Comprehensive Age-Stratified Impact of NPM1 Mutation in Acute Myeloid Leukemia: A Real-World Experience
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Cohort Selection
2.2. Genetic Studies
2.3. Statistical Analysis
3. Results
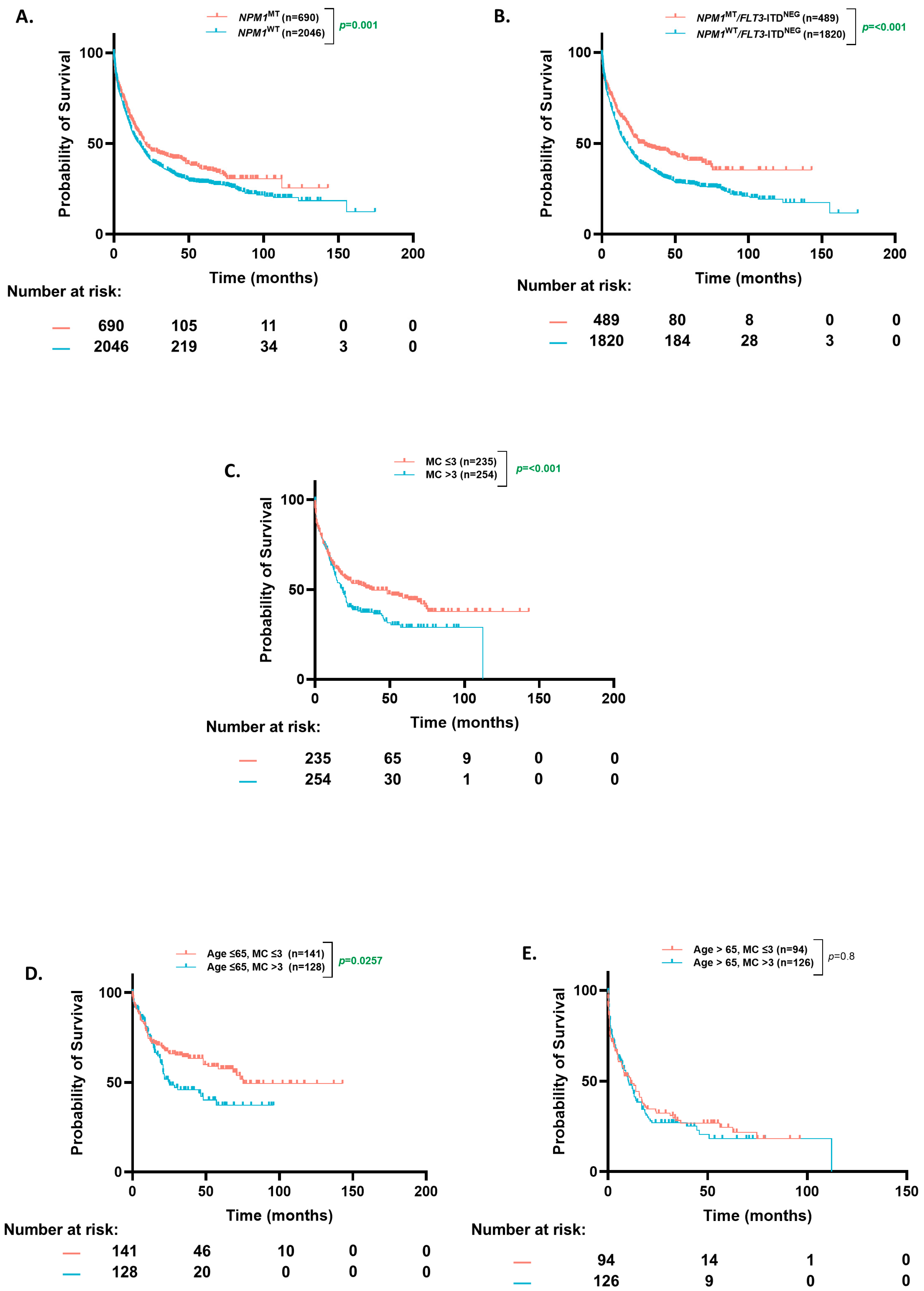
3.1. Cohort Overview and OS Based on Co-Occurring Mutational Count
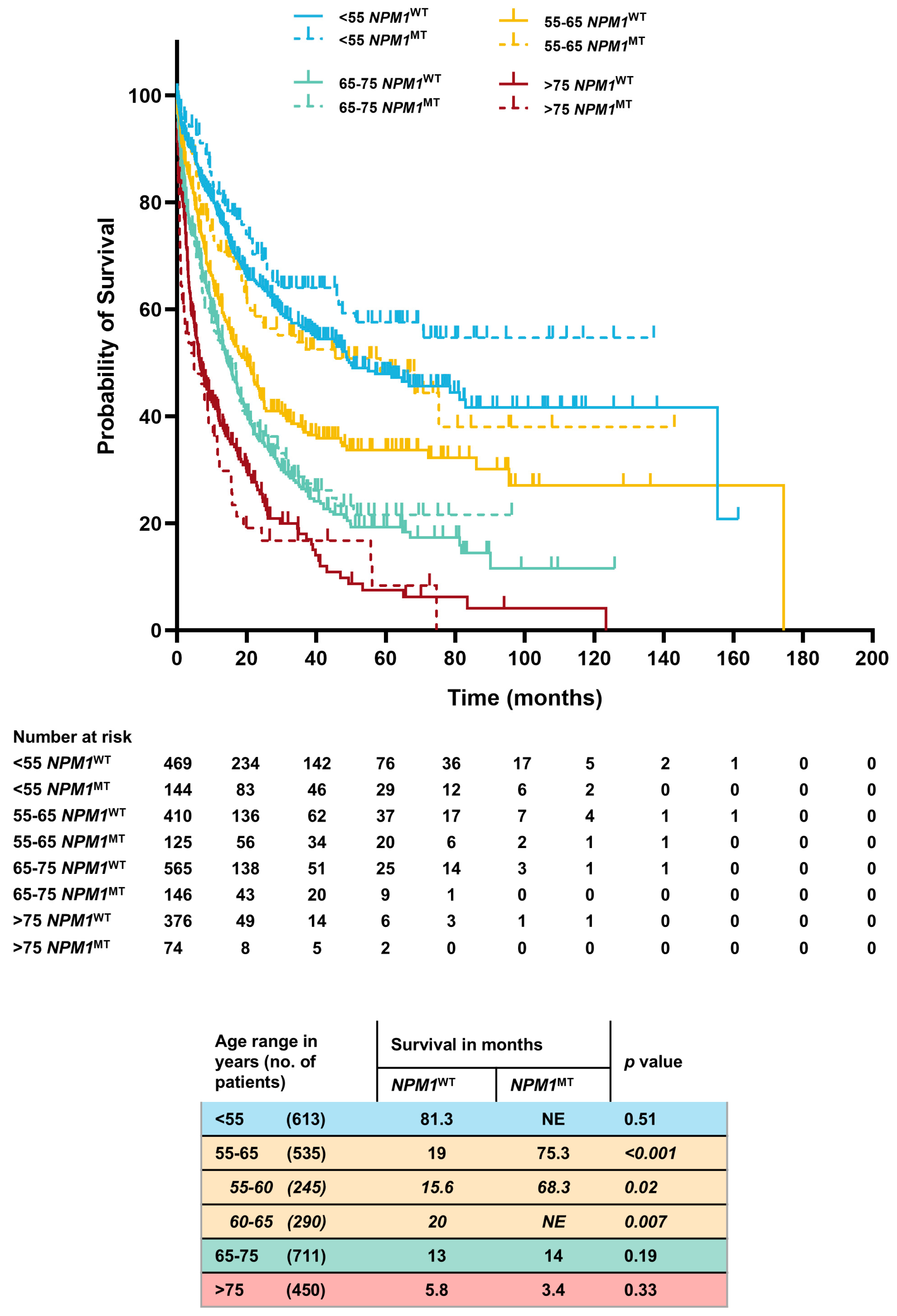
3.2. Age-Stratified OS (Based on NPM1 Status)
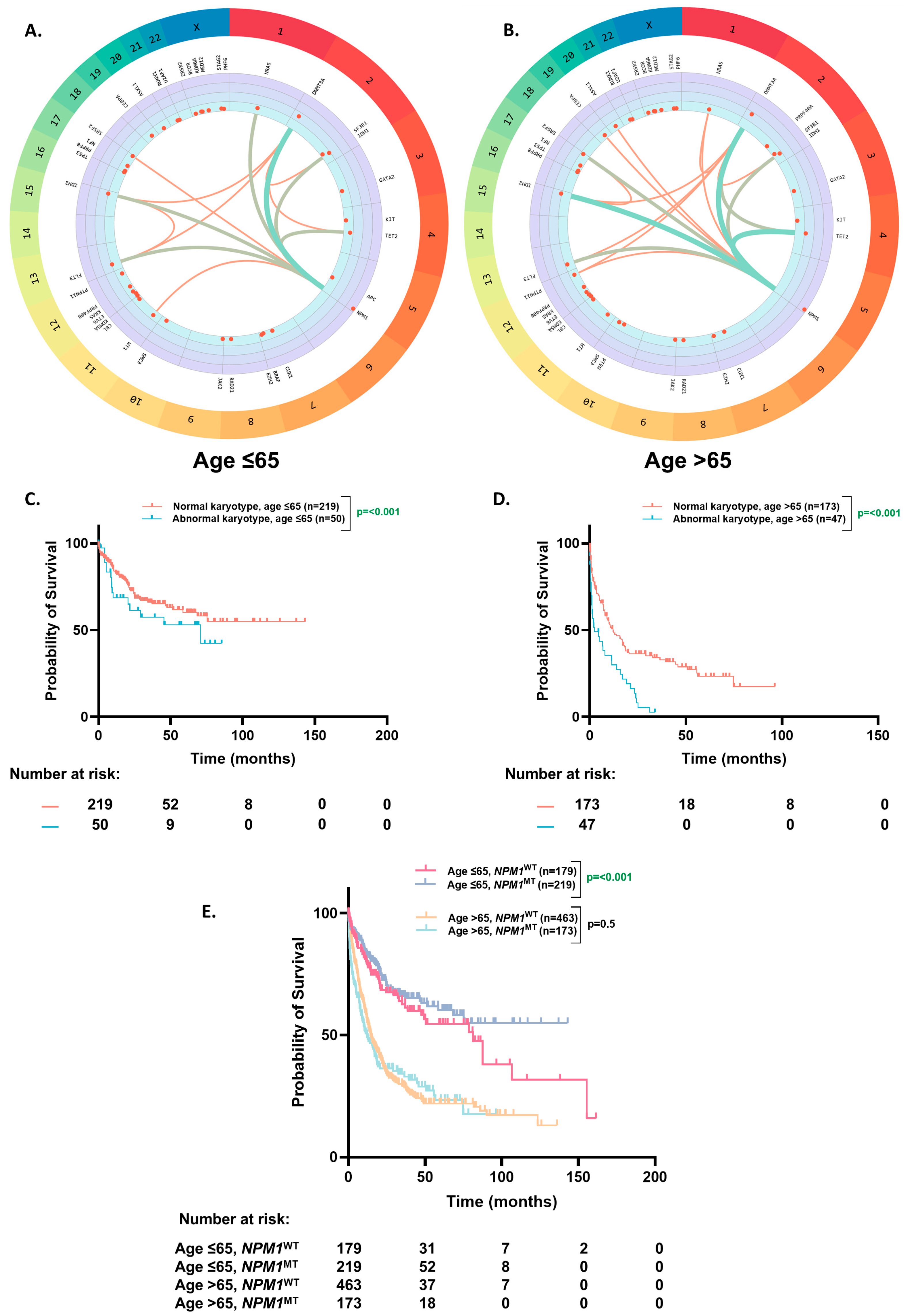
3.3. Molecular Profile of NPM1MT Patients
3.4. OS Based on Karyotype
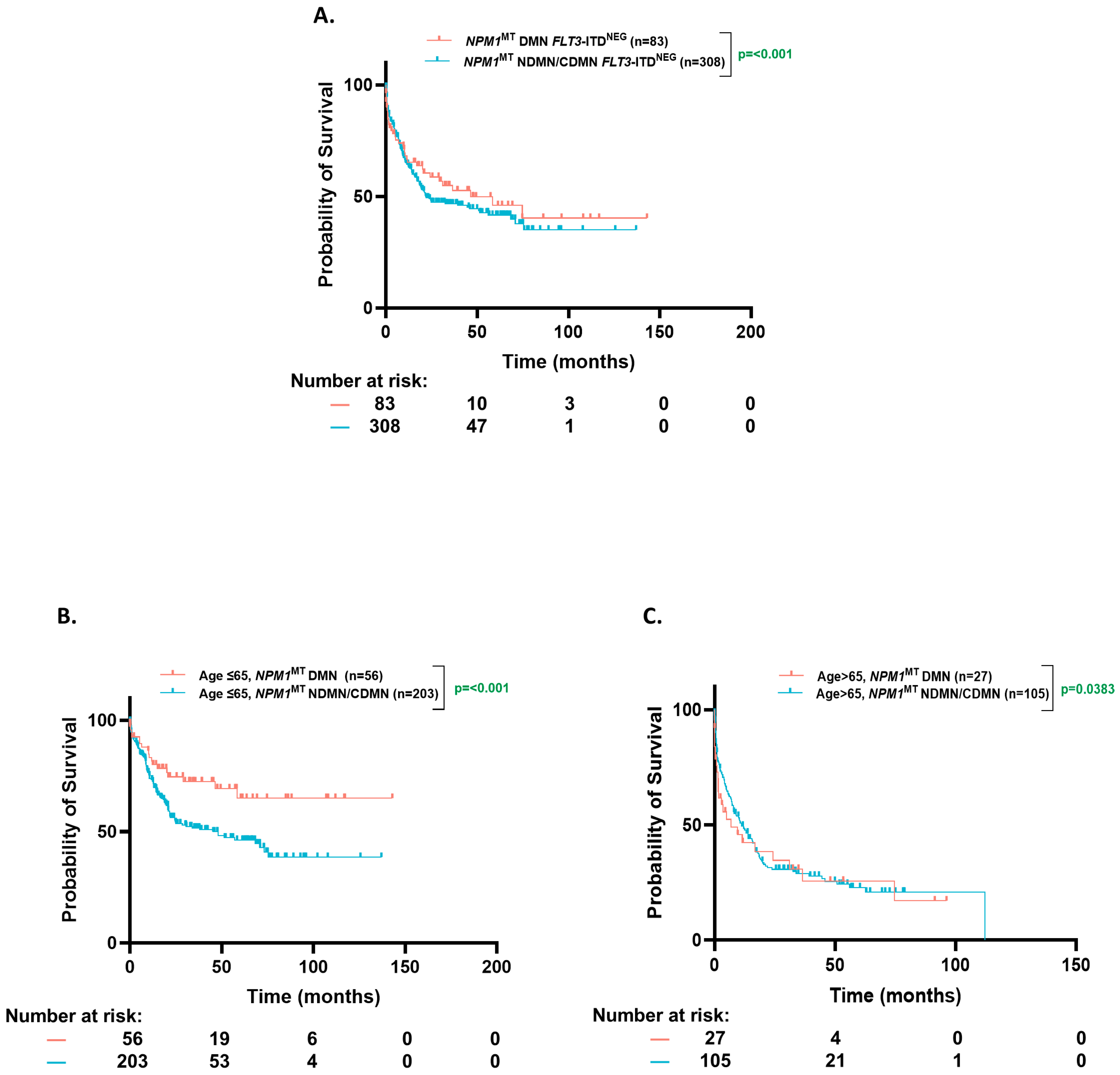
3.5. OS Based on Clonal Dominance
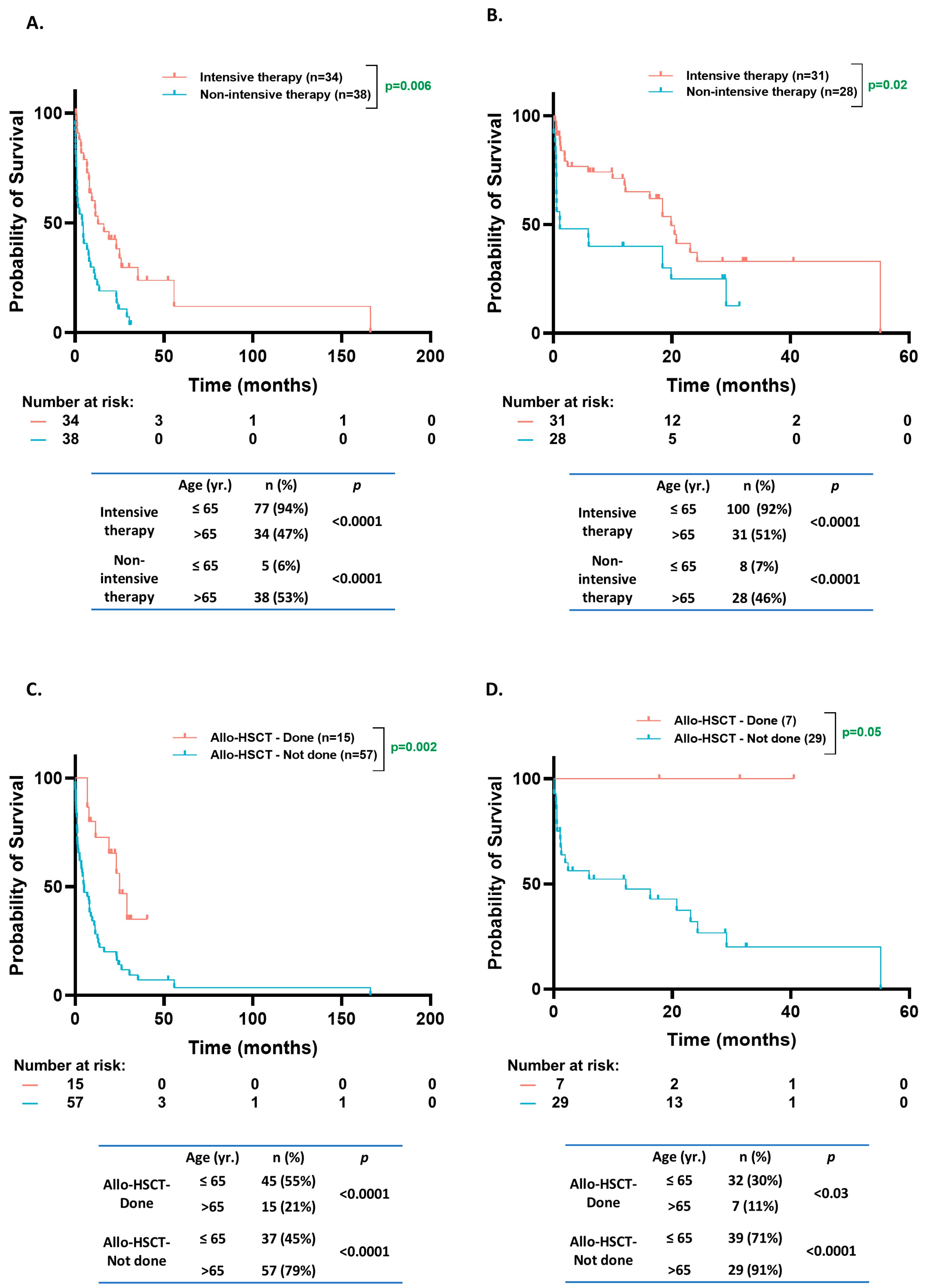
3.6. OS Based on Treatment Intensity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Suzuki, T.; Kiyoi, H.; Ozeki, K.; Tomita, A.; Yamaji, S.; Suzuki, R.; Kodera, Y.; Miyawaki, S.; Asou, N.; Kuriyama, K. Clinical characteristics and prognostic implications of NPM1 mutations in acute myeloid leukemia. Blood 2005, 106, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood J. Am. Soc. Hematol. 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood J. Am. Soc. Hematol. 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.; Chan, S.; Minden, M.; Murphy, T.; Shlush, L.; Schimmer, A. Biological and clinical consequences of NPM1 mutations in AML. Leukemia 2017, 31, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Kayser, S.; Levis, M.J. The clinical impact of the molecular landscape of acute myeloid leukemia. Haematologica 2023, 108, 308. [Google Scholar] [CrossRef]
- Döhner, K.; Schlenk, R.F.; Habdank, M.; Scholl, C.; Rücker, F.G.; Corbacioglu, A.; Bullinger, L.; Fröhling, S.; Döhner, H.; Group, A.S. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: Interaction with other gene mutations. Blood 2005, 106, 3740–3746. [Google Scholar] [CrossRef]
