; Curiel-Olmo, S.; Derdak, S.; Beltran, S.; Santibañez, M.; Martínez, N.; Castillo-Trujillo, A.; Gut, M.; Sánchez-Pacheco, R.; Almaraz, C.; et al. Shared Oncogenic Pathways Implicated in Both Virus-Positive and UV-Induced Merkel Cell Carcinomas. J. Investig. Dermatol. 2017, 137, 197–206. [Google Scholar] [CrossRef]
- Ricci, C.; Morandi, L.; Righi, A.; Gibertoni, D.; Maletta, F.; Ambrosi, F.; Agostinelli, C.; Uccella, S.; Asioli, S.; Sessa, F.; et al. PD-1 (PDCD1) Promoter Methylation in Merkel Cell Carcinoma: Prognostic Relevance and Relationship with Clinico-Pathological Parameters. Mod. Pathol. 2019, 32, 1359–1372. [Google Scholar] [CrossRef]
- Ricci, C.; Morandi, L.; Ambrosi, F.; Righi, A.; Gibertoni, D.; Maletta, F.; Agostinelli, C.; Corradini, A.G.; Uccella, S.; Asioli, S.; et al. Intron 4-5 HTERT DNA Hypermethylation in Merkel Cell Carcinoma: Frequency, Association with Other Clinico-Pathological Features and Prognostic Relevance. Endocr. Pathol. 2021, 32, 385–395. [Google Scholar] [CrossRef]
- Martin, B.; Poblet, E.; Rios, J.J.; Kazakov, D.; Kutzner, H.; Brenn, T.; Calonje, E. Merkel Cell Carcinoma with Divergent Differentiation: Histopathological and Immunohistochemical Study of 15 Cases with PCR Analysis for Merkel Cell Polyomavirus. Histopathology 2013, 62, 711–722. [Google Scholar] [CrossRef]
- Gould, E.; Albores-Saavedra, J.; Dubner, B.; Smith, W.; Payne, C.M. Eccrine and Squamous Differentiation in Merkel Cell Carcinoma. An Immunohistochemical Study. Am. J. Surg. Pathol. 1988, 12, 768–772. [Google Scholar] [CrossRef]
- Plaza, J.A.; Suster, S. The Toker Tumor: Spectrum of Morphologic Features in Primary Neuroendocrine Carcinomas of the Skin (Merkel Cell Carcinoma). Ann. Diagn. Pathol. 2006, 10, 376–385. [Google Scholar] [CrossRef]
- Covello, R.; Licci, S.; Ferrari, A.; Morelli, L.; Catricalà, C. Merkel Cell Carcinoma of the Thumb with Squamous and Leiomyosarcomatous Differentiation. Eur. J. Dermatol. 2010, 20, 529–530. [Google Scholar] [CrossRef]
- Tilling, T.; Moll, I. Which Are the Cells of Origin in Merkel Cell Carcinoma? J. Skin. Cancer 2012, 2012, 680410. [Google Scholar] [CrossRef] [PubMed]
- Divergent Differentiation in Endocrine and Nonendocrine Tumors of the Skin—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/10839616/ (accessed on 5 January 2025).
- Walsh, N.M.G. Primary Neuroendocrine (Merkel Cell) Carcinoma of the Skin: Morphologic Diversity and Implications Thereof. Hum. Pathol. 2001, 32, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.-B.; Murali, R.; Karim, R.Z.; Dutta, B.; Dutta, R.; McCarthy, S.W.; Scolyer, R.A. Merkel Cell Carcinoma with Fibrosarcomatous Differentiation. Pathology 2008, 40, 314–316. [Google Scholar] [CrossRef]
- Gamissans, M.; Muntaner-Virgili, C.; Penín, R.-M.; Marcoval, J. Characteristics of Spontaneous Regression in Merkel Cell Carcinoma: A Retrospective Analysis of Patient and Tumor Features. Clin. Exp. Dermatol. 2024, in proof. [Google Scholar] [CrossRef]
- Takenaka, H.; Kishimoto, S.; Shibagaki, R.; Nagata, M.; Yasuno, H. Merkel Cell Carcinoma with Partial Spontaneous Regression: An Immunohistochemical, Ultrastructural, and TUNEL Labeling Study. Am. J. Dermatopathol. 1997, 19, 614–618. [Google Scholar] [CrossRef]
- Cassarino, D.S. Insulinoma-Associated 1: A Sensitive Marker of Neuroendocrine Differentiation in Cutaneous and Metastatic Neuroendocrine Tumors. J. Cutan. Pathol. 2021, 48, 8–10. [Google Scholar] [CrossRef]
- Smoller, B.R.; Bichakjian, C.; Brown, J.A.; Crowson, A.N.; Divaris, D.; Frishberg, D.P.; Gao, L.; Gershenwald, J.; Mcniff, J.M.; Nghiem, P.; et al. Protocol for the Examination of Specimens From Patients with Merkel Cell Carcinoma of the Skin; College of American Pathologists: Northfield, IL, USA, 2017. [Google Scholar]
- Jour, G.; Aung, P.P.; Rozas-Muñoz, E.; Curry, J.L.; Prieto, V.; Ivan, D. Intraepidermal Merkel Cell Carcinoma: A Case Series of a Rare Entity with Clinical Follow Up. J. Cutan. Pathol. 2017, 44, 684–691. [Google Scholar] [CrossRef]
- LeBoit, P.E.; Crutcher, W.A.; Shapiro, P.E. Pagetoid Intraepidermal Spread in Merkel Cell (Primary Neuroendocrine) Carcinoma of the Skin. Am. J. Surg. Pathol. 1992, 16, 584–592. [Google Scholar] [CrossRef]
- Merkel Cell Carcinoma Treatment (PDQ®)—NCI. Available online: https://www.cancer.gov/types/skin/hp/merkel-cell-treatment-pdq (accessed on 28 February 2025).
