Secondary Oral Cancer after Systemic Treatment of Hematological Malignancies and Oral GVHD: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
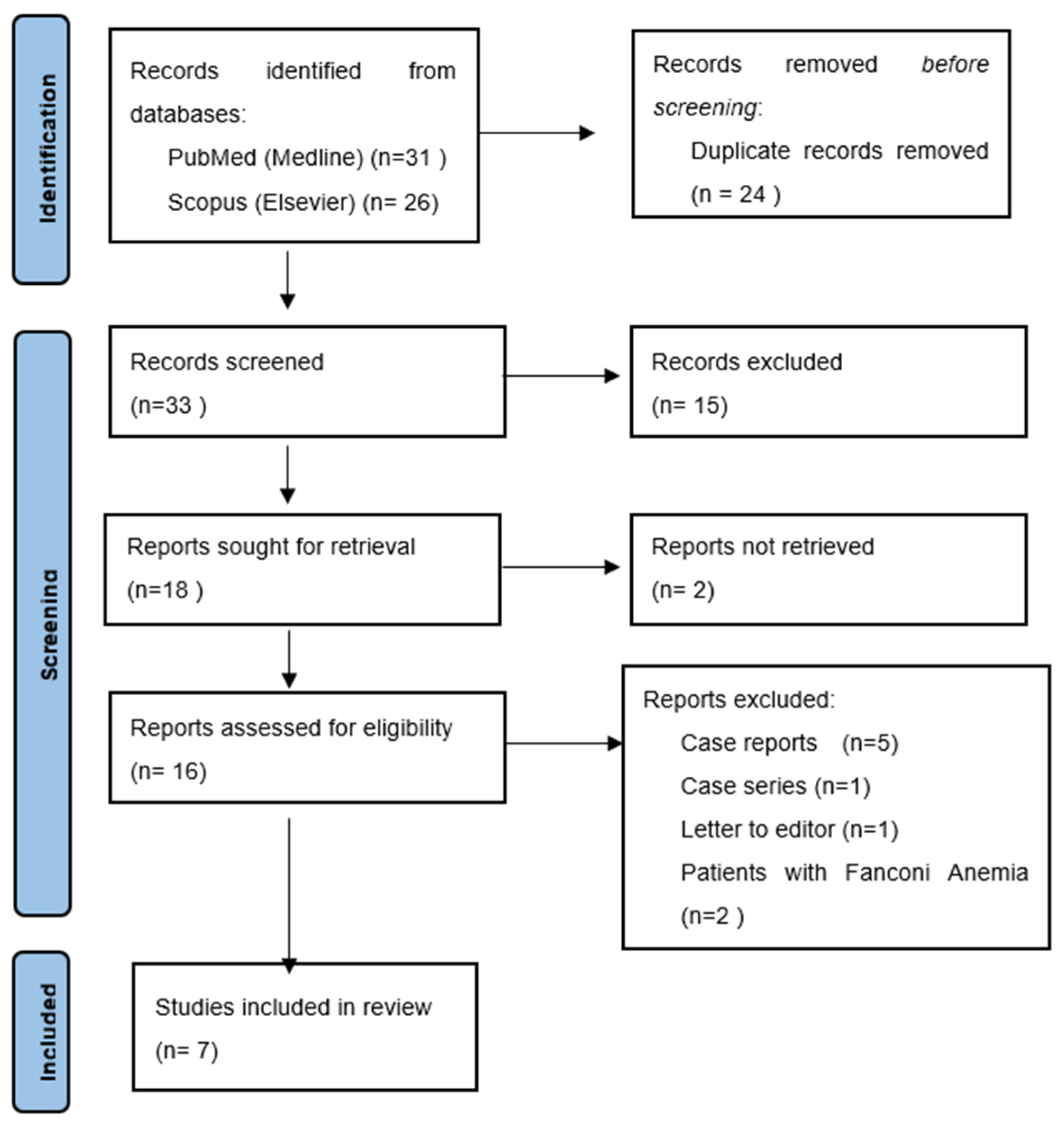
2.2. Searching and Data Screening
2.3. Eligibility Criteria
2.4. Data Extraction and Processing
3. Results
3.1. Databases Search Results
3.2. Included Studies Characteristics
3.3. Risk of Bias Assessment
3.4. Results of Data Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Lv, M.; Zhang, X.; Shen, Y.; Wang, F.; Yang, J.; Wang, B.; Chen, Z.; Li, P.; Zhang, X.; Li, S.; et al. Clinical Analysis and Prognosis of Synchronous and Metachronous Multiple Primary Malignant Tumors. Medicine 2017, 96, e6799. [Google Scholar] [CrossRef] [PubMed]
- Janowiak-Majeranowska, A.; Lebiedziński, F.; Majeranowski, A. Bone Marrow Donation in Poland: 2021 Update, and the Impact of the Coronavirus Disease 2019 Pandemic on Haematopoietic Stem Cell Transplantation. Clin. Ethics 2021, 17, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Barriga, F.; Ramírez, P.; Wietstruck, A.; Rojas, N. Hematopoietic Stem Cell Transplantation: Clinical Use and Perspectives. Biol. Res. 2012, 45, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mays, J.W.; Fassil, H.; Edwards, D.A.; Pavletic, S.Z.; Bassim, C.W. Oral Chronic Graft-versus-Host Disease: Current Pathogenesis, Therapy, and Research. Oral Dis. 2013, 19, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.E.; Kansu, E.; Sullivan, K.M. Pathophysiology and Treatment of Graft-versus-Host Disease. Hematol. Oncol. Clin. N. Am. 1999, 13, 1091–1112, viii–ix. [Google Scholar] [CrossRef]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. Meta-Anal. 2021, 372, n71. [Google Scholar] [CrossRef]
- Matchar, D.B. Chapter 1: Introduction to the Methods Guide for Medical Test Reviews. J. Gen. Intern. Med. 2012, 27, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A.L.D.; Grätz, K.W. Oral Carcinoma after Hematopoietic Stem Cell Transplantation—A New Classification Based on a Literature Review over 30 Years. Head Neck Oncol. 2009, 1, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, C.M.; Jethwa, A.R.; Hasan, W.; Liu, A.; Gilbert, R.; Goldstein, D.; De Almedia, J.; Lipton, J.; Irish, J.C. Long-Term Survival of Head and Neck Squamous Cell Carcinoma after Bone Marrow Transplant. Head Neck 2020, 42, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http//www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 February 2022).
