Health-Related Quality of Life in Multiple Myeloma Patients Treated with High- or Low-Dose Lenalidomide Maintenance Therapy after Autologous Stem Cell Transplantation—Results from the LenaMain Trial (NCT00891384)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Treatment
2.2. HR-QoL Assessments and Endpoint
3. Results
3.1. Patient Population
3.2. Compliance
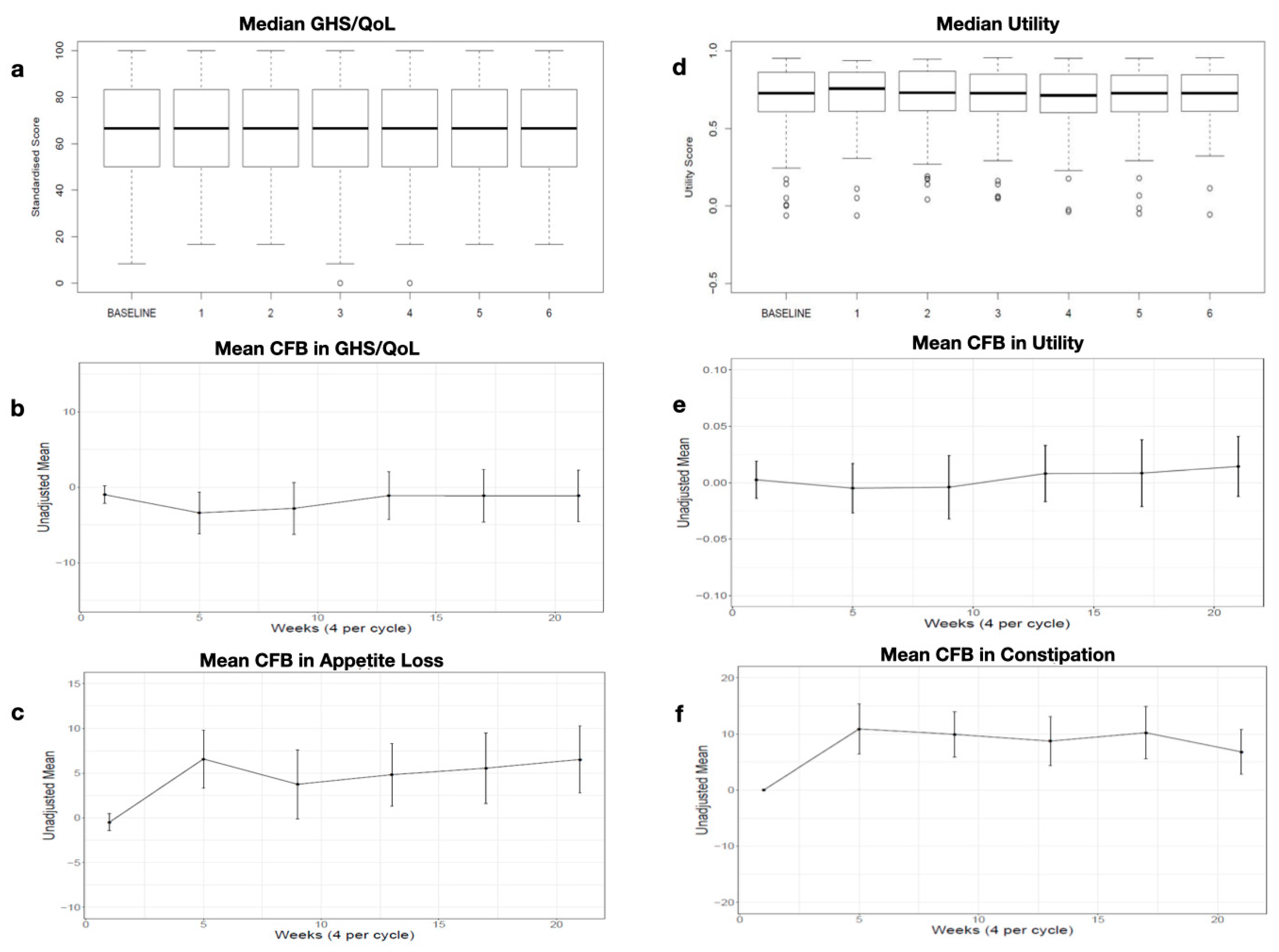
3.3. Quality of Life during the Consolidation Phase
3.3.1. Global Health Status/Quality of Life (GHS/QoL) and Utility Values
3.3.2. EORTC QLQ-C30 Domain Scores
3.4. Quality of Life during the Maintenance Period
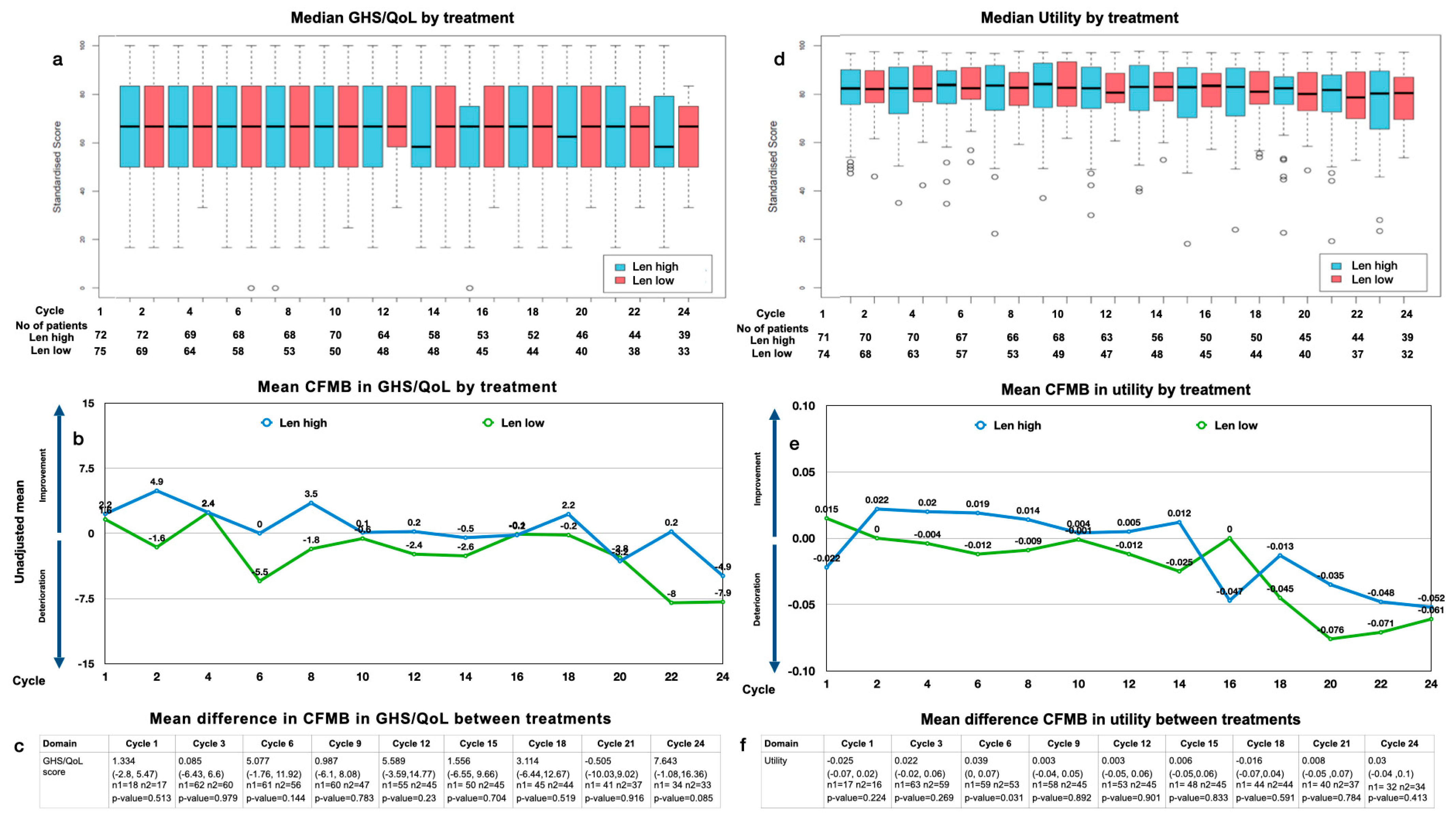
3.4.1. GHS/QoL
3.4.2. Utility Values
3.4.3. Function and Symptom Scales, Single Items
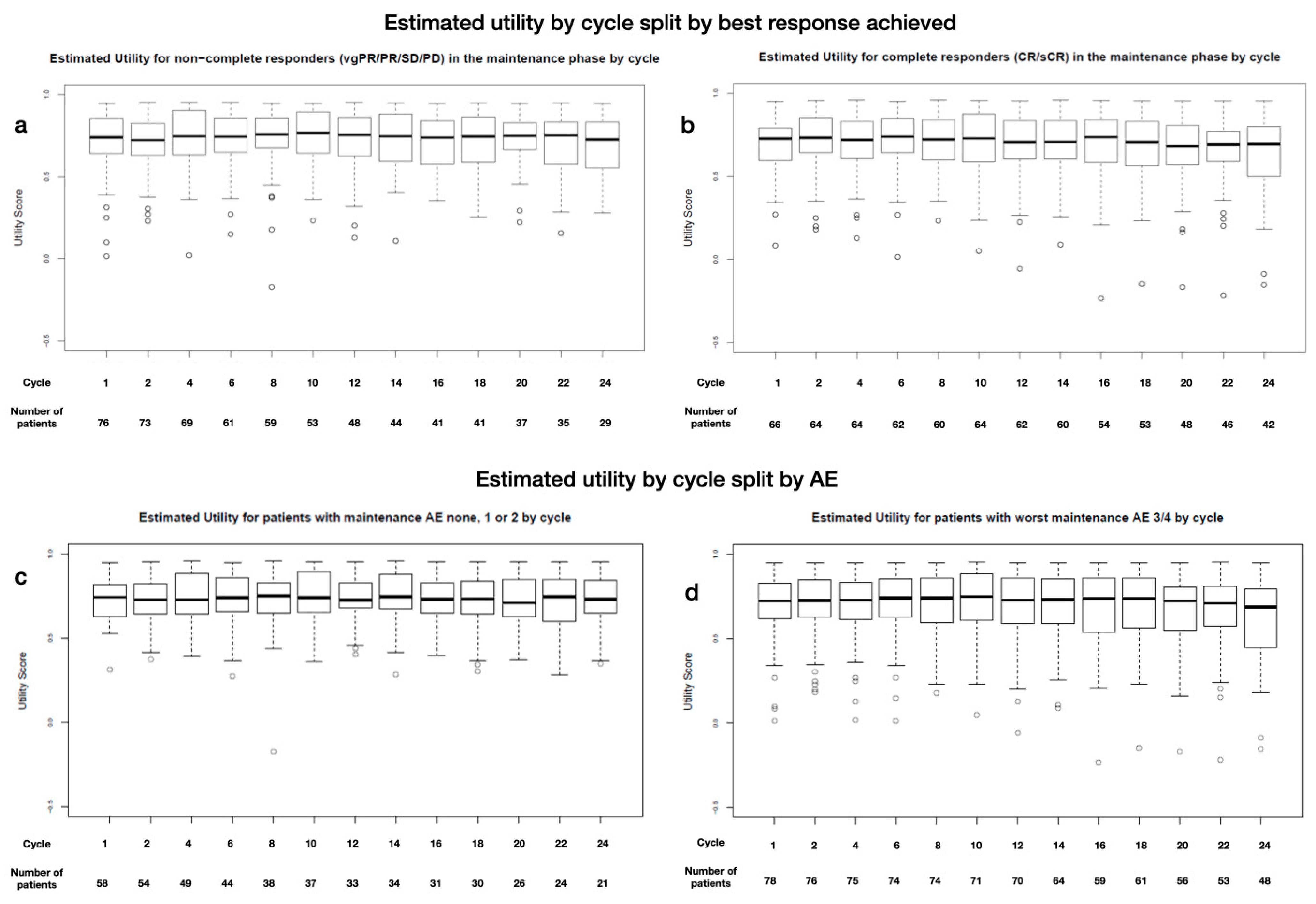
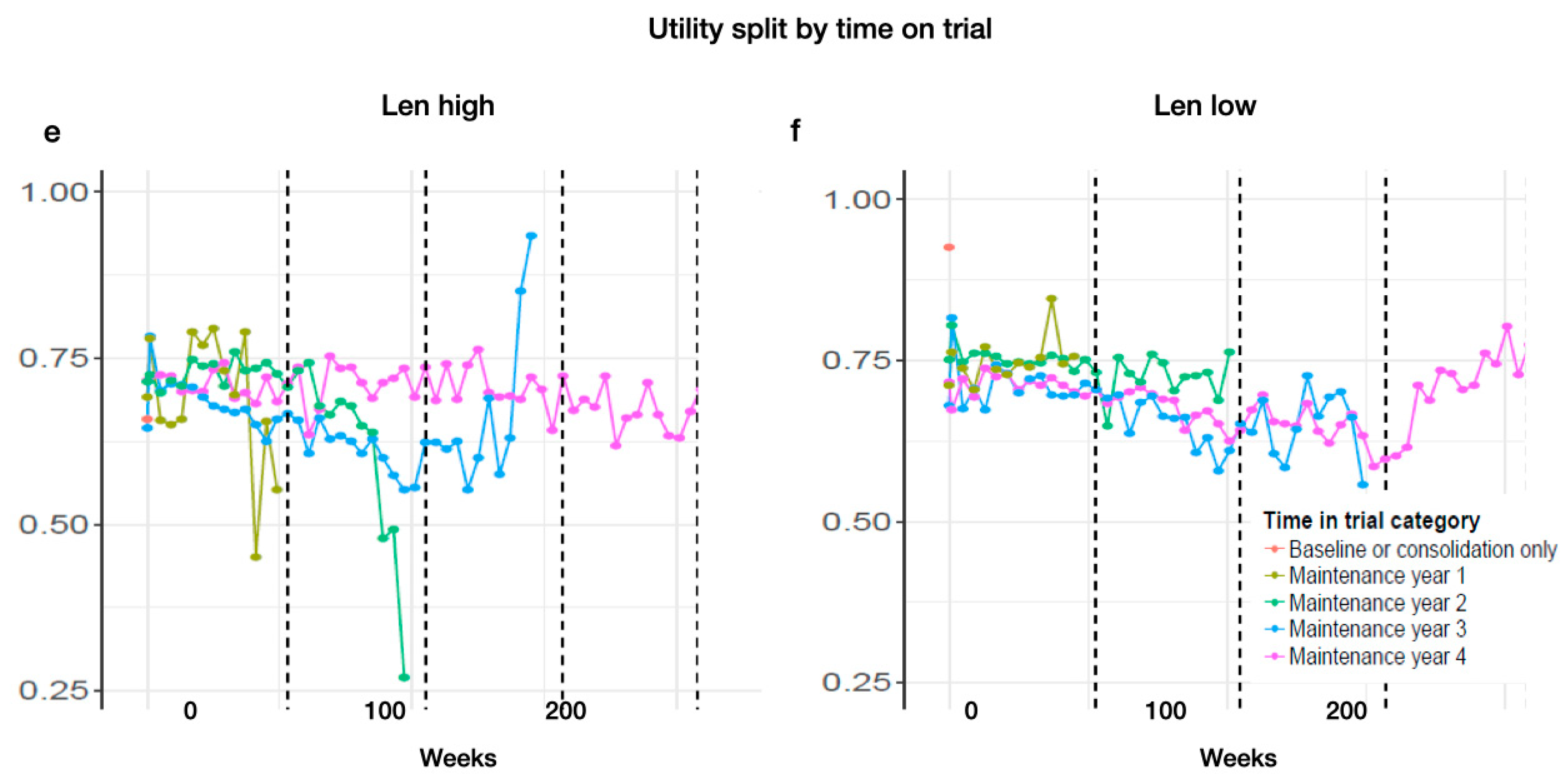
3.5. Subgroup Analysis
3.6. Missing Data
4. Discussion
5. Relevance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Materials and Methods
Appendix A.1. Study Design and Treatment
Appendix A.2. HR-QoL Assessments and Endpoint
Appendix A.3. Assessing Raw Data
Appendix A.4. Calculating QLQ-C30 Scores
| Category | Scale | Code | Range (r) | EORTC QLQ-C30–Corresponding Questions |
|---|---|---|---|---|
| Global health status/QoL | Global health status/QoL | QL | 6 | 29, 30 |
| Functional scales | Physical functioning | PF | 3 | 1–5 |
| Role functioning | RF | 3 | 6, 7 | |
| Emotional functioning | EF | 3 | 21–24 | |
| Cognitive functioning | CF | 3 | 20, 25 | |
| Social functioning | SF | 3 | 26, 27 | |
| Symptom scales | Fatigue | FA | 3 | 10, 12, 18 |
| Nausea and vomiting | NV | 3 | 14, 15 | |
| Pain | PA | 3 | 9, 19 | |
| Single items | Dyspnea | DY | 3 | 8 |
| Insomnia | SL | 3 | 11 | |
| Appetite loss | AP | 3 | 13 | |
| Constipation | CO | 3 | 16 | |
