Reduced Taurine Synthesis Underlies Morphine-Promoted Lung Metastasis of Triple-Negative Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Reagents
2.2. Cell Culture, Animal Handling, and Ethics Statement
2.3. Cell Proliferation and Invasion Assays
2.4. Real-Time Quantitative PCR
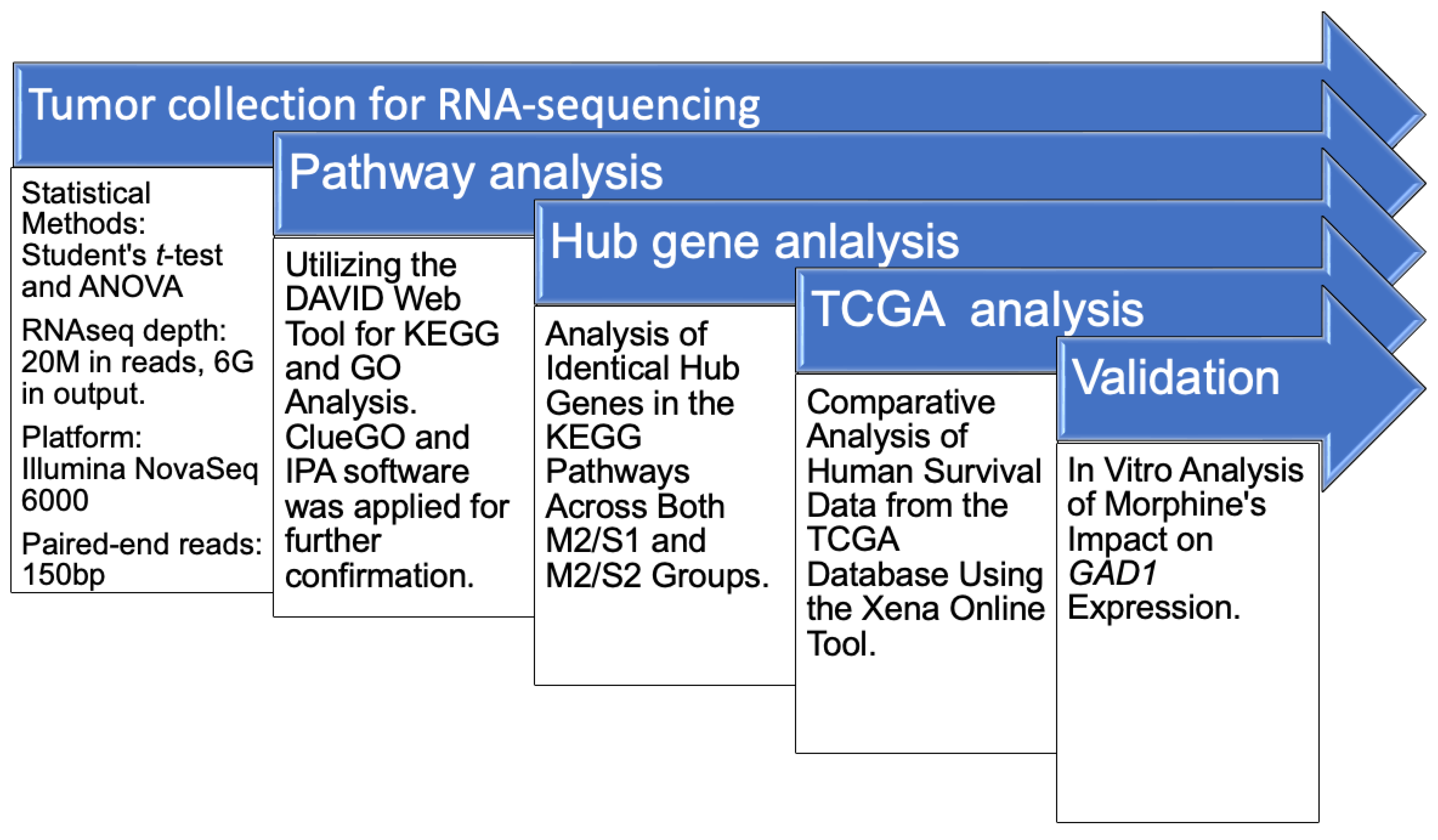
2.5. RNA Sequencing and Data Analysis
2.6. Statistical Analysis
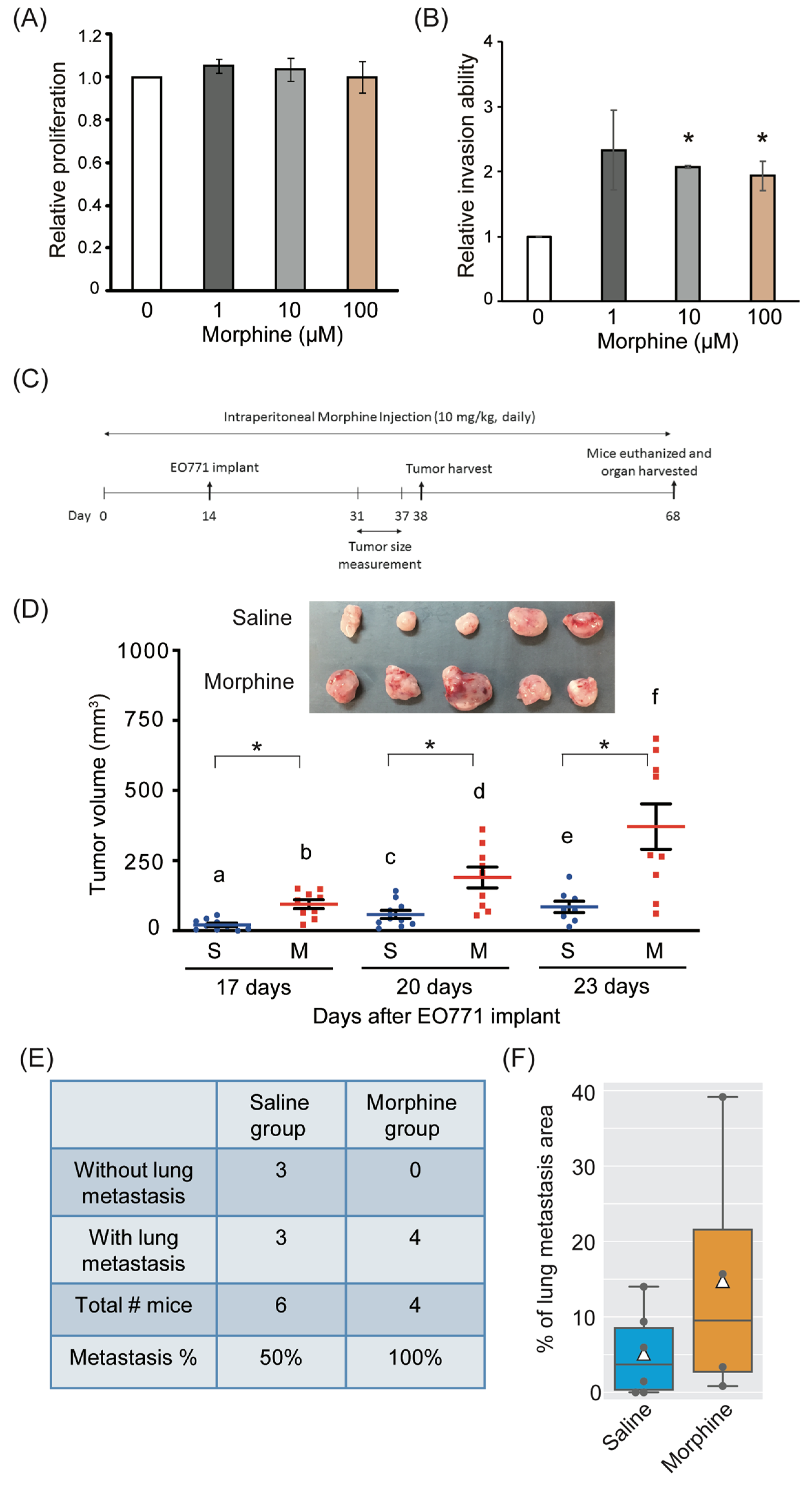
3. Results
3.1. Effect of Morphine on Cell Viability, Invasion, Tumor Growth, and Metastasis
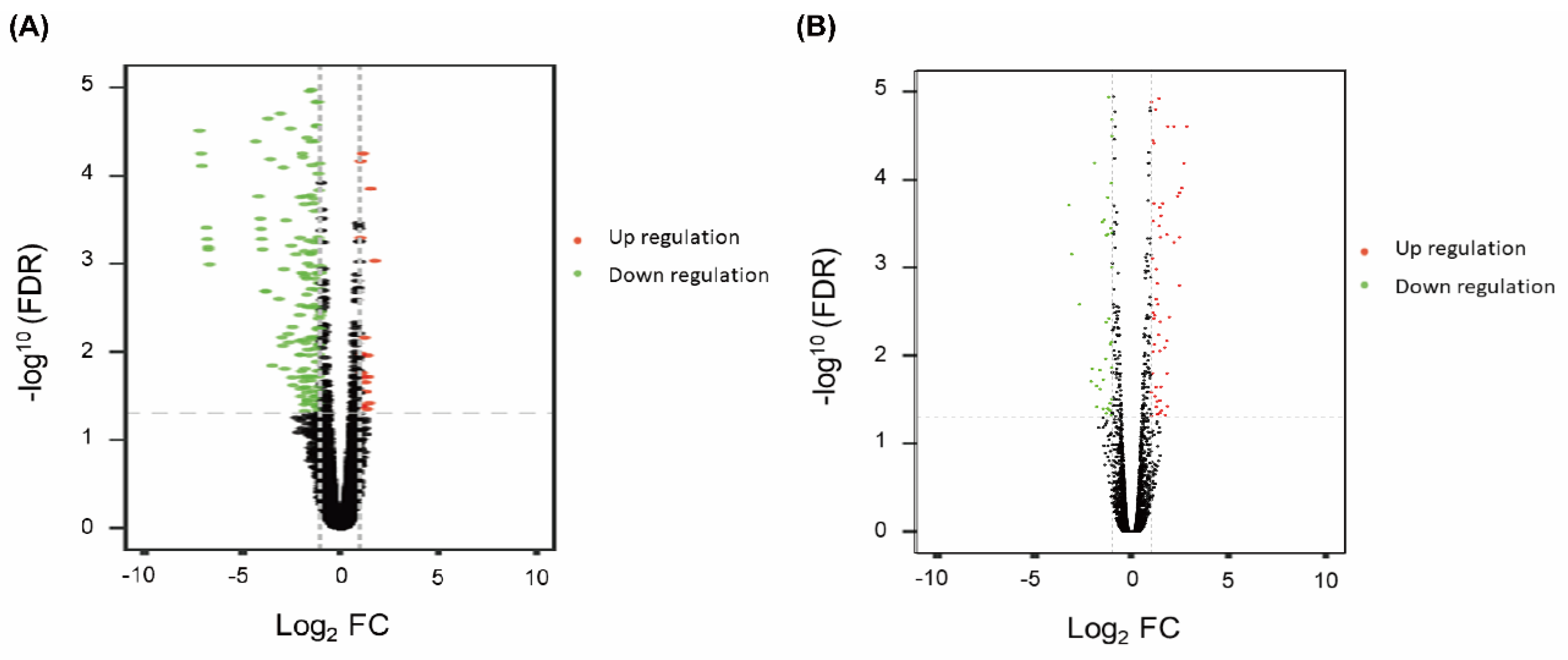
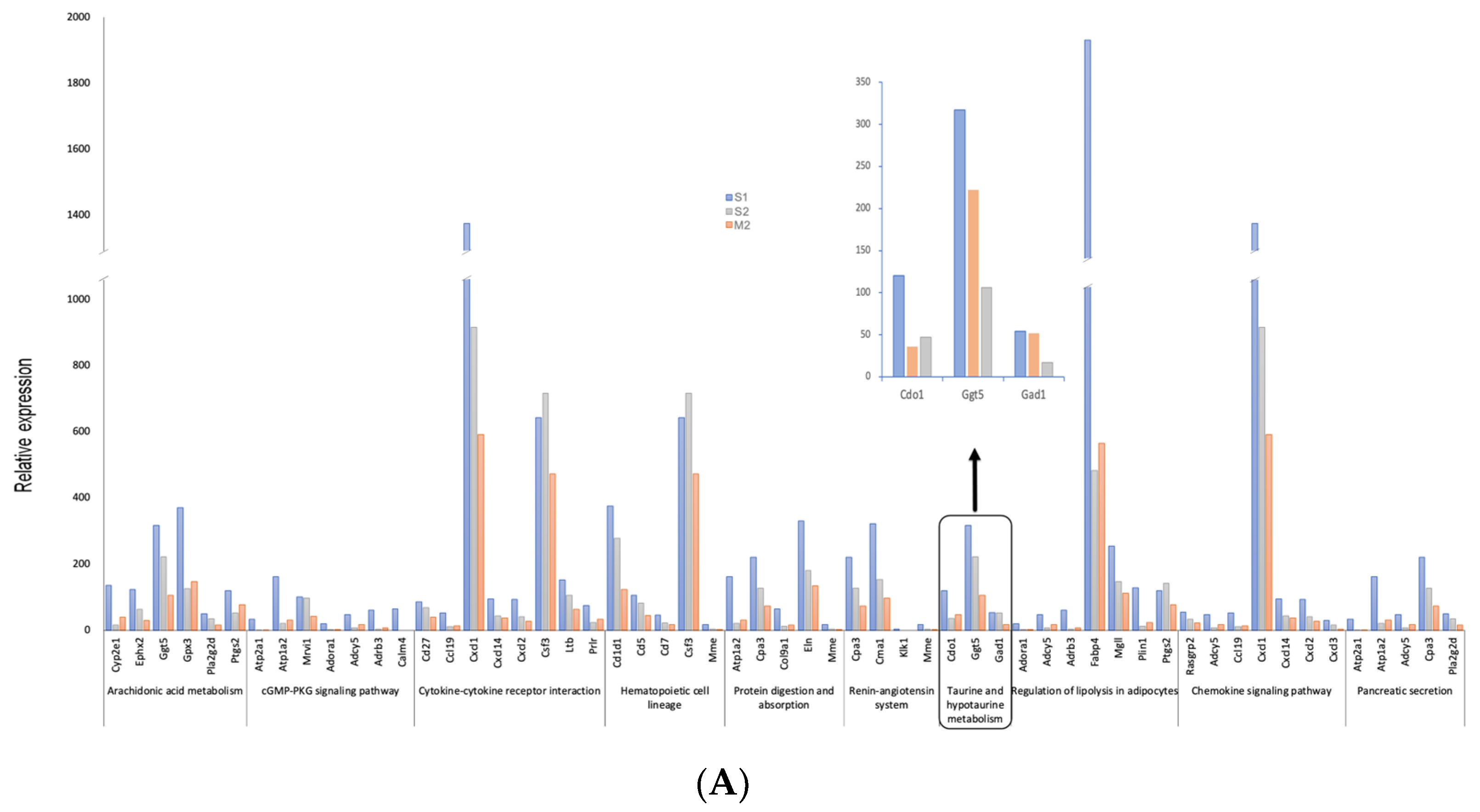
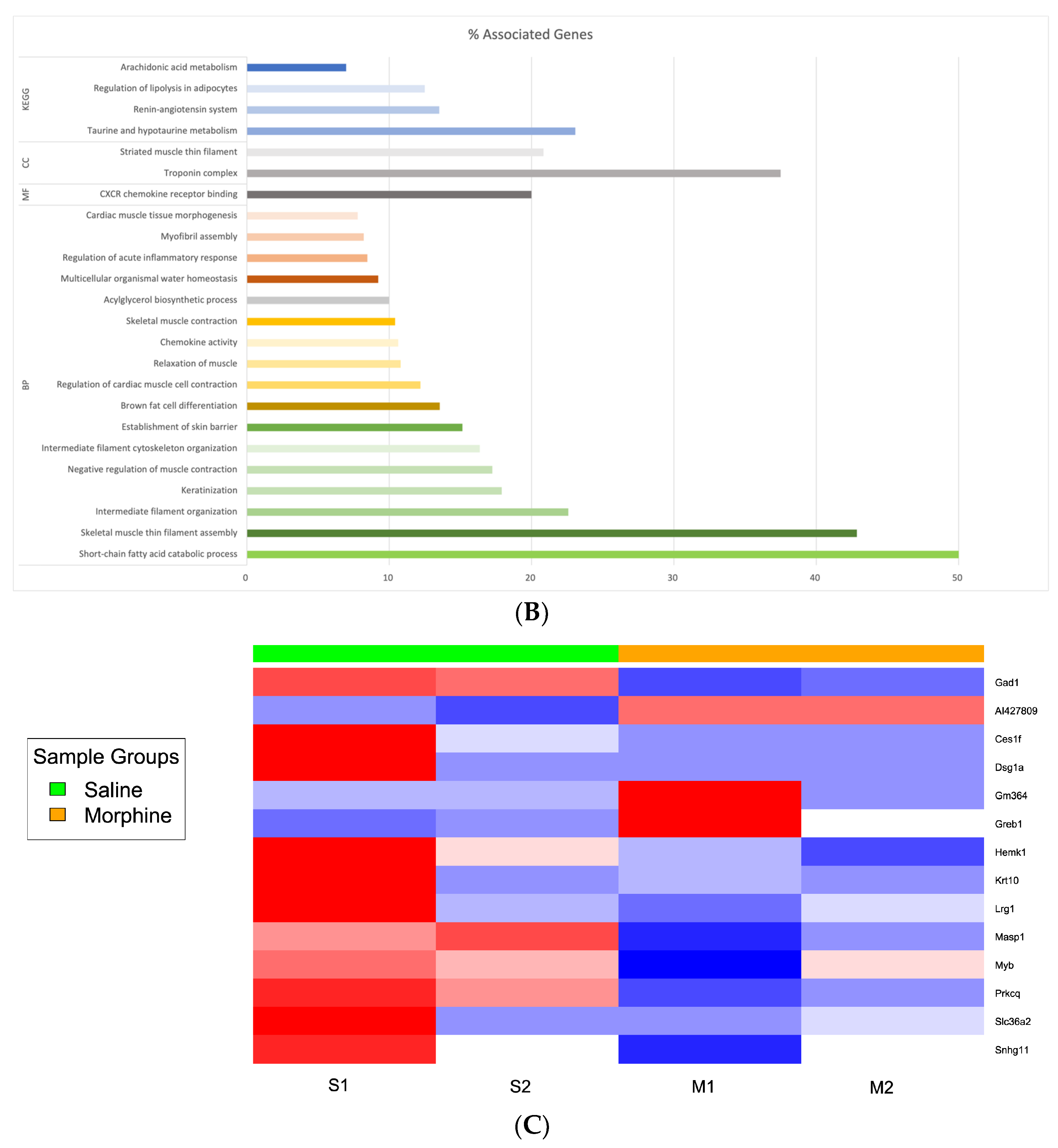
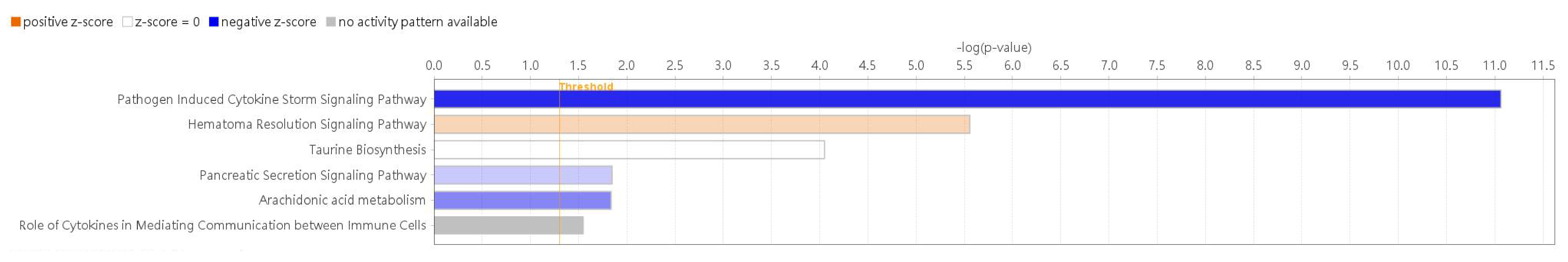
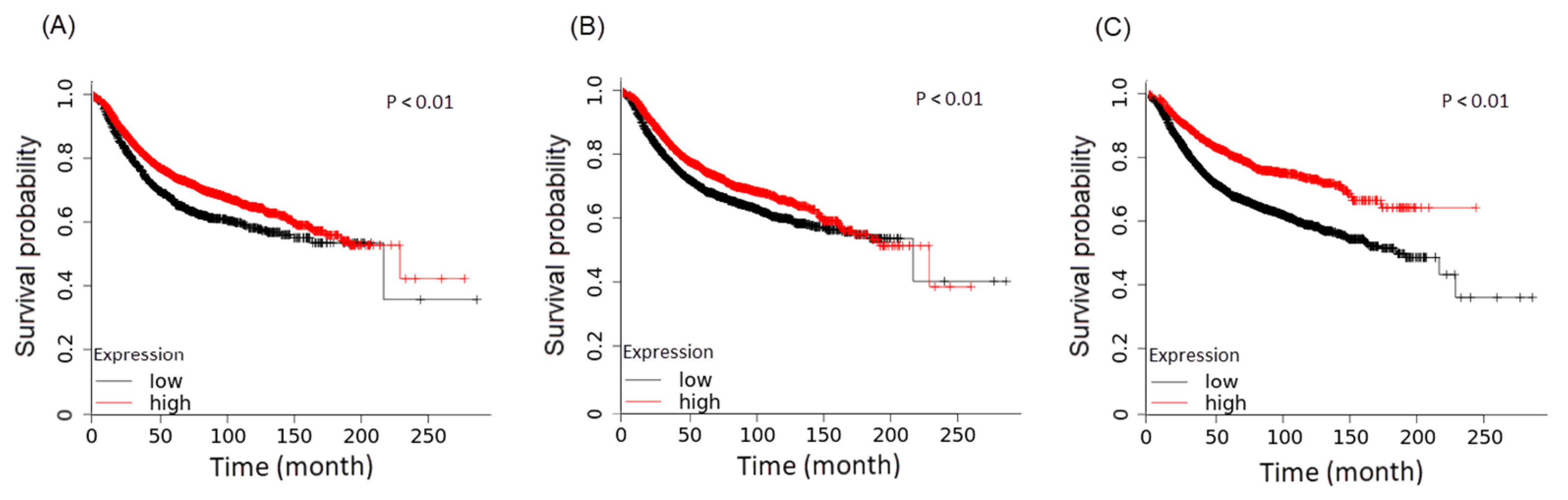
3.2. Differential Expression Genes and Functional and Pathway Enrichment Analysis
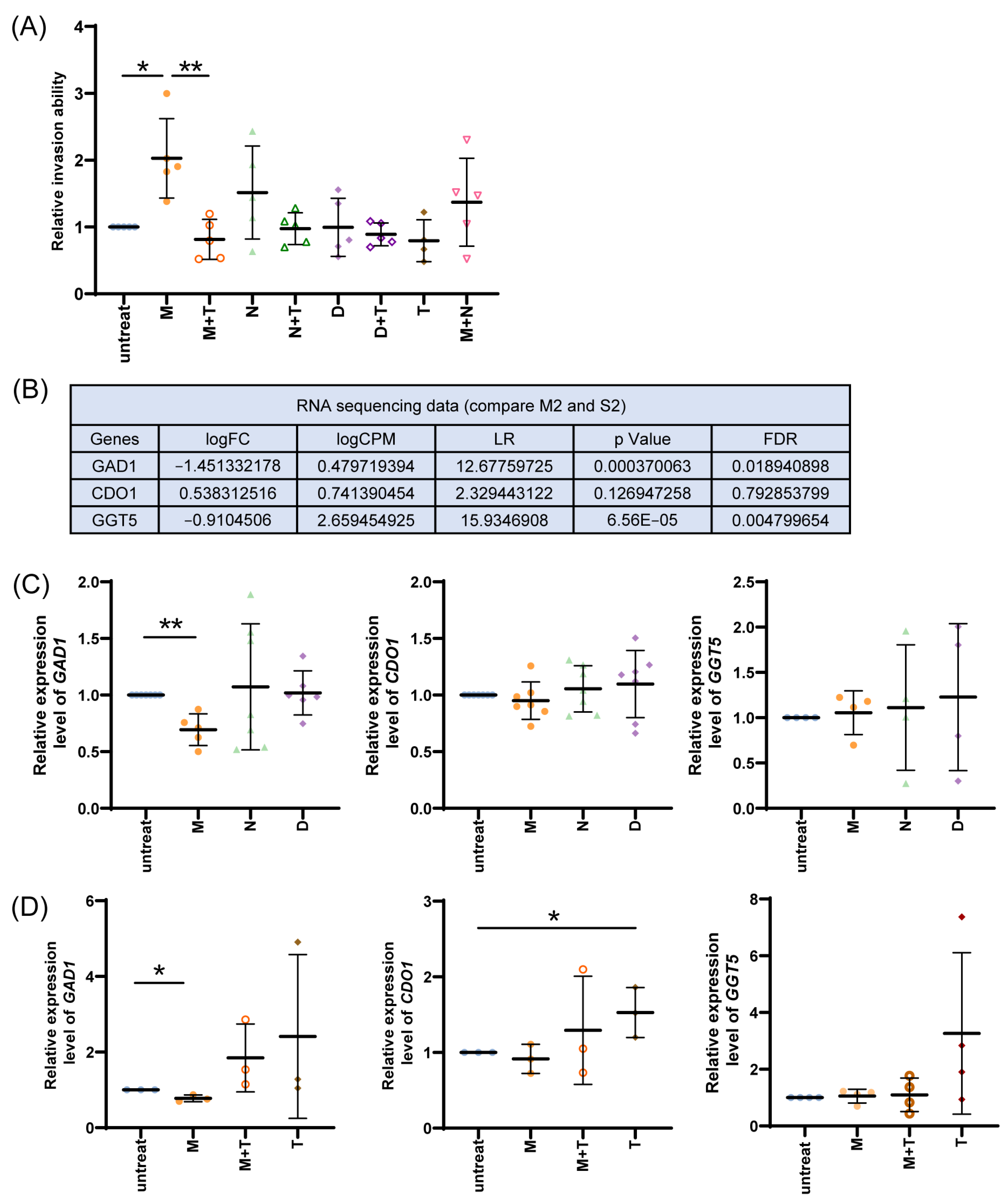
3.3. Effect of Taurine on Morphine-Mediated Cell Invasion
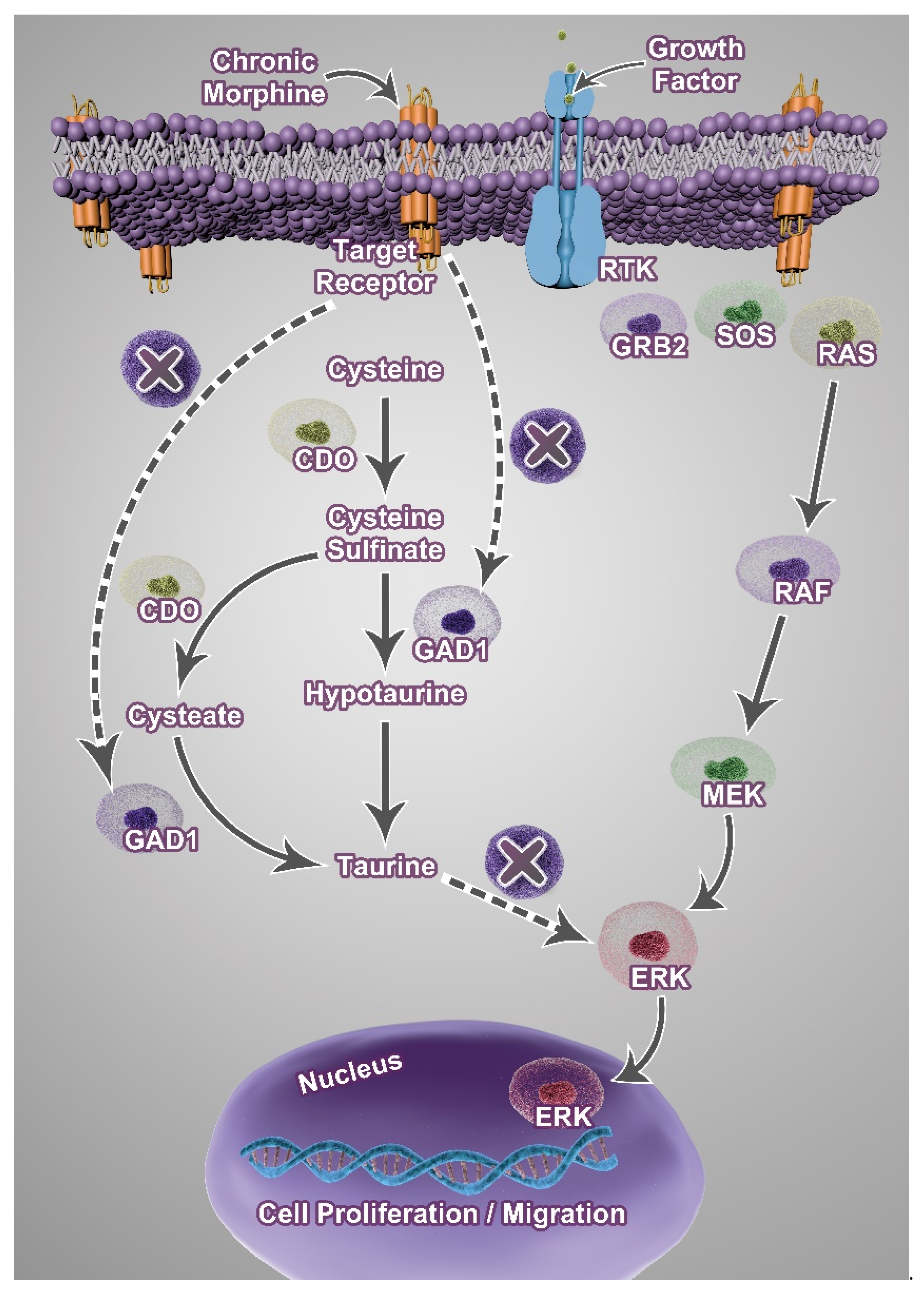
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GPCR | G-protein-coupled receptor |
| MOR | μ-opioid receptor |
| NK | natural killer |
| FBS | fetal bovine serum |
| MTT | 3-(4,5 dimethylthiazol-2-tl)-2,3-diphenyltetrazolium bromide |
| BSA | Bovine serum albumin |
| NTHU | National Tsing Hua University |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| FDR | False Discovery Rate |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| DEGs | Differentially expressed genes |
| DAVID | Database for Annotation, Visualization, and Integrated Discovery |
| TCGA | Cancer Genome Atlas |
| GAD1 | Glutamate decarboxylase 1 |
| BP | Biological process |
| MF | Molecular function |
| CC | Cellular compartment |
| CDO1 | Cysteine dioxygenase 1 |
| CD1d1 | Cluster of Differentiation 1-d1 |
| Hspa1b | Heat Shock Protein a1b |
| Mrvi1 | Murine Retrovirus Integration site 1 |
References