- Juliusson, G.; Jädersten, M.; Deneberg, S.; Lehmann, S.; Möllgård, L.; Wennström, L.; Antunovic, P.; Cammenga, J.; Lorenz, F.; Ölander, E. The prognostic impact of FLT3-ITD and NPM1 mutation in adult AML is age-dependent in the population-based setting. Blood Adv. 2020, 4, 1094–1101. [Google Scholar] [CrossRef]
- Falini, B.; Mecucci, C.; Tiacci, E.; Alcalay, M.; Rosati, R.; Pasqualucci, L.; La Starza, R.; Diverio, D.; Colombo, E.; Santucci, A. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 2005, 352, 254–266. [Google Scholar] [CrossRef]
- Becker, H.; Marcucci, G.; Maharry, K.; Margeson, D.; Radmacher, M.; Whitman, S.; Mrózek, K.; Baer, M.; Larson, R.; Bloomfield, C. NPM1 mutations as an independent prognosticator for older cytogenetically normal acute myeloid leukemia (CN AML). J. Clin. Oncol. 2009, 27, 7000. [Google Scholar] [CrossRef]
- Ostronoff, F.; Othus, M.; Lazenby, M.; Estey, E.; Appelbaum, F.R.; Evans, A.; Godwin, J.; Gilkes, A.; Kopecky, K.J.; Burnett, A. Prognostic significance of NPM1 mutations in the absence of FLT3–internal tandem duplication in older patients with acute myeloid leukemia: A SWOG and UK National Cancer Research Institute/Medical Research Council Report. J. Clin. Oncol. 2015, 33, 1157–1164. [Google Scholar] [CrossRef]
- Falini, B.; Brunetti, L.; Sportoletti, P.; Martelli, M.P. NPM1-mutated acute myeloid leukemia: From bench to bedside. Blood J. Am. Soc. Hematol. 2020, 136, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Awada, H.; Durmaz, A.; Gurnari, C.; Kishtagari, A.; Meggendorfer, M.; Kerr, C.M.; Kuzmanovic, T.; Durrani, J.; Shreve, J.; Nagata, Y. Machine learning integrates genomic signatures for subclassification beyond primary and secondary acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2021, 138, 1885–1895. [Google Scholar] [CrossRef]
- Nagata, Y.; Makishima, H.; Kerr, C.M.; Przychodzen, B.P.; Aly, M.; Goyal, A.; Awada, H.; Asad, M.F.; Kuzmanovic, T.; Suzuki, H. Invariant patterns of clonal succession determine specific clinical features of myelodysplastic syndromes. Nat. Commun. 2019, 10, 5386. [Google Scholar] [CrossRef] [PubMed]