| Diarrhea | DI | 3 | 17 | |
| Financial difficulties | FI | 3 | 28 |
- Raw scores (RSs) are calculated as the mean of the responses that belong to each scale or item (e.g., for the physical function, this would be the mean of the responses for questions 1–5);
- Standardized scores (Ss) are calculated using a transformation such that they vary at an interval from 0 to 100.
- 1.
- For functional scales: ;
- 2.
- For symptom scales, single items, global health status/QoL scales: .
- 3.
- If at least half of the items that contribute to a scale have been answered, then calculate the score as above, ignoring the missing items;
- 4.
- If more than half are missing, then the scale is set to missing;
- 5.
- Single items will always be set to missing.
Appendix A.5. Utility Mapping
Appendix A.6. Analyses Performed
Appendix A.7. Change from Baseline
Appendix A.8. Minimally Important Difference (MID)
Appendix A.9. Subgroup Analysis
Appendix A.10. Study Discontinuations
| 25 mg | 5 mg | |
|---|---|---|
| Discontinuation | 61 (65%) | 74 (78%) |
| 27 (29%) | 42 (45%) |
| 27 (29%) | 26 (28%) |
| 3 (3%) | 1 (1%) |
| 4 (4%) | 5 (5%) |
| Median time (range) until EOT (months) | 26.8 (0.5–87) | 22.9 (0.3–69) |
Appendix A.11. Dose Reductions
| Dose Reduction Due to | N = 337 |
|---|---|
| Neutropenia | 187 (56) |
| Thrombocytopenia | 31 (9) |
| Constitutional symptoms | 24 (7) |
| Infection | 17 (5) |
| Dermatological AEs | 14 (4) |
| New primary malignancy | 13 (4) |
| Neurological AEs | 13 (4) |
| Patient request | 9 (3) |
| Anemia | 9 (3) |
| Renal AEs | 6 (2) |
| Gastrointestinal + Liver AEs | 5 (2) |
| Death | 4 (1) |
| Pulmonal AEs | 3 (1) |
| Cardiac AEs | 2 (1) |
| Bleeding | 0 (0) |
Appendix B. Compliance
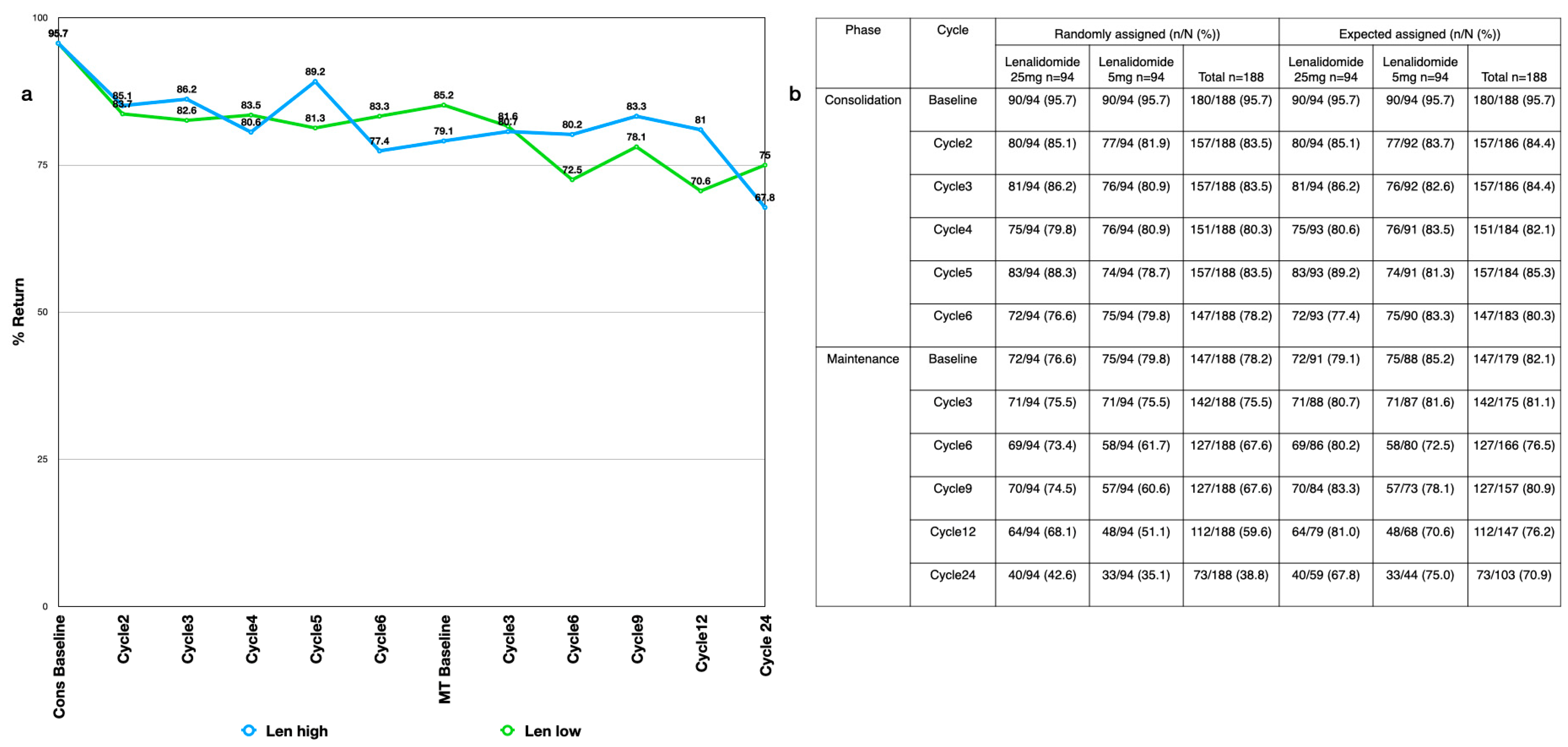
Appendix C. Consolidation