- Ingbar, D.H. Cardiogenic pulmonary edema: Mechanisms and treatment—An intensivist’s view. Curr. Opin. Crit. Care 2019, 25, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Kassop, D. Acute Coronary Syndrome: Management. FP Essent. 2020, 490, 20–28. [Google Scholar] [PubMed]
- Sullivan, M.D.; Edlund, M.J.; Fan, M.Y.; DeVries, A.; Braden, J.B.; Martin, B.C. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain 2010, 150, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Gallagher, R.M.; Gracely, E.; Knowlton, C.; Wescules, D. The terminal cancer patient: Effects of age, gender, and primary tumor site on opioid dose. Pain Med. 2003, 4, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Schuettrumpf, J.; Zou, J.; Zhang, Y.; Schlachterman, A.; Liu, Y.L.; Edmonson, S.; Xiao, W.; Arruda, V.R. The inhibitory effects of anticoagulation on in vivo gene transfer by adeno-associated viral or adenoviral vectors. Mol. Ther. 2006, 13, 88–97. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Lucia, M.; Luca, T.; Federica, D.P.; Cecilia, G.; Chiara, M.; Laura, D.M.; Carlo, D.R.; Grazia, P.M. Opioids and Breast Cancer Recurrence: A Systematic Review. Cancers 2021, 13, 5499. [Google Scholar] [CrossRef]
- Mathew, B.; Lennon, F.E.; Siegler, J.; Mirzapoiazova, T.; Mambetsariev, N.; Sammani, S.; Gerhold, L.M.; LaRiviere, P.J.; Chen, C.T.; Garcia, J.G.; et al. The novel role of the mu opioid receptor in lung cancer progression: A laboratory investigation. Anesth. Analg. 2011, 112, 558–567. [Google Scholar] [CrossRef]
- Aich, A.; Afrin, L.B.; Gupta, K. Mast Cell-Mediated Mechanisms of Nociception. Int. J. Mol. Sci. 2015, 16, 29069–29092. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, Z.; Ma, S.; Yan, W.; He, M.; Wang, D.; Ding, P. Morphine enhances renal cell carcinoma aggressiveness through promotes survivin level. Ren. Fail. 2017, 39, 258–264. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Y.J.; Li, Q.; Hou, Y.Y.; Hong, M.H.; Cao, Y.L.; Chi, Z.Q.; Liu, J.G. Chronic high-dose morphine treatment promotes SH-SY5Y cell apoptosis via c-Jun N-terminal kinase-mediated activation of mitochondria-dependent pathway. FESB J. 2009, 276, 2022–2036. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Nunez, V.; Noriega-Prieto, J.A.; Rodríguez, R.E. Morphine modulates cell proliferation through mir133b &mir128 in the neuroblastoma SH-SY5Y cell line. Biochim. Biophys. Acta 2014, 1842, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Min, T.J.; Park, S.H.; Ji, Y.H.; Lee, Y.S.; Kim, T.W.; Kim, J.H.; Kim, W.Y.; Park, Y.C. Morphine attenuates endothelial cell adhesion molecules induced by the supernatant of LPS-stimulated colon cancer cells. J. Korean Med. Sci. 2011, 26, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Grandhi, R.K.; Lee, S.; Abd-Elsayed, A. Does Opioid Use Cause Angiogenesis and Metastasis? Pain Med. 2017, 18, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Khabbazi, S.; Nassar, Z.D.; Gregory, K.; Vithanage, T.; Anand-Apte, B.; Cabot, P.J.; Sturgess, D.; Shaw, P.N.; Parat, M.O. Morphine alters the circulating proteolytic profile in mice: Functional consequences on cellular migration and invasion. FESB J. 2017, 31, 5208–5216. [Google Scholar] [CrossRef]
- Baliou, S.; Kyriakopoulos, A.M.; Spandidos, D.A.; Zoumpourlis, V. Role of taurine, its haloamines and its lncRNA TUG1 in both inflammation and cancer progression. On the road to therapeutics? (Review). Int. J. Oncol. 2020, 57, 631–664. [Google Scholar] [CrossRef]
- Hiller, K.; Metallo, C.M. Profiling metabolic networks to study cancer metabolism. Curr. Opin. Biotechnol. 2013, 24, 60–68. [Google Scholar] [CrossRef]
- Tuerxun, H.; Cui, J. The dual effect of morphine on tumor development. Clin. Transl. Oncol. 2019, 21, 695–701. [Google Scholar] [CrossRef]