- Tyner, J.W.; Tognon, C.E.; Bottomly, D.; Wilmot, B.; Kurtz, S.E.; Savage, S.L.; Long, N.; Schultz, A.R.; Traer, E.; Abel, M. Functional genomic landscape of acute myeloid leukaemia. Nature 2018, 562, 526–531. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.L.; Schumacher, J.A.; Frizzell, K.; Sorrells, S.; Shen, W.; Clayton, A.; Jattani, R.; Kelley, T.W. Coexisting and cooperating mutations in NPM1-mutated acute myeloid leukemia. Leuk. Res. 2017, 56, 7–12. [Google Scholar] [CrossRef]
- Arber, D.A.; Erba, H.P. Diagnosis and treatment of patients with acute myeloid leukemia with myelodysplasia-related changes (AML-MRC). Am. J. Clin. Pathol. 2020, 154, 731–741. [Google Scholar] [CrossRef]
- Othman, J.; Potter, N.; Ivey, A.; Tazi, Y.; Papaemmanuil, E.; Jovanovic, J.; Freeman, S.D.; Gilkes, A.; Gale, R.; Rapoz-D’Silva, T. Molecular, clinical, and therapeutic determinants of outcome in NPM1-mutated AML. Blood 2024, 144, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-C.; Chou, S.-C.; Liu, C.-Y.; Chen, C.-Y.; Hou, H.-A.; Kuo, Y.-Y.; Lee, M.-C.; Ko, B.-S.; Tang, J.-L.; Yao, M. TET2 mutation is an unfavorable prognostic factor in acute myeloid leukemia patients with intermediate-risk cytogenetics. Blood J. Am. Soc. Hematol. 2011, 118, 3803–3810. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Cassady, K.; Zou, Z.; Zhang, X. TET2 function in hematopoietic malignancies, immune regulation, and DNA repair. Front. Oncol. 2019, 9, 210. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Z.; Li, T.; Li, Y.; Wang, W.; Hao, Q.; Xie, X.; Wan, D.; Jiang, Z.; Wang, C. Mutational spectrum and prognosis in NRAS-mutated acute myeloid leukemia. Sci. Rep. 2020, 10, 12152. [Google Scholar] [CrossRef]