Appendix D. Subgroup Analysis
Results
References
- Engelhardt, M.; Ihorst, G.; Singh, M.; Rieth, A.; Saba, G.; Pellan, M.; Lebioda, A. Real-World Evaluation of Health-Related Quality of Life in Patients with Multiple Myeloma from Germany. Clin. Lymphoma Myeloma Leuk. 2020, S2152–S2650. [Google Scholar] [CrossRef]
- Despiégel, N.; Touboul, C.; Flinois, A.; Saba, G.; Suzan, F.; Gonzalez-McQuire, S.; Bonnetain, F. Health-Related Quality of Life of Patients with Multiple Myeloma Treated in Routine Clinical Practice in France. Clin. Lymphoma Myeloma Leuk. 2019, 19, e13–e28. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Vij, R.; Norkin, M.; Buadi, F.; Kindwall-Keller, T.L.; Roberts, J.S.; White, D.J.; Wood, R.P.; Blanthorn-Hazell, S.E.; Rossi, A.C.; et al. Health related quality of life for multiple myeloma patients according to treatment strategy after autologous stem cell transplant: A cross-sectional study using EORTC, EQ-5D and MY-20 scales. Leuk. Lymphoma 2019, 60, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Weisel, K.; Ludwig, H.; Rieth, A.; Lebioda, A.; Goldschmidt, H. Health-related quality of life of carfilzomib- and daratumumab-based therapies in patients with relapsed/refractory multiple myeloma, based on German benefit assessment data. Qual. Life Res. 2020, 29, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Moreau, P.; Dimopoulos, M.A.; Mateos, M.V.; Kaiser, M.; Hajek, R.; Feng, S.; Cocks, K.; Buchanan, J.; Weisel, K. Health-related quality of life in the ENDEAVOR study: Carfilzomib-dexamethasone vs. bortezomib-dexamethasone in relapsed/refractory multiple myeloma. Blood Cancer J. 2019, 22, 23. [Google Scholar] [CrossRef]