- Servy, A.; Maubec, E.; Sugier, P.E.; Grange, F.; Mansard, S.; Lesimple, T.; Marinho, E.; Couturaud, B.; Girod, A.; Albert, S.; et al. Merkel Cell Carcinoma: Value of Sentinel Lymph-Node Status and Adjuvant Radiation Therapy. Ann. Oncol. 2016, 27, 914–919. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Griffith, K.A.; Lowe, L.; Wong, S.L.; McLean, S.A.; Fullen, D.R.; Lao, C.D.; Hayman, J.A.; Bradford, C.R.; Rees, R.S.; et al. Features Predicting Sentinel Lymph Node Positivity in Merkel Cell Carcinoma. J. Clin. Oncol. 2011, 29, 1036–1041. [Google Scholar] [CrossRef]
- Mbous, Y.P.V.; Mohamed, R.; Sambamoorthi, U.; Bharmal, M.; Kamal, K.M.; LeMasters, T.; Kolodney, J.; Kelley, G.A. Effectiveness and Safety of Treatments for Early-Stage Merkel Cell Carcinoma: A Systematic Review and Meta-Analysis of Randomized and Non-Randomized Studies. Cancer Med. 2025, 14, e70553. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Remulla, D.; Wehrle, C.; Woo, K.P.; Dahdaleh, F.S.; Joyce, D.; Naffouje, S.A. The Role of Neoadjuvant Immunotherapy in the Management of Merkel Cell Carcinoma with Clinically Detected Regional Lymph Node Metastasis. Ann. Surg. Oncol. 2024, 31, 6079–6087. [Google Scholar] [CrossRef] [PubMed]
- Fojnica, A.; Ljuca, K.; Akhtar, S.; Gatalica, Z.; Vranic, S. An Updated Review of the Biomarkers of Response to Immune Checkpoint Inhibitors in Merkel Cell Carcinoma: Merkel Cell Carcinoma and Immunotherapy. Cancers 2023, 15, 5084. [Google Scholar] [CrossRef]
- Bénigni, P.; Guénolé, M.; Bonsang, B.; Marcorelles, P.; Schick, U.; Uguen, A. Foci of Programmed Cell Death-Ligand 1 (PD-L1)-Positive Tumor Areas With Tumor-Infiltrating Leukocytes (TILs) Evocative of a PD-1/PD-L1-Related Adaptive Immune Resistance Are Frequent in Merkel Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 17–22. [Google Scholar] [CrossRef]
- Bhatia, S.; Nghiem, P.; Veeranki, S.P.; Vanegas, A.; Lachance, K.; Tachiki, L.; Chiu, K.; Boller, E.; Bharmal, M. Real-World Clinical Outcomes with Avelumab in Patients with Merkel Cell Carcinoma Treated in the USA: A Multicenter Chart Review Study. J. Immunother. Cancer 2022, 10, e004904. [Google Scholar] [CrossRef]
- Averbuch, I.; Stoff, R.; Miodovnik, M.; Fennig, S.; Bar-Sela, G.; Yakobson, A.; Daliot, J.; Asher, N.; Fenig, E. Avelumab for the Treatment of Locally Advanced or Metastatic Merkel Cell Carcinoma-A Multicenter Real-World Experience in Israel. Cancer Med. 2023, 12, 12065–12070. [Google Scholar] [CrossRef]
- Spassova, I.; Ugurel, S.; Kubat, L.; Zimmer, L.; Terheyden, P.; Mohr, A.; Björn Andtback, H.; Villabona, L.; Leiter, U.; Eigentler, T.; et al. Clinical and Molecular Characteristics Associated with Response to Therapeutic PD-1/PD-L1 Inhibition in Advanced Merkel Cell Carcinoma. J. Immunother. Cancer 2022, 10, e003198. [Google Scholar] [CrossRef]
- De Moraes, F.C.A.; Kreuz, M.; de Lara, I.C.A.; Lôbo, A.d.O.M.; Burbano, R.M.R. Efficacy and Safety of PD-1/PD-L1 Inhibitors in Patients with Merkel Cell Carcinoma: A Systematic Review and Meta-Analysis. BMC Cancer 2024, 24, 1357. [Google Scholar] [CrossRef]
- Spassova, I.; Ugurel, S.; Terheyden, P.; Sucker, A.; Hassel, J.C.; Ritter, C.; Kubat, L.; Habermann, D.; Farahpour, F.; Saeedghalati, M.; et al. Predominance of Central Memory T Cells with High T-Cell Receptor Repertoire Diversity Is Associated with Response to PD-1/PD-L1 Inhibition in Merkel Cell Carcinoma. Clin. Cancer Res. 2020, 26, 2257–2267. [Google Scholar] [CrossRef]