- Sezer, G.; Caner, A.; Önal, M.G.; Cumaoğlu, A. Morphine counteracts the effects of paclitaxel in triple-negative breast cancer cells. Indian J. Med. Res. 2022, 156, 70–76. [Google Scholar] [CrossRef]
- Afsharimani, B.; Cabot, P.; Parat, M.-O. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. 2011, 30, 225–238. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Rea, D.; Palma, G.; Luciano, A.; Cuomo, A.; Arra, C.; Izzo, F. Morphine Promotes Tumor Angiogenesis and Increases Breast Cancer Progression. Biomed. Res. Int. 2015, 2015, 161508. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.E.; Laster, W.R., Jr.; Ross, G.L. Sustained enhanced growth of carcinoma EO771 in C57 black mice. Proc. Soc. Exp. Biol. Med. 1951, 77, 358–362. [Google Scholar] [CrossRef]
- Ewens, A.; Mihich, E.; Ehrke, M.J. Distant metastasis from subcutaneously grown E0771 medullary breast adenocarcinoma. Anticancer Res. 2005, 25, 3905–3915. [Google Scholar] [PubMed]
- Johnstone, C.N.; Smith, Y.E.; Cao, Y.; Burrows, A.D.; Cross, R.S.; Ling, X.; Redvers, R.P.; Doherty, J.P.; Eckhardt, B.L.; Natoli, A.L.; et al. Functional and molecular characterisation of EO771.LMB tumours, a new C57BL/6-mouse-derived model of spontaneously metastatic mammary cancer. Dis. Model. Mech. 2015, 8, 237–251. [Google Scholar] [CrossRef]
- Gades, N.M.; Danneman, P.J.; Wixson, S.K.; Tolley, E.A. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp. Top. Lab. Anim. Sci. 2000, 39, 8–13. [Google Scholar] [PubMed]
- Becker, G.L.; Gerak, L.R.; Li, J.X.; Koek, W.; France, C.P. Precipitated and conditioned withdrawal in morphine-treated rats. Psychopharmacology 2010, 209, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.S.; Erasso, D.M.; Zhuang, G.Z.; Upadhyay, U.; Ozdemir, M.; Wiltshire, T.; Sarantopoulos, K.D.; Smith, S.B.; Maixner, W.; Martin, E.R.; et al. Impact of human CA8 on thermal antinociception in relation to morphine equivalence in mice. Neuroreport 2017, 28, 1215–1220. [Google Scholar] [CrossRef]