- Siegel, D.; Weisel, K.; Zahlten-Kumeli, A.; Medhekar, R.; Ding, B.; Leleu, X. Health-related quality of life outcomes from the CANDOR study in patients with relapsed or refractory multiple myeloma. Leuk. Lymphoma 2021, 62, 3002–3010. [Google Scholar] [CrossRef]
- Delforge, M.; Minuk, L.; Eisenmann, J.C.; Arnulf, B.; Canepa, L.; Fragasso, A.; Leyvraz, S.; Langer, C.; Ezaydi, Y.; Vogl, D.T.; et al. Health-related quality of life in patients with newly diagnosed multiple myeloma ineligible for stem cell transplantation: Results from the randomized phase III ALCYONE trial. BMC Cancer 2021, 21, 659. [Google Scholar]
- Dimopoulos, M.A.; Jakubowiak, A.J.; McCarthy, P.L.; Orlowski, R.Z.; Attal, M.; Bladé, J.; Goldschmidt, H.; Weisel, K.C.; Ramasamy, K.; Zweegman, S.; et al. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J. 2020, 10, 17. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Hemasphere 2021, 5, e528. [Google Scholar]
- Chakraborty, R.; Hamilton, B.K.; Hashmi, S.K.; Kumar, S.K.; Majhail, N.S. Health-Related Quality of Life after Autologous Stem Cell Transplantation for Multiple Myeloma. Biol. Blood Marrow Transplant. 2018, 24, 1546–1553. [Google Scholar] [CrossRef]
- Roussel, M.; Hebraud, B.; Hulin, C.; Perrot, A.; Caillot, D.; Stoppa, A.M.; Macro, M.; Escoffre, M.; Arnulf, B.; Belhadj, K.; et al. Health-related quality of life results from the IFM 2009 trial: Treatment with lenalidomide, bortezomib, and dexamethasone in transplant-eligible patients with newly diagnosed multiple myeloma. Leuk. Lymphoma 2020, 61, 1323–1333. [Google Scholar] [CrossRef]
- Palumbo, A.; Cavallo, F.; Gay, F.; Di Raimondo, F.; Ben Yehuda, D.; Petrucci, M.T.; Pezzatti, S.; Caravita, T.; Cerrato, C.; Ribakovsky, E.; et al. Autologous transplantation and maintenance therapy in multiple myeloma. N. Engl. J. Med. 2014, 371, 895–905. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Stege, C.; Lissenberg-Witte, B.; Van Der Holt, B.; Mellqvist, U.H.; Salomo, M.; Bos, G.; Levin, M.D.; Visser-Wisselaar, H.; Hansson, M.; et al. Health-related quality of life in transplant ineligible newly diagnosed multiple myeloma patients treated with either thalidomide or lenalidomide-based regimen until progression: A prospective, open-label, multicenter, randomized, phase 3 study. Haematologica 2020, 105, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Verelst, S.G.; Lewis, P.; Gray-Schopfer, V.; Hutchings, A.; Nixon, A.; Petrucci, M.T. Review of health-related quality of life data in multiple myeloma patients treated with novel agents. Leukemia 2013, 27, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Merz, M.; Dechow, T.; Scheytt, M.; Schmidt, C.; Hackanson, B.; Knop, S. The clinical management of lenalidomide-based therapy in patients with newly diagnosed multiple myeloma. Ann. Hematol. 2020, 99, 1709–1725. [Google Scholar] [CrossRef] [PubMed]
- Fenk, R.; Giagounidis, A.; Goldschmidt, H.; Heinsch, M.; Rummel, M.; Kroger, N.; Boquoi, A.; Lopez, D.; Gerrlich, C.; Baier, J.; et al. Efficacy and Tolerability of High- versus Low-dose Lenalidomide Maintenance Therapy of Multiple Myeloma after Autologous Blood Stem Cell Transplantation. Clin. Cancer Res. 2020, 26, 5879–5886. [Google Scholar] [CrossRef]
- Abonour, R.; Wagner, L.; Durie, B.G.; Jagannath, S.; Narang, M.; Terebelo, H.R.; Gasparetto, C.J.; Toomey, K.; Hardin, J.W.; Kitali, A.; et al. Impact of post-transplantation maintenance therapy on health-related quality of life in patients with multiple myeloma: Data from the Connect® MM Registry. Ann. Hematol. 2018, 97, 2425–2436. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Jarden, M.; Andersen, C.L.; Frederiksen, H.; Abildgaard, N. A systematic review of health-related quality of life in longitudinal studies of myeloma patients. Eur. J. Haematol. 2017, 99, 3–17. [Google Scholar] [CrossRef]