- Bacher, U.; Haferlach, T.; Schoch, C.; Kern, W.; Schnittger, S. Implications of NRAS mutations in AML: A study of 2502 patients. Blood 2006, 107, 3847–3853. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.-A.; Huang, T.-C.; Lin, L.-I.; Liu, C.-Y.; Chen, C.-Y.; Chou, W.-C.; Tang, J.-L.; Tseng, M.-H.; Huang, C.-F.; Chiang, Y.-C. WT1 mutation in 470 adult patients with acute myeloid leukemia: Stability during disease evolution and implication of its incorporation into a survival scoring system. Blood J. Am. Soc. Hematol. 2010, 115, 5222–5231. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Mengge, G.; Wang, K.; Wang, Y.; Kong, J.; Sun, Y.; Zhao, X.; Huang, X.J. Prognostic impact of WT1 mutation on AML of different risk groups based on 2022 European Leukemianet (ELN) risk classification. Blood 2022, 140, 3216–3217. [Google Scholar] [CrossRef]
- Awada, H.; Durmaz, A.; Gurnari, C.; Kishtagari, A.; Zawit, M.; Pagliuca, S.; Visconte, V. Friend or foe? The case of Wilms’ Tumor 1 (WT1) mutations in acute myeloid leukemia. Blood Cells Mol. Dis. 2021, 88, 102549. [Google Scholar] [CrossRef]
- Chan, O.; Al Ali, N.; Tashkandi, H.; Ellis, A.; Ball, S.; Zhang, L.; Hussaini, M.; Song, J.; Yun, S.; Talati, C. Mutations highly specific for secondary AML are associated with poor outcomes in patients with NPM1-mutated ELN favorable risk AML. Blood 2021, 138, 686. [Google Scholar] [CrossRef]
- Zhao, D.; Zarif, M.; Eladl, E.; Capo-Chichi, J.-M.; Smith, A.C.; Atenafu, E.G.; Tierens, A.; Minden, M.D.; Schuh, A.; Chang, H. NPM1-mutated AML-MRC diagnosed on the basis of history of MDS or MDS/MPN frequently harbours secondary-type mutations and confers inferior outcome compared to AML with mutated NPM1. Leuk. Res. 2022, 118, 106869. [Google Scholar] [CrossRef]
- Eckardt, J.-N.; Bill, M.; Rausch, C.; Metzeler, K.; Spiekermann, K.; Stasik, S.; Sauer, T.; Scholl, S.; Hochhaus, A.; Crysandt, M. Secondary-type mutations do not impact outcome in NPM1-mutated acute myeloid leukemia–implications for the European LeukemiaNet risk classification. Leukemia 2023, 37, 2282–2285. [Google Scholar] [CrossRef]