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, G.J.; Kofman, E.R.; Shazib, M.A.; Woo, S.B.; Reardon, B.; Treister, N.S.; Haddad, R.I.; Cutler, C.S.; Antin, J.H.; Van Allen, E.M.; et al. Integrated Genomic Characterization of Oral Carcinomas in Post-Hematopoietic Stem Cell Transplantation Survivors. Oral Oncol. 2018, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leuci, S.; Coppola, N.; Blasi, A.; Ruoppo, E.; Bizzoca, M.E.; Lo Muzio, L.; Marano, L.; Risitano, A.M.; Mignogna, M.D. Oral Dysplastic Complications after HSCT: Single Case Series of Multidisciplinary Evaluation of 80 Patients. Life 2020, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Mawardi, H.; Elad, S.; Correa, M.E.; Stevenson, K.; Woo, S.B.; Almazrooa, S.; Haddad, R.; Antin, J.H.; Soiffer, R.; Treister, N. Oral Epithelial Dysplasia and Squamous Cell Carcinoma Following Allogeneic Hematopoietic Stem Cell Transplantation: Clinical Presentation and Treatment Outcomes. Bone Marrow Transplant. 2011, 46, 884–891. [Google Scholar] [CrossRef] [Green Version]
- Atsuta, Y.; Suzuki, R.; Yamashita, T.; Fukuda, T.; Miyamura, K.; Taniguchi, S.; Iida, H.; Uchida, T.; Ikegame, K.; Takahashi, S.; et al. Continuing Increased Risk of Oral/Esophageal Cancer after Allogeneic Hematopoietic Stem Cell Transplantation in Adults in Association with Chronic Graft-versus-Host Disease. Ann. Oncol. 2014, 25, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Santarone, S.; Natale, A.; Angelini, S.; Papalinetti, G.; Vaddinelli, D.; Di Bartolomeo, A.; Di Bartolomeo, P. Secondary Oral Cancer Following Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2021, 56, 1038–1046. [Google Scholar] [CrossRef]
- Baker, K.S.; DeFor, T.E.; Burns, L.J.; Ramsay, N.K.C.; Neglia, J.P.; Robison, L.L. New Malignancies after Blood or Marrow Stem-Cell Transplantation in Children and Adults: Incidence and Risk Factors. J. Clin. Oncol. 2003, 21, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, W.; Pond, G.R.; Rifkind, J.T.; Messner, H.A.; Lau, A.; Daly, A.S.; Kiss, T.L.; Kotchetkova, N.; Galal, A.; Lipton, J.H. Long-Term Follow-up of Secondary Malignancies in Adults after Allogeneic Bone Marrow Transplantation. Bone Marrow Transplant. 2005, 35, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janowiak–Majeranowska, A.; Majeranowski, A. Regarding Chronic Graft-versus-Host Disease in Children and Adolescents with Thalassemia after Hematopoietic Stem Cell Transplantation. Int. J. Hematol. 2021, 114, 147–148. [Google Scholar] [CrossRef]
- Otsubo, H.; Yokoe, H.; Miya, T.; Atsuta, F.; Miura, N.; Tanzawa, H.; Sato, K. Gingival Squamous Cell Carcinoma in a Patient with Chronic Graft-versus-Host Disease. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1997, 84, 171–174. [Google Scholar] [CrossRef]