- Gulbrandsen, N.; Hjermstad, M.J.; Wisløff, F.; Nordic Myeloma Study Group. Interpretation of quality of life scores in multiple myeloma by comparison with a reference population and assessment of the clinical importance of score differences. Eur. J. Haematol. 2004, 72, 172–180. [Google Scholar] [CrossRef]
- Leleu, X.; Petrucci, M.T.; Welslau, M.; Broek, I.V.; Murphy, P.T.; Bottomley, A.; Bacon, P.; Lewis, P.; Gilet, H.; Arnould, B.; et al. Psychometric performance of the EORTC Quality-of-Life Core questionnaire (QLQ-C30) and QLQ-Multiple Myeloma (QLQ-MY20) in relapsed/refractory multiple myeloma (RRMM). Blood 2013, 122, 1721. [Google Scholar] [CrossRef]
- Kvam, A.K.; Wisløff, F.; Fayers, P.M. Minimal important differences and response shift in health-related quality of life; a longitudinal study in patients with multiple myeloma. Health Qual. Life Outcomes 2010, 8, 79. [Google Scholar] [CrossRef]
- Cocks, K.; King, M.T.; Velikova, G.; de Castro, G., Jr.; Martyn St-James, M.; Fayers, P.M.; Brown, J.M. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur. J. Cancer. 2012, 48, 1713–1721. [Google Scholar] [CrossRef]
- Cocks, K.; King, M.T.; Velikova, G.; Martyn St-James, M.; Fayers, P.M.; Brown, J.M. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J. Clin. Oncol. 2011, 29, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.W.; Fayers, P.M.; Aaronson, N.K.; Bottomley, A.; de Graeff, A.; Groenvold, M.; Gundy, C.; Koller, M.; Petersen, M.A.; Sprangers, M.A.G.; et al. EORTC QLQ-C30 Reference Values. July 2008. Available online: http://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf (accessed on 20 January 2018).