- Lachowiez, C.; DiNardo, C.D.; Morita, K.; Furudate, K.; Wang, F.; Tanaka, T.; Wang, S.A.; Kadia, T.M.; Daver, N.; Short, N.J. Longitudinal next generation sequencing reveals the clonal hierarchy of IDH mutated clones and impact on survival in NPM1 mutated AML. Blood 2021, 138, 607. [Google Scholar] [CrossRef]
- Benard, B.; Leak, L.; Azizi, A.; Thomas, D.; Gentles, A.; Majeti, R. Clonal Architecture and Variant Allele Frequency Predict Clinical Outcomes and Drug Response in Acute Myeloid Leukemia. Blood 2020, 136, 2. [Google Scholar] [CrossRef]
- Benard, B.A.; Leak, L.B.; Azizi, A.; Thomas, D.; Gentles, A.J.; Majeti, R. Clonal architecture predicts clinical outcomes and drug sensitivity in acute myeloid leukemia. Nat. Commun. 2021, 12, 7244. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, L.; Shen, C.; Zhu, S.; Lang, W.; Luo, Y.; Zhang, H.; Yang, W.; Han, Y.; Ma, L. Impact of mutational variant allele frequency on prognosis in myelodysplastic syndromes. Am. J. Cancer Res. 2020, 10, 4476. [Google Scholar] [PubMed]
- Abbas, H.A.; Ravandi, F.; Loghavi, S.; Patel, K.P.; Borthakur, G.; Kadia, T.M.; Jabbour, E.; Takahashi, K.; Cortes, J.; Issa, G.C. NPM1 mutant variant allele frequency correlates with leukemia burden but does not provide prognostic information in NPM1-mutated AML. Am. J. Hematol. 2019, 94, E158. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg-Thurley, M.; Herold, T.; Görlich, D.; Sauerland, C.; Janke, H.; Prassek, V.V.; Konstandin, N.P.; Dufour, A.M.; Schneider, S.; Ksienzyk, B. NPM1 variant allele frequency and outcomes in AML. Blood 2018, 132, 1486. [Google Scholar] [CrossRef]
- Sorror, M.L.; Storer, B.E.; Fathi, A.T.; Brunner, A.; Gerds, A.T.; Sekeres, M.A.; Mukherjee, S.; Medeiros, B.C.; Wang, E.S.; Vachhani, P. Multisite 11-year experience of less-intensive vs intensive therapies in acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2021, 138, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Guyatt, G.H.; Teich, T.; Dawdy, J.L.; Shahid, S.; Altman, J.K.; Stone, R.M.; Sekeres, M.A.; Mukherjee, S.; LeBlanc, T.W. Intensive versus less-intensive antileukemic therapy in older adults with acute myeloid leukemia: A systematic review. PLoS ONE 2021, 16, e0249087. [Google Scholar] [CrossRef]