- Tabachnick-Cherny, S.; Pulliam, T.; Rodriguez, H.J.; Fan, X.; Hippe, D.S.; Jones, D.C.; Moshiri, A.S.; Smythe, K.S.; Kulikauskas, R.M.; Zaba, L.C.; et al. Characterization of Immunosuppressive Myeloid Cells in Merkel Cell Carcinoma: Correlation with Resistance to PD-1 Pathway Blockade. Clin. Cancer Res. 2024, 30, 1189–1199. [Google Scholar] [CrossRef]
- Martins, C.; Rasbach, E.; Heppt, M.V.; Singh, P.; Kulcsar, Z.; Holzgruber, J.; Chakraborty, A.; Mucciarone, K.; Kleffel, S.; Brandenburg, A.; et al. Tumor Cell–Intrinsic PD-1 Promotes Merkel Cell Carcinoma Growth by Activating Downstream MTOR-Mitochondrial ROS Signaling. Sci. Adv. 2024, 10, eadi2012. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Lebbé, C.; Nghiem, P.; Brohl, A.S.; Mrowiec, T.; Leslie, T.; Georges, S.; Güzel, G.; Shah, P. Biomarker Analyses Investigating Disease Biology and Associations with Outcomes in the JAVELIN Merkel 200 Trial of Avelumab in Metastatic Merkel Cell Carcinoma. Clin. Cancer Res. 2024, 30, OF1–OF11. [Google Scholar] [CrossRef]
- Ramadoss, T.; Nichols, M.; Palacios, C.; Eroglu, Z.; Markowitz, J.; Karapetyan, L.; Tarhini, A.A.; Wuthrick, E.J.; Sondak, V.K.; Khushalani, N.I.; et al. Durability of Response to Immune Checkpoint Blockade Following Treatment Discontinuation and Efficacy of Rechallenge in Advanced Merkel Cell Carcinoma. J. Immunother. Cancer 2024, 12, e009816. [Google Scholar] [CrossRef]
- Strong, J.; Hallaert, P.; Brownell, I. Merkel Cell Carcinoma. Hematol. Oncol. Clin. N. Am. 2024, 38, 1133–1147. [Google Scholar] [CrossRef]
- Nakhaei, P.; Hiscott, J.; Lin, R. STING-Ing the Antiviral Pathway. J. Mol. Cell Biol. 2010, 2, 110–112. [Google Scholar] [CrossRef]
- Liu, W.; Alameh, M.G.; Yang, J.F.; Xu, J.R.; Lin, P.J.C.; Tam, Y.K.; Weissman, D.; You, J. Lipid Nanoparticles Delivering Constitutively Active STING MRNA to Stimulate Antitumor Immunity. Int. J. Mol. Sci. 2022, 23, 14504. [Google Scholar] [CrossRef]
- Liu, W.; Kim, G.B.; Krump, N.A.; Zhou, Y.; Riley, J.L.; You, J. Selective Reactivation of STING Signaling to Target Merkel Cell Carcinoma. Proc. Natl. Acad. Sci. USA 2020, 117, 13730–13739. [Google Scholar] [CrossRef]
- Pulliam, T.; Jani, S.; Goff, P.H.; Bhakuni, R.; Tabachnick-Cherny, S.; Smythe, K.; Seaton, B.W.; Tachiki, L.; Kulikauskas, R.; Church, C.; et al. Intratumoral STING Agonist Reverses Immune Evasion in PD-(L)1-Refractory Merkel Cell Carcinoma: Mechanistic Insights from Detailed Biomarker Analyses. J. Immunother. Cancer 2024, 12, e009803. [Google Scholar] [CrossRef]
- Wang, R.; Yang, J.F.; Senay, T.E.; Liu, W.; You, J. Characterization of the Impact of Merkel Cell Polyomavirus-Induced Interferon Signaling on Viral Infection. J. Virol. 2023, 97, e0190722. [Google Scholar] [CrossRef]
- Wong, M.K.; Yee, C. Polyomavirus-Positive Merkel Cell Carcinoma: The Beginning of the Beginning. J. Clin. Investig. 2024, 134. [Google Scholar] [CrossRef]
- Asano, Y.; Veatch, J.; McAfee, M.; Bakhtiari, J.; Lee, B.; Martin, L.; Zhang, S.; Mazziotta, F.; Paulson, K.G.; Schmitt, T.M.; et al. Tumor Regression Following Engineered Polyomavirus-Specific T Cell Therapy in Immune Checkpoint Inhibitor-Refractory Merkel Cell Carcinoma. medRxiv 2024. [Google Scholar] [CrossRef]
- Buchta Rosean, C.; Leyder, E.C.; Hamilton, J.; Carter, J.J.; Galloway, D.A.; Koelle, D.M.; Nghiem, P.; Heiland, T. LAMP1 Targeting of the Large T Antigen of Merkel Cell Polyomavirus Results in Potent CD4 T Cell Responses and Tumor Inhibition. Front. Immunol. 2023, 14, 1253568. [Google Scholar] [CrossRef]