- Chen, L.K.; Shih, C.H.; Chen, S.S.; Huang, Z.X.; Chang, Y.J.; Chen, L.; Chuang, T.H.; Chen, K.B. Ketamine promotes breast tumor growth in a mouse breast tumor model involving with high expression of miR-27b-3p and EGFR. Investig. New Drugs 2022, 40, 1165–1172. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Bo Li, C.N.D. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Huang, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2013, 30, 523–530. [Google Scholar] [CrossRef]
- Schumacher, B.L.a.M. Maximally Selected Rank Statistics. Biometrics 1992, 18, 73–85. [Google Scholar]
- Dent, R.; Hanna, W.M.; Trudeau, M.; Rawlinson, E.; Sun, P.; Narod, S.A. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res. Treat. 2009, 115, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, A.; Rossary, A.; Vasson, M.P. EO771, is it a well-characterized cell line for mouse mammary cancer model? Limit and uncertainty. Cancer Med. 2020, 9, 8074–8085. [Google Scholar] [CrossRef] [PubMed]
- Fitts, D.A. Ethics and animal numbers: Informal analyses, uncertain sample sizes, inefficient replications, and type I errors. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 445–453. [Google Scholar] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Simon, R.H.; Arbo, T.E. Morphine increases metastatic tumor growth. Brain Res. Bull. 1986, 16, 363–367. [Google Scholar] [CrossRef]
- Sergeeva, M.G.; Grishina, Z.V.; Varfolomeyev, S.D. Morphine effect on proliferation of normal and tumor cells of immune origin. Immunol. Lett. 1993, 36, 215–218. [Google Scholar] [CrossRef]
- Ang Chen, Y.Z.; Sun, D.; Xu, Y.; Guo, Y.; Wang, X. Investigation of the content differences of arachidonic acid metabolites in a mouse model of breast cancer by using LC–MS/MS. J. Pharm. Biomed. Anal. 2021, 194, 113763. [Google Scholar]