- Mohty, M.; Richardson, P.G.; McCarthy, P.L.; Attal, M. Consolidation and maintenance therapy for multiple myeloma after autologous transplantation: Where do we stand? Bone Marrow Transplant. 2015, 50, 1024–1029. [Google Scholar] [CrossRef]
- Al-Ani, F.; Louzada, M. Post-transplant consolidation plus lenalidomide maintenance vs lenalidomide maintenance alone in multiple myeloma: A systematic review. Eur. J. Haematol. 2017, 99, 479–488. [Google Scholar] [CrossRef]
- Cavo, M.; Gay, F.; Beksac, M.; Pantani, L.; Petrucci, M.T.; Dimopoulos, M.A.; Dozza, L.; van der Holt, B.; Zweegman, S.; Oliva, S.; et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): A multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020, 7, e456–e468. [Google Scholar] [PubMed]
- Cohen, O.C.; Counsell, N.; Rabin, N.; Popat, R.; Owen, R.G.; Popova, B.; Schofield, O.; Clifton-Hadley, L.; Lyons-Lewis, J.; Rawstron, A.; et al. Bortezomib consolidation post-ASCT as frontline therapy for multiple myeloma deepens disease response and MRD-negative rate whilst maintaining QOL and response to re-treatment at relapse. Br. J. Haematol. 2019, 185, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Nunnelee, J.; Cottini, F.; Zhao, Q.; Faisal, M.S.; Elder, P.; Rosko, A.; Bumma, N.; Khan, A.; Umyarova, E.; Devarakonda, S.; et al. Early Versus Late Discontinuation of Maintenance Therapy in Multiple Myeloma. Blood 2021, 138, 3796. [Google Scholar] [CrossRef]
- Pawlyn, C.; Khan, M.S.; Muls, A.; Sriskandarajah, P.; Kaiser, M.F.; Davies, F.E.; Morgan, G.J.; Andreyev, H.J. Lenalidomide-induced diarrhea in patients with myeloma is caused by bile acid malabsorption that responds to treatment. Blood 2014, 124, 2467–2468. [Google Scholar] [CrossRef]
- Boland, E.; Eiser, C.; Ezaydi, Y.; Greenfield, D.M.; Ahmedzai, S.H.; Snowden, J.A. Living with advanced but stable multiple myeloma: A study of the symptom burden and cumulative effects of disease and intensive (hematopoietic stem cell transplant-based) treatment on health-related quality of life. J. Pain. Symptom Manag. 2013, 46, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.; Ishak, K.J.; Lewis, P.; Proskorovsky, I.; Payne, K.A.; Lordan, N.; Kyriakou, C.; Davies, F.; Peters, S.; Williams, C.D. Determinants of Global QOL and Physical and Social Functionality in Multiple Myeloma. Blood 2010, 116, 934. [Google Scholar] [CrossRef]
- Majithia, N.; Rajkumar, S.V.; Lacy, M.Q.; Buadi, F.K.; Dispenzieri, A.; Gertz, M.A.; Hayman, S.R.; Dingli, D.; Kapoor, P.; Hwa, L.; et al. Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia 2016, 30, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Royle, K.L.; Gregory, W.M.; Cairns, D.A.; Bell, S.E.; Cook, G.; Owen, R.G.; Drayson, M.T.; Davies, F.E.; Jackson, G.H.; Morgan, G.J.; et al. Quality of life during and following sequential treatment of previously untreated patients with multiple myeloma: Findings of the Medical Research Council Myeloma IX randomised study. Br. J. Haematol. 2018, 182, 816–829. [Google Scholar] [CrossRef]
- Vogl, D.T.; Delforge, M.; Song, K.; Guo, S.; Gibson, C.J.; Ervin-Haynes, A.; Facon, T. Long-term health-related quality of life in transplant-ineligible patients with newly diagnosed multiple myeloma receiving lenalidomide and dexamethasone. Leuk. Lymphoma 2018, 59, 398–405. [Google Scholar] [CrossRef]
- Kesireddy, M.; Holstein, S.A. The era of lenalidomide maintenance therapy in multiple myeloma: Settings for achieving best outcomes. Expert. Rev. Clin. Pharmacol. 2022, 15, 19–31. [Google Scholar] [CrossRef]
- Richardson, P.G.; Jacobus, S.J.; Weller, E.A.; Hassoun, H.; Lonial, S.; Raje, N.S.; Medvedova, E.; McCarthy, P.L.; Libby, E.N.; Voorhees, P.M.; et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N. Engl. J. Med. 2022, 387, 132–147. [Google Scholar] [CrossRef]

| Characteristic | Level | Overall (n = 188) | Lenalidomide 25 mg n = 94 | Lenalidomide 5 mg n = 94 | |||
|---|---|---|---|---|---|---|---|