- Khattri, M.; Amako, Y.; Gibbs, J.R.; Collura, J.L.; Arora, R.; Harold, A.; Li, M.Y.; Harms, P.W.; Ezhkova, E.; Shuda, M. Methyltransferase-Independent Function of Enhancer of Zeste Homologue 2 Maintains Tumorigenicity Induced by Human Oncogenic Papillomavirus and Polyomavirus. Tumour Virus Res. 2023, 16, 200264. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.A.; Drouin, A.; Mouchard, A.; Durand, L.; Esnault, C.; Berthon, P.; Tallet, A.; Le Corre, Y.; Hainaut-Wierzbicka, E.; Blom, A.; et al. Distinct Regulation of EZH2 and Its Repressive H3K27me3 Mark in Polyomavirus-Positive and -Negative Merkel Cell Carcinoma. J. Investig. Dermatol. 2023, 143, 1937–1946.e7. [Google Scholar] [CrossRef]
- Imon, R.R.; Samad, A.; Alam, R.; Alsaiari, A.A.; Talukder, M.E.K.; Almehmadi, M.; Ahammad, F.; Mohammad, F. Computational Formulation of a Multiepitope Vaccine Unveils an Exceptional Prophylactic Candidate against Merkel Cell Polyomavirus. Front. Immunol. 2023, 14, 1160260. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, S.; He, Y.; Jin, X.; Zhao, G.; Wang, B. Development of a Therapeutic Vaccine Targeting Merkel Cell Polyomavirus Capsid Protein VP1 against Merkel Cell Carcinoma. npj Vaccines 2021, 6, 119. [Google Scholar] [CrossRef]
- Gambichler, T.; Schrama, D.; Käpynen, R.; Weyer-Fahlbusch, S.S.; Becker, J.C.; Susok, L.; Kreppel, F.; Abu Rached, N. Current Progress in Vaccines against Merkel Cell Carcinoma: A Narrative Review and Update. Vaccines 2024, 12, 533. [Google Scholar] [CrossRef]
- Davies, S.I.; Barrett, J.; Wong, S.; Chang, M.J.; Muranski, P.J.; Brownell, I. Robust Production of Merkel Cell Polyomavirus Oncogene Specific T Cells From Healthy Donors for Adoptive Transfer. Front. Immunol. 2020, 11, 592721. [Google Scholar] [CrossRef]
- Tarabadkar, E.S.; Thomas, H.; Blom, A.; Parvathaneni, U.; Olencki, T.; Nghiem, P.; Bhatia, S. Clinical Benefit from Tyrosine Kinase Inhibitors in Metastatic Merkel Cell Carcinoma: A Case Series of 5 Patients. Am. J. Case Rep. 2018, 19, 505. [Google Scholar] [CrossRef]
- Chan, J.A.; Kulke, M.H. Progress in the Treatment of Neuroendocrine Tumors. Curr. Oncol. Rep. 2009, 11, 193. [Google Scholar] [CrossRef]
- Bob, A.; Nielen, F.; Krediet, J.; Schmitter, J.; Freundt, D.; Terhorst, D.; Röwert-Huber, J.; Kanitakis, J.; Stockfleth, E.; Ulrich, C.; et al. Tumor Vascularization and Clinicopathologic Parameters as Prognostic Factors in Merkel Cell Carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.; Beer, T.W.; Murray, K. Vascular Density Has Prognostic Value in Merkel Cell Carcinoma. Am. J. Dermatopathol. 2008, 30, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Thurnher, D.; Pammer, J.; Geleff, S.; Heiduschka, G.; Reinisch, C.M.; Petzelbauer, P.; Erovic, B.M. Expression of VEGF-A/C, VEGF-R2, PDGF-α/Β, c-Kit, EGFR, Her-2/Neu, Mcl-1 and Bmi-1 in Merkel Cell Carcinoma. Mod. Pathol. 2008, 21, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Kervarrec, T.; Gaboriaud, P.; Tallet, A.; Leblond, V.; Arnold, F.; Berthon, P.; Schweinitzer, S.; Larcher, T.; Guyétant, S.; Schrama, D.; et al. VEGF-A Inhibition as a Potential Therapeutic Approach in Merkel Cell Carcinoma. J. Investig. Dermatol. 2019, 139, 736–739. [Google Scholar] [CrossRef]
- Raimondi, C.; Fantin, A.; Lampropoulou, A.; Denti, L.; Chikh, A.; Ruhrberg, C. Imatinib Inhibits VEGF-Independent Angiogenesis by Targeting Neuropilin 1–Dependent ABL1 Activation in Endothelial Cells. J. Exp. Med. 2014, 211, 1167. [Google Scholar] [CrossRef]