- Lazenby, M.; Gilkes, A.; Marrin, C.; Evans, A.; Hills, R.K.; Burnett, A. The prognostic relevance of flt3 and npm1 mutations on older patients treated intensively or non-intensively: A study of 1312 patients in the UK NCRI AML16 trial. Leukemia 2014, 28, 1953–1959. [Google Scholar] [CrossRef]
- Scholl, S.; Theuer, C.; Scheble, V.; Kunert, C.; Heller, A.; Mügge, L.O.; Fricke, H.J.; Höffken, K.; Wedding, U. Clinical impact of nucleophosmin mutations and Flt3 internal tandem duplications in patients older than 60 yr with acute myeloid leukaemia. Eur. J. Haematol. 2008, 80, 208–215. [Google Scholar] [CrossRef]
- Jentzsch, M.; Grimm, J.; Bill, M.; Goldmann, K.; Schulz, J.; Niederwieser, D.; Platzbecker, U.; Schwind, S. Outcomes of older patients with NPM1 mutated and FLT3-ITD negative acute myeloid leukemia receiving allogeneic transplantation. HemaSphere 2020, 4, e326. [Google Scholar] [CrossRef]
- Simcock, R.; Wright, J. Beyond performance status. Clin. Oncol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Weisdorf, D. How old is too old for a transplant? Best Pract. Res. Clin. Haematol. 2021, 34, 101243. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.A.; Guyatt, G.; Abel, G.; Alibhai, S.; Altman, J.K.; Buckstein, R.; Choe, H.; Desai, P.; Erba, H.; Hourigan, C.S. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020, 4, 3528–3549. [Google Scholar] [CrossRef]
- Wang, E.S. Treating acute myeloid leukemia in older adults. Hematol. Am. Soc. Hematol. Educ. Program 2014, 2014, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Loghavi, S.; Kadia, T.M.; Daver, N.; Borthakur, G.; Pemmaraju, N.; Naqvi, K.; Alvarado, Y.; Yilmaz, M.; Short, N. Outcomes of older patients with NPM1-mutated AML: Current treatments and the promise of venetoclax-based regimens. Blood Adv. 2020, 4, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Brunetti, L.; Martelli, M.P. How I diagnose and treat NPM1-mutated AML. Blood J. Am. Soc. Hematol. 2021, 137, 589–599. [Google Scholar]