- Borin, T.F.; Angara, K.; Rashid, M.H.; Achyut, B.R.; Arbab, A.S. Arachidonic Acid Metabolite as a Novel Therapeutic Target in Breast Cancer Metastasis. Int. J. Mol. Sci. 2017, 18, 2661. [Google Scholar] [CrossRef]
- Brodin, E.; Gazelius, B.; Panopoulos, P.; Olgart, L. Morphine inhibits substance P release from peripheral sensory nerve endings. Acta Physiol. Scand. 1983, 117, 567–570. [Google Scholar]
- Kis, B.; Mezei, Z.; Gecse, A.; Telegdy, G. The effect of substance P on the arachidonate cascade of rat platelets. Acta Physiol. Hung. 1997, 85, 89–97. [Google Scholar]
- Guan, Y.; Zhu, X.; Liang, J.; Wei, M.; Huang, S.; Pan, X. Upregulation of HSPA1A/HSPA1B/HSPA7 and Downregulation of HSPA9 Were Related to Poor Survival in Colon Cancer. Front. Oncol. 2021, 11, 749673. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, K.; Jiang, W.; Li, J.; Zhang, J.A.; Zhu, X. MRVI1 and NTRK3 Are Potential Tumor Suppressor Genes Commonly Inactivated by DNA Methylation in Cervical Cancer. Front. Oncol. 2021, 11, 802068. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, S.; Zhang, J.; Krovi, S.H.; Bedel, R.; Tuttle, K.D.; Veerapen, N.; Besra, G.S.; Khandokar, Y.; Praveena, T.; Le Nours, J.; et al. Differing roles of CD1d2 and CD1d1 proteins in type I natural killer T cell development and function. Proc. Natl. Acad. Sci. USA 2018, 115, E1204–E1213. [Google Scholar] [CrossRef] [PubMed]
- Hix, L.M.; Shi, Y.H.; Brutkiewicz, R.R.; Stein, P.L.; Wang, C.R.; Zhang, M. CD1d-expressing breast cancer cells modulate NKT cell-mediated antitumor immunity in a murine model of breast cancer metastasis. PLoS ONE 2011, 6, e20702. [Google Scholar] [CrossRef]
- Sommers, S.W.; Tolle, H.J.; Trinkley, K.E.; Johnston, C.G.; Dietsche, C.L.; Eldred, S.V.; Wick, A.T.; Hoppe, J.A. Clinical Decision Support to Increase Emergency Department Naloxone Coprescribing: Implementation Report. JMIR Med. Inform. 2024, 12, e58276. [Google Scholar] [CrossRef]