- Savage, D.G.; Antman, K.H. Imatinib Mesylate—A New Oral Targeted Therapy. N. Engl. J. Med. 2002, 346, 683–693. [Google Scholar] [CrossRef]
- Loader, D.E.; Feldmann, R.; Baumgartner, M.; Breier, F.; Schrama, D.; Becker, J.C.; Steiner, A. Clinical Remission of Merkel Cell Carcinoma after Treatment with Imatinib. J. Am. Acad. Dermatol. 2013, 69, e181–e183. [Google Scholar] [CrossRef]
- Samlowski, W.E.; Moon, J.; Tuthill, R.J.; Heinrich, M.C.; Balzer-Haas, N.S.; Merl, S.A.; DeConti, R.C.; Thompson, J.A.; Witter, M.T.; Flaherty, L.E.; et al. A Phase II Trial of Imatinib Mesylate in Merkel Cell Carcinoma (Neuroendocrine Carcinoma of the Skin): A Southwest Oncology Group Study (S0331). Am. J. Clin. Oncol. 2010, 33, 495. [Google Scholar] [CrossRef]
- O’Toole, T.J.; Sharma, S. Physiology, Somatostatin; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fagerstedt, K.W.; Vesterinen, T.; Leijon, H.; Sihto, H.; Böhling, T.; Arola, J. Somatostatin Receptor Expression in Merkel Cell Carcinoma: Correlation with Clinical Data. Acta Oncol. 2023, 62, 1001–1007. [Google Scholar] [CrossRef]
- Rogoza, O.; Megnis, K.; Kudrjavceva, M.; Gerina-Berzina, A.; Rovite, V. Role of Somatostatin Signalling in Neuroendocrine Tumours. Int. J. Mol. Sci. 2022, 23, 1447. [Google Scholar] [CrossRef]
- Buder, K.; Lapa, C.; Kreissl, M.C.; Schirbel, A.; Herrmann, K.; Schnack, A.; Bröcker, E.-B.; Goebeler, M.; Buck, A.K.; Becker, J.C. Somatostatin Receptor Expression in Merkel Cell Carcinoma as Target for Molecular Imaging. BMC Cancer 2014, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Fakiha, M.; Letertre, P.; Vuillez, J.P.; Lebeau, J. Remission of Merkel Cell Tumor after Somatostatin Analog Treatment. J. Cancer Res. Ther. 2010, 6, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.; D’Alò, A.; Mangia, A.; Di Pinto, F.; Sonnessa, M.; Albano, A.; Sciacovelli, A.; Asabella, A.N.; Fucci, L. Somatostatin Receptors in Merkel-Cell Carcinoma: A Therapeutic Opportunity Using Somatostatin Analog Alone or in Association With Checkpoint Inhibitors Immunotherapy. A Case Report. Front. Oncol. 2020, 10, 01073. [Google Scholar] [CrossRef]
- Akaike, T.; Qazi, J.; Anderson, A.; Behnia, F.S.; Shinohara, M.M.; Akaike, G.; Hippe, D.S.; Thomas, H.; Takagishi, S.R.; Lachance, K.; et al. High Somatostatin Receptor Expression and Efficacy of Somatostatin Analogues in Patients with Metastatic Merkel Cell Carcinoma. Br. J. Dermatol. 2021, 184, 319–327. [Google Scholar] [CrossRef]
- Long, G.V.; Carlino, M.S.; Au-Yeung, G.; Spillane, A.J.; Shannon, K.F.; Gyorki, D.E.; Hsiao, E.; Kapoor, R.; Thompson, J.R.; Batula, I.; et al. Neoadjuvant Pembrolizumab, Dabrafenib and Trametinib in BRAFV600-Mutant Resectable Melanoma: The Randomized Phase 2 NeoTrio Trial. Nat. Med. 2024, 30, 2540–2548. [Google Scholar] [CrossRef]
- Yang, J.F.; Liu, W.; You, J. Characterization of Molecular Mechanisms Driving Merkel Cell Polyomavirus Oncogene Transcription and Tumorigenic Potential. PLoS Pathog. 2023, 19, e1011598. [Google Scholar] [CrossRef]
- Shiver, M.B.; Mahmoud, F.; Gao, L. Response to Idelalisib in a Patient with Stage IV Merkel-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1580. [Google Scholar] [CrossRef]
- Fang, B.; Kannan, A.; Zhao, S.; Nguyen, Q.H.; Ejadi, S.; Yamamoto, M.; Camilo Barreto, J.; Zhao, H.; Gao, L. Inhibition of PI3K by Copanlisib Exerts Potent Antitumor Effects on Merkel Cell Carcinoma Cell Lines and Mouse Xenografts. Sci. Rep. 2020, 10, 8867. [Google Scholar] [CrossRef]