- Wang, R.; Xu, P.; Chang, L.-L.; Zhang, S.-Z.; Zhu, H.-H. Targeted therapy in NPM1-mutated AML: Knowns and unknowns. Front. Oncol. 2022, 12, 972606. [Google Scholar] [CrossRef]
- Issa, G.C.; Aldoss, I.; DiPersio, J.; Cuglievan, B.; Stone, R.; Arellano, M.; Thirman, M.J.; Patel, M.R.; Dickens, D.S.; Shenoy, S. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature 2023, 615, 920–924. [Google Scholar] [CrossRef]
- Erba, H.P.; Fathi, A.T.; Issa, G.C.; Altman, J.K.; Montesinos, P.; Patnaik, M.M.; Foran, J.M.; De Botton, S.; Baer, M.R.; Schiller, G.J. Update on a phase 1/2 first-in-human study of the menin-KMT2A (MLL) inhibitor ziftomenib (KO-539) in patients with relapsed or refractory acute myeloid leukemia. Blood 2022, 140, 153–156. [Google Scholar] [CrossRef]
| Total Number of Patients (n = 2309) | NPM1WT (n = 1820) | NPM1MT (n = 489) | p Value | |
|---|---|---|---|---|
| Median age at diagnosis—years. (range) | 66.9 (9.9–100) | 63.8 (20.2–91) | 0.0375 | |
| Sex—n (%) | Female | 960 (52.8%) | 249 (50.9%) | 0.49 |
| Male | 860 (47.8%) | 240 (49.1%) | ||
| AML type—n (%) | pAML | 1340 (73.6%) | 461 (94.2%) | 0.0001 |
| sAML | 480 (26.4%) | 28 (5.8%) | ||
| Karyotype—n (%) | Abnormal | 1178 (64.7%) | 97 (19.8%) | <0.0001 |
| Normal | 642 (35.1%) | 392 (80.2%) | ||
| ELN 2022 risk classification—n (%) | Favorable | 169 (9.3%) | 91 (18.5%) | |
| Intermediate | 724 (39.8%) | 363 (74%) | 0.0001 | |
| Adverse | 927 (50.9%) | 35 (7.1%) | 0.0001 | |
| Bone marrow blasts—% (range) | 51.0 (2.5–99%) | 73.0 (8.5–99%) | <0.0001 | |
| WBC count—×1000/mm3 (range) | 5.65 (0–600) | 25.6 (400–321) | <0.0001 | |
| Hemoglobin—g/dL (range) | 9.3 (2.3–19) | 9.2 (2.8–16.8) | 0.69 | |
| Platelet count—×1000/mm3 (range) | 55 (0–2366) | 61.5 (7–493) | 0.3109 | |
| Mutation (n) | Age ≤ 65 | Age > 65 | p Value |