| Age median, range | 59.00 (34.00–73.00) | 59.00 (34.00–72.00) | 59.00 (34.00–73.00) | ||||
| Gender n (%) | Male | 116 (61.70) | 56 (59.57) | 60 (63.83) | |||
| β-2-Microglobulin levels n (%) | ≥2.5 | 54 (28.72) | 28 (29.79) | 26 (27.66) | |||
| <2.5 | 134 (71.28) | 66 (70.21) | 68 (72.34) | ||||
| Prior therapies n (%) | 1 | 156 (82.98) | 76 (80.85) | 80 (85.11) | |||
| 2 | 32 (17.02) | 18 (19.15) | 14 (14.89) | ||||
| Radiotherapy n (%) | 1 | 65 (34.57) | 30 (31.91) | 35 (37.23) | |||
| 2 | 123 (65.43) | 64 (68.09) | 59 (62.77) | ||||
| EORTC QLQ-C30 domain or utility | Baseline | Maintenance Baseline | |||||
| Overall | Lenalidomide 25 mg | Lenalidomide 5 mg | Overall | Lenalidomide 25 mg | Lenalidomide 5 mg | ||
| Utility—mean (SD) | Utility | 0.70 (0.20) | 0.69 (0.21) | 0.71 (0.20) | 0.71 (0.18) | 0.69 (0.20) | 0.72 (0.15) |
| GHQ/Qol score—mean (SD) | GHS/QoL | 65.78 (19.90) | 64.81 (19.34) | 66.76 (20.51) | 65.25 (19.66) | 63.08 (21.30) | 67.33 (17.85) |
| Functional domain scores—mean (SD) | Cognitive functioning | 84.72 (20.31) | 84.81 (22.71) | 84.63 (17.71) | 78.23 (23.38) | 79.63 (22.42) | 76.89 (24.34) |
| Emotional functioning | 75.15 (23.77) | 76.73 (23.21) | 73.58 (24.35) | 72.05 (22.39) | 71.99 (23.71) | 72.11(21.20) | |
| Physical functioning | 77.01 (20.38) | 76.00 (20.96) | 78.02 (19.84) | 77.14 (18.22) | 76.94 (19.54) | 77.33 (16.98) | |
| Role functioning | 66.11 (30.41) | 65.00 (29.78) | 67.22 (31.15) | 64.17 (26.27) | 64.12 (26.78) | 64.22 (25.95) | |
| Social functioning | 69.17 (27.94) | 69.44 (29.38) | 68.89 (26.58) | 71.88 (26.66) | 70.83 (29.57) | 72.89 (23.69) | |
| Symptom domain scores—mean (SD) | Fatigue | 31.64 (24.03) | 32.35(23.77) | 30.93 (24.40) | 32.99 (23.57) | 34.26 (23.86) | 31.78 (23.38) |
| Nausea and Vomiting | 3.61 (12.37) | 2.41 (9.20) | 4.81 (14.83) | 4.76 (11.21) | 3.70 (9.37) | 5.78 (12.70) | |
| Pain | 29.17 (27.85) | 33.15 (28.65) | 25.19 (26.59) | 29.02 (24.21) | 31.02 (25.53) | 27.11 (22.89) | |
| Single item scores—mean (SD) | Appetite Loss | 7.96 (19.42) | 7.04 (19.02) | 8.89 (19.86) | 8.84 (18.43) | 10.65 (20.80) | 7.11 (15.78) |
| Constipation | 7.22 (19.39) | 7.41 (20.47) | 7.04 (18.36) | 14.29 (26.46) | 17.59 (30.11) | 11.11(22.15) | |
| Diarrhoea | 8.70 (19.09) | 7.04 (16.94) | 10.37 (20.99) | 12.70 (22.86) | 9.72 (21.26) | 15.56 (24.10) | |
| Dyspnoea | 20.86 (26.19) | 20.37 (26.77) | 21.35 (25.75) | 22.90 (24.61) | 20.37 (22.76) | 25.33 (26.19) | |
| Financial Difficulties | 23.22 (30.23) | 20.97 (28.60) | 25.47 (31.79) | 22.15 (30.14) | 18.06 (26.79) | 26.13 (32.77) | |
| Insomnia | 23.15 (29.08) | 23.33 (31.39) | 22.96 (26.74) | 28.08 (28.95) | 28.64 (30.50) | 27.56 (27.60) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boquoi, A.; Giagounidis, A.; Goldschmidt, H.; Heinsch, M.; Rummel, M.J.; Kröger, N.; Mai, E.K.; Strapatsas, J.; Haas, R.; Kobbe, G. Health-Related Quality of Life in Multiple Myeloma Patients Treated with High- or Low-Dose Lenalidomide Maintenance Therapy after Autologous Stem Cell Transplantation—Results from the LenaMain Trial (NCT00891384). Cancers 2023, 15, 5157. https://doi.org/10.3390/cancers15215157
Boquoi A, Giagounidis A, Goldschmidt H, Heinsch M, Rummel MJ, Kröger N, Mai EK, Strapatsas J, Haas R, Kobbe G. Health-Related Quality of Life in Multiple Myeloma Patients Treated with High- or Low-Dose Lenalidomide Maintenance Therapy after Autologous Stem Cell Transplantation—Results from the LenaMain Trial (NCT00891384). Cancers. 2023; 15(21):5157. https://doi.org/10.3390/cancers15215157
Chicago/Turabian StyleBoquoi, Amelie, Aristoteles Giagounidis, Hartmut Goldschmidt, Michael Heinsch, Mathias J. Rummel, Nicolaus Kröger, Elias K. Mai, Judith Strapatsas, Rainer Haas, and Guido Kobbe. 2023. "Health-Related Quality of Life in Multiple Myeloma Patients Treated with High- or Low-Dose Lenalidomide Maintenance Therapy after Autologous Stem Cell Transplantation—Results from the LenaMain Trial (NCT00891384)" Cancers 15, no. 21: 5157. https://doi.org/10.3390/cancers15215157