- Kannan, A.; Lin, Z.; Shao, Q.; Zhao, S.; Fang, B.; Moreno, M.A.; Vural, E.; Stack, B.C.; Suen, J.Y.; Kannan, K.; et al. Dual MTOR Inhibitor MLN0128 Suppresses Merkel Cell Carcinoma (MCC) Xenograft Tumor Growth. Oncotarget 2015, 7, 6576. [Google Scholar] [CrossRef]
- Nammour, H.M.; Madrigal, K.; Starling, C.T.; Doan, H.Q. Advancing Treatment Options for Merkel Cell Carcinoma: A Review of Tumor-Targeted Therapies. Int. J. Mol. Sci. 2024, 25, 11055. [Google Scholar] [CrossRef]
- Secchiero, P.; Bosco, R.; Celeghini, C.; Zauli, G. Recent Advances in the Therapeutic Perspectives of Nutlin-3. Curr. Pharm. Des. 2011, 17, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Oliner, J.D.; Pietenpol, J.A.; Thiagalingam, S.; Gyuris, J.; Kinzler, K.W.; Vogelstein, B. Oncoprotein MDM2 Conceals the Activation Domain of Tumour Suppressor P53. Nature 1993, 362, 857–860. [Google Scholar] [CrossRef]
- Harris, S.L.; Levine, A.J. The P53 Pathway: Positive and Negative Feedback Loops. Oncogene 2005, 24, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Ananthapadmanabhan, V.; Frost, T.C.; Soroko, K.M.; Knott, A.; Magliozzi, B.J.; Gokhale, P.C.; Tirunagaru, V.G.; Doebele, R.C.; DeCaprio, J.A. Milademetan Is a Highly Potent MDM2 Inhibitor in Merkel Cell Carcinoma. JCI Insight 2022, 7, e160513. [Google Scholar] [CrossRef]
- Zhang, K.; Wong, P.; Zhang, L.; Jacobs, B.; Borden, E.C.; Aster, J.C.; Bedogni, B. A Notch1–Neuregulin1 Autocrine Signaling Loop Contributes to Melanoma Growth. Oncogene 2012, 31, 4609–4618. [Google Scholar] [CrossRef]
- Iwasaki, T.; Hayashi, K.; Matsushita, M.; Nonaka, D.; Kohashi, K.; Kuwamoto, S.; Umekita, Y.; Oda, Y. Merkel Cell Polyomavirus–Negative Merkel Cell Carcinoma Is Associated with JAK-STAT and MEK-ERK Pathway Activation. Cancer Sci. 2022, 113, 251–260. [Google Scholar] [CrossRef]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in Angiogenesis and Lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604. [Google Scholar] [CrossRef]
- Reyes-González, J.M.; Rajkumar, H.; Lee, W.; Baidoo, K.E.; Edinger, R.S.; Diehl, G.; Nambiar, D.; Okada, R.; Edmondson, E.F.; Fayn, S.; et al. Evaluation of VLA-4 (Integrin A4β1) as a Shared Target for Radiopharmaceutical Therapy across Solid Tumors. Mol. Cancer Ther. 2025, OF1–OF11. [Google Scholar] [CrossRef]
- Testa, A.; Quaglia, F.; Naranjo, N.M.; Verrillo, C.E.; Shields, C.D.; Lin, S.; Pickles, M.W.; Hamza, D.F.; Von Schalscha, T.; Cheresh, D.A.; et al. Targeting the AVβ3/NgR2 Pathway in Neuroendocrine Prostate Cancer. Matrix Biol. 2023, 124, 49. [Google Scholar] [CrossRef]
- Xiong, J.; Xiao, R.; Zhao, J.; Zhao, Q.; Luo, M.; Li, F.; Zhang, W.; Wu, M. Matrix Stiffness Affects Tumor-Associated Macrophage Functional Polarization and Its Potential in Tumor Therapy. J. Transl. Med. 2024, 22, 85. [Google Scholar] [CrossRef]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights Into the Role of Matrix Metalloproteinases in Cancer and Its Various Therapeutic Aspects: A Review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Tumor Angiogenesis: MMP-Mediated Induction of Intravasation- and Metastasis-Sustaining Neovasculature. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef]