|---|---|---|---|
| DNMT3A (199) | 107 (46%) | 92 (39%) | 0.27 |
| FLT3-ITD (138) | 81 (31%) | 57 (19%) | 0.16 |
| IDH2 (112) | 58 (22%) | 54 (25%) | 0.61 |
| TET2 (91) | 34 (13%) | 57 (26%) | 0.0001 |
| IDH1 (82) | 49 (19%) | 33 (15%) | 0.25 |
| NRAS (49) | 33 (13%) | 16 (7%) | 0.03 |
| SRSF2 (44) | 12 (5%) | 32 (15%) | 0.0002 |
| FLT3-TKD (43) | 24 (9%) | 19 (9%) | 1 |
| WT1 (33) | 25 (10%) | 8 (4%) | 0.01 |
| PTPN11 (30) | 12 (5%) | 18 (8%) | 0.18 |
| CEBPA (24) | 12 (5%) | 12 (6%) | 0.63 |
| ASXL1 (19) | 3 (1%) | 16 (7%) | 0.0006 |
| KRAS (19) | 11 (4%) | 8 (4%) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhillon, V.; Khan, A.M.; Aguilar, J.J.M.; Nanja Reddy, S.; Aly, M.M.; Kewan, T.; Bahaj, W.; Gurnari, C.; Visconte, V.; Carr, D.; et al. Comprehensive Age-Stratified Impact of NPM1 Mutation in Acute Myeloid Leukemia: A Real-World Experience. Cancers 2025, 17, 1020. https://doi.org/10.3390/cancers17061020
Dhillon V, Khan AM, Aguilar JJM, Nanja Reddy S, Aly MM, Kewan T, Bahaj W, Gurnari C, Visconte V, Carr D, et al. Comprehensive Age-Stratified Impact of NPM1 Mutation in Acute Myeloid Leukemia: A Real-World Experience. Cancers. 2025; 17(6):1020. https://doi.org/10.3390/cancers17061020
Chicago/Turabian StyleDhillon, Vikram, Abdul Moiz Khan, Jeff Justin M. Aguilar, Sushmitha Nanja Reddy, Mai M. Aly, Tariq Kewan, Waled Bahaj, Carmelo Gurnari, Valeria Visconte, David Carr, and et al. 2025. "Comprehensive Age-Stratified Impact of NPM1 Mutation in Acute Myeloid Leukemia: A Real-World Experience" Cancers 17, no. 6: 1020. https://doi.org/10.3390/cancers17061020
APA StyleDhillon, V., Khan, A. M., Aguilar, J. J. M., Nanja Reddy, S., Aly, M. M., Kewan, T., Bahaj, W., Gurnari, C., Visconte, V., Carr, D., Boerner, J., Yang, J., Dyson, G., Maciejewski, J., & Balasubramanian, S. K. (2025). Comprehensive Age-Stratified Impact of NPM1 Mutation in Acute Myeloid Leukemia: A Real-World Experience. Cancers, 17(6), 1020. https://doi.org/10.3390/cancers17061020