- José Vicente Negrete-Díaz, K.S.; Real, M.Á.; Medina-Luque, J.; Valderrama-Carvajal, A.; Flores, G.; Rodríguez-Moreno, A.; Rivera, A. Pharmacological activation of dopamine D 4 receptor modulates morphine-induced changes in the expression of GAD 65/67 and GABA B receptors in the basal ganglia. Neuropharmacology 2019, 152, 22–29. [Google Scholar] [CrossRef]
- Weibel, S.; Rücker, G.; Eberhart, L.H.; Pace, N.L.; Hartl, H.M.; Jordan, O.L.; Mayer, D.; Riemer, M.; Schaefer, M.S.; Raj, D.; et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: A network meta-analysis. Cochrane Database Syst. Rev. 2020, 10, Cd012859. [Google Scholar] [CrossRef]
- Gupta, K.; Kshirsagar, S.; Chang, L.; Schwartz, R.; Law, P.Y.; Yee, D.; Hebbel, R.P. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002, 62, 4491–4498. [Google Scholar]
- Ninković, J.; Roy, S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 2013, 45, 9–24. [Google Scholar] [CrossRef]
- Esch, T.; Kream, R.M.; Stefano, G.B. Emerging regulatory roles of opioid peptides, endogenous morphine, and opioid receptor subtypes in immunomodulatory processes: Metabolic, behavioral, and evolutionary perspectives. Immunol. Lett. 2020, 227, 28–33. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, H.; Wang, Y.; Liu, C.; Zhu, W.; Zheng, S.; Wan, F. Taurine induces the apoptosis of breast cancer cells by regulating apoptosis-related proteins of mitochondria. Int. J. Mol. Med. 2015, 35, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Kim, Y.S.; Choi, E.J.; Hwang, Y.J.; Yun, Y.S.; Bae, S.M.; Park, P.J.; Kim, E.K. Taurine Attenuates Epithelial-Mesenchymal Transition-Related Genes in Human Prostate Cancer Cells. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Zhang, W.; Wang, Q.; Wang, C.; Ma, W.; Zhang, C.; Ge, M.; Tian, M.; Yu, J.; Jiao, A.; et al. Cancer SLC6A6-mediated taurine uptake transactivates immune checkpoint genes and induces exhaustion in CD8(+) T cells. Cell 2024, 187, 2288–2304.e2227. [Google Scholar] [CrossRef]