- Kapoor, C.; Vaidya, S.; Wadhwan, V.; Kaur, G.; Pathak, A. Seesaw of Matrix Metalloproteinases (MMPs). J. Cancer Res. Ther. 2016, 12, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Rydlova, M.; Holubec, L.; Ludvikova, M.; Kalfert, D.; Franekova, J.; Povysil, C.; Ludvikova, M. Biological Activity and Clinical Implications of the Matrix Metalloproteinases. Anticancer. Res. 2008, 28, 1389–1397. [Google Scholar]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures into Future Successes. Mol. Cancer Ther. 2018, 17, 1147. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, X.S.; Yang, Z.Y.; Wei, M.; Zhu, X.C.; Wang, P.; Liu, B.Y.; Gu, Q.L. Upregulated Expression of LOX Is a Novel Independent Prognostic Marker of Worse Outcome in Gastric Cancer Patients after Curative Surgery. Oncol. Lett. 2013, 5, 896–902. [Google Scholar] [CrossRef]
- Cetin, M.; Saatci, O.; Rezaeian, A.H.; Rao, C.N.; Beneker, C.; Sreenivas, K.; Taylor, H.; Pederson, B.; Chatzistamou, I.; Buckley, B.; et al. A Highly Potent Bi-Thiazole Inhibitor of LOX Rewires Collagen Architecture and Enhances Chemoresponse in Triple-Negative Breast Cancer. Cell Chem. Biol. 2024, 31, 1926–1941.e11. [Google Scholar] [CrossRef]
- Cao, Z.; Quazi, S.; Arora, S.; Osellame, L.D.; Burvenich, I.J.; Janes, P.W.; Scott, A.M. Cancer-Associated Fibroblasts as Therapeutic Targets for Cancer: Advances, Challenges, and Future Prospects. J. Biomed. Sci. 2025, 32, 7. [Google Scholar] [CrossRef]
- Tobin, R.P.; Cogswell, D.T.; Cates, V.M.; Davis, D.M.; Borgers, J.S.W.; Van Gulick, R.J.; Katsnelson, E.; Couts, K.L.; Jordan, K.R.; Gao, D.; et al. Targeting MDSC Differentiation Using ATRA: A Phase I/II Clinical Trial Combining Pembrolizumab and All-Trans Retinoic Acid for Metastatic Melanoma. Clin. Cancer Res. 2023, 29, 1209–1219. [Google Scholar] [CrossRef]
| Signaling Pathway | Involvement in MCC |
|---|---|
| PI3K/AKT/mTOR | -Activating mutations in PI3KCA identified in MCC [49]; -AKT phosphorylation at threonine 308 [50]; -Screening for PIK3CA mutations could help identify patients who might benefit from PI3K pathway inhibitors [51]; -MCPyV-negative MCCs exhibit a higher p-AKT activation frequency [52]; -Genes upregulated in deceased patients were primarily associated with the PI3K/AKT pathway [53]; -Inhibition of AKT results in the inactivation of mTOR and glycogen synthase kinase 3 pathway proteins, upregulation of p16 expression, and modulation of the phosphorylation of the B-cell lymphoma-2-associated death promoter, leading to suppression of MCC cell proliferation [54]; -Chemical dual mTORC1/mTORC2 inhibition suppresses MCC cell growth in vitro and in vivo [55]. |
| MAPK/ERK | -A subset of genes upregulated in deceased MCC patients was linked to MAPK pathway activation, independently of the viral status [52]; -A gene expression profiling study identified 2 distinct molecular subtypes of MCC, where Subtype II is associated with overexpression of genes involved in the TNF signaling and MAPK signaling pathways [56]; -MCPyV-ST activates p38 MAPK signaling, driving cell migration and motility [57]; Genes upregulated in deceased patients were primarily associated with angiogenesis and the MAPK pathway [53]. |
| Notch | -NOTCH1 membrane and cytoplasmic expression identified in MCC [58]; -NOTCH1 and NOTCH2 expression is not correlated with MCPyV status or prognosis [59]; -NOTCH3 expression is higher in MCPyV-positive MCCs and it is associated with enhanced prognosis (NOTCH3 expression serves as a prognostic MCC marker) [59]; -Expression of JAG1 (Notch ligand) is higher in MCPyV-negative MCCs [59]; -Decreased Notch expression correlated with increased necrosis and apoptosis of MCPyV-negative tumor cells [60]; -Higher expression of EMT-related genes is observed in MCPyV-negative MCCs, which were enriched in Notch signaling [61]. |
| TP53 | -MCPyV-negative MCCs present inactivating mutations or deletions of TP53 [12]; -MCPyV-positive MCCs exhibit downregulated activity of TP53 [12]; -UVB-specific mutations were identified in the p53 pathway [62,63]; -TP53 mutations mainly present in MCPyV-DNA-negative MCCs [64]; -The p53 pathway is activated in Positive MCCs via the binding of MCPyV-LT to pRB, while the MCPyV-ST downregulates p53 by increasing the levels of MDM2 and CKα [65]; -p53 inactivating mutations are generally low in MCCs (10–14%) [66,67]. |
| RB | -MCPyV-LT targets pRB and binds strongly and inactivates RB1, promoting tumor growth [68]; -RB1 gene copy loss found on over 60% of MCPyV-negative MCCs [69]; -In a study incuding 13 MCPyV-positive and 13 MCPyV-negative tumors, RB expression was significantly higher in MCPyV-positive MCCs and was associated with better prognosis [70]; -The RB1 promoter is hypermethylated in all MCCs, regardless of their RB expression [70]. |
| Wnt/β-Catenin | -UV exposure along with advanced age triggers the Wnt/β-catenin pathway [38]; -Accumulation of β-catenin is infrequent in MCC [71,72]. |
| Integrins | -Identified in MCC cell line-derived exosomes [38]; -Integrin β1 is involved in MCC filopodia formation [35]; -Integrin β5 is highly expressed in adherent MCC cell lines, which are mainly MCPyV-negative [73]. |
| ECM Remodeling Enzymes | -MCPyV-ST enhances MMP-9 and Snail, while MMP9 inhibition reduces MCPyV-ST-driven cell migration and invasion [74]; -WNT/β-catenin signaling pathway and other growth factors induce MMP1, MMP3, MMP7, MMP9, MMP10, MMP11, and MMP13 gene expression, which in turn enhances MCPyV infection [38]; -High levels of MMP7 and MMP10/2 correlate with MCC’s metastatic capacity [40]; -Distinct expression patterns of MMP-10, MMP-21, MMP-26, and MMP-28 have been identified in primary MCCs and lymph node metastases. MMP-28 was found in tumor cells, particularly in smaller tumors (<2 cm) [39]; -MMP26 was expressed in stromal cells, associated with larger tumors (>2cm) and poor prognosis [39]; -MMP10 is the most frequently expressed and prominent in metastatic lymph nodes matrix metalloproteinase [39]. |
| Therapy Targeting Small Molecule Inhibitors | Concept and Available Evidence |
|---|---|
| |
|
|
| |
|
|
|
|
| |
| |
| |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantaraki, M.; Berdiaki, A.; Neagu, M.; Zurac, S.; Krasagakis, K.; Nikitovic, D. Understanding Merkel Cell Carcinoma: Pathogenic Signaling, Extracellular Matrix Dynamics, and Novel Treatment Approaches. Cancers 2025, 17, 1212. https://doi.org/10.3390/cancers17071212
Konstantaraki M, Berdiaki A, Neagu M, Zurac S, Krasagakis K, Nikitovic D. Understanding Merkel Cell Carcinoma: Pathogenic Signaling, Extracellular Matrix Dynamics, and Novel Treatment Approaches. Cancers. 2025; 17(7):1212. https://doi.org/10.3390/cancers17071212
Chicago/Turabian StyleKonstantaraki, Maria, Aikaterini Berdiaki, Monica Neagu, Sabina Zurac, Konstantinos Krasagakis, and Dragana Nikitovic. 2025. "Understanding Merkel Cell Carcinoma: Pathogenic Signaling, Extracellular Matrix Dynamics, and Novel Treatment Approaches" Cancers 17, no. 7: 1212. https://doi.org/10.3390/cancers17071212
APA StyleKonstantaraki, M., Berdiaki, A., Neagu, M., Zurac, S., Krasagakis, K., & Nikitovic, D. (2025). Understanding Merkel Cell Carcinoma: Pathogenic Signaling, Extracellular Matrix Dynamics, and Novel Treatment Approaches. Cancers, 17(7), 1212. https://doi.org/10.3390/cancers17071212