- Gordon, R.E.; Heller, R.F.; Heller, R.F. Taurine protection of lungs in hamster models of oxidant injury: A morphologic time study of paraquat and bleomycin treatment. Adv. Exp. Med. Biol. 1992, 315, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Choueiri, M.; Shen, J.P.; Randall, J.M.; Ideker, T.; Hofree, M. Association of methylation of genes in the taurine/hypotaurine pathway with worse prognosis in renal cell carcinoma. J. Clin. Oncol. 2013, 31, e15562. [Google Scholar] [CrossRef]
- Koodie, L.; Ramakrishnan, S.; Roy, S. Morphine Suppresses Tumor Angiogenesis through a HIF-1α/p38MAPK Pathway. Am. J. Pathol. 2010, 177, 984–997. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-H.; Ke, T.-L.; Shih, C.-H.; Hsiung, C.-N.; Chen, K.-C.; Huang, Z.-X.; Chuang, T.-H.; Chen, L.-K.; Chen, L. Reduced Taurine Synthesis Underlies Morphine-Promoted Lung Metastasis of Triple-Negative Breast Cancer. Cancers 2025, 17, 1086. https://doi.org/10.3390/cancers17071086
Chen S-H, Ke T-L, Shih C-H, Hsiung C-N, Chen K-C, Huang Z-X, Chuang T-H, Chen L-K, Chen L. Reduced Taurine Synthesis Underlies Morphine-Promoted Lung Metastasis of Triple-Negative Breast Cancer. Cancers. 2025; 17(7):1086. https://doi.org/10.3390/cancers17071086
Chicago/Turabian StyleChen, Shih-Hong, Ting-Ling Ke, Chien-Hung Shih, Chia-Ni Hsiung, Kuo-Chin Chen, Zi-Xuan Huang, Tsung-Hsien Chuang, Li-Kuei Chen, and Linyi Chen. 2025. "Reduced Taurine Synthesis Underlies Morphine-Promoted Lung Metastasis of Triple-Negative Breast Cancer" Cancers 17, no. 7: 1086. https://doi.org/10.3390/cancers17071086
APA StyleChen, S.-H., Ke, T.-L., Shih, C.-H., Hsiung, C.-N., Chen, K.-C., Huang, Z.-X., Chuang, T.-H., Chen, L.-K., & Chen, L. (2025). Reduced Taurine Synthesis Underlies Morphine-Promoted Lung Metastasis of Triple-Negative Breast Cancer. Cancers, 17(7), 1086. https://doi.org/10.3390/cancers17071086