- Fall-Dickson, J.M.; Pavletic, S.Z.; Mays, J.W.; Schubert, M.M. Oral Complications of Chronic Graft-Versus-Host Disease. JNCI Monogr. 2019, 2019, lgz007. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, C.P.; Sprague, K.A.; Pilichowska, M.; Cowan, J.; Klein, A.K.; Kaul, E.; Miller, K.B. Clinicopathologic Characteristics of Secondary Squamous Cell Carcinoma of Head and Neck in Survivors of Allogeneic Hematopoietic Stem Cell Transplantation for Hematologic Malignancies. Bone Marrow Transplant. 2019, 54, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.T.; Wu, E.; Wein, R.O. Oral Squamous Cell Carcinoma in Post-Transplant Patients. Am. J. Otolaryngol.-Head Neck Med. Surg. 2013, 34, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Chang, P.M.; Li, W.Y.; Hsiao, L.T.; Hong, Y.C.; Liu, C.Y.; Gau, J.P.; Liu, J.H.; Chen, P.M.; Chiou, T.J.; et al. High Incidence of Oral Squamous Cell Carcinoma Independent of HPV Infection after Allogeneic Hematopoietic SCT in Taiwan. Bone Marrow Transplant. 2011, 46, 567–572. [Google Scholar] [CrossRef]
- Kolb, H.J.; Socié, G.; Duell, T.; Van Lint, M.T.; Tichelli, A.; Apperley, J.F.; Nekolla, E.; Ljungman, P.; Jacobsen, N.; van Weel, M.; et al. Malignant Neoplasms in Long-Term Survivors of Bone Marrow Transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann. Intern. Med. 1999, 131, 738–744. [Google Scholar] [CrossRef]
- Lee, S.J.; Wolff, D.; Kitko, C.; Koreth, J.; Inamoto, Y.; Jagasia, M.; Pidala, J.; Olivieri, A.; Martin, P.J.; Przepiorka, D.; et al. Measuring Therapeutic Response in Chronic Graft-versus-Host Disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2014 Response Criteria Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 984–999. [Google Scholar] [CrossRef] [Green Version]
- Rose, B.; Wilkins, D.; Li, W.; Tran, N.; Thompson, C.; Cossart, Y.; McGeechan, K.; O’Brien, C.; Eris, J. Human Papillomavirus in the Oral Cavity of Patients with and without Renal Transplantation. Transplantation 2006, 4, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, A.K. Current Aspects on Oral Squamous Cell Carcinoma. Open Dent. J. 2012, 6, 126. [Google Scholar] [CrossRef]
- Tamma, R.; Limongelli, L.; Maiorano, E.; Pastore, D.; Cascardi, E.; Tempesta, A.; Carluccio, P.; Mastropasqua, M.G.; Capodiferro, S.; Covelli, C.; et al. Vascular Density and Inflammatory Infiltrate in Primary Oral Squamous Cell Carcinoma and after Allogeneic Hematopoietic Stem Cell Transplantation. Ann. Hematol. 2019, 98, 979–986. [Google Scholar] [CrossRef]
- Hayashida, J.-N.; Nakamura, S.; Toyoshima, T.; Moriyama, M.; Sasaki, M.; Kawamura, E.; Ohyama, Y.; Kumamaru, W.; Shirasuna, K. Possible Involvement of Cytokines, Chemokines and Chemokine Receptors in the Initiation and Progression of Chronic GVHD. Bone Marrow Transplant. 2013, 48, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.F.; Lan, J.H.; Chao, P.J.; Ting, H.M.; Chen, H.C.; Hsu, H.C.; Lee, T.F. Radiation-Induced Secondary Malignancies for Nasopharyngeal Carcinoma: A Pilot Study of Patients Treated via IMRT or VMAT. Cancer Manag. Res. 2018, 10, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Fang, Q.; Li, P.; Wu, J.; Zhang, X. Secondary Squamous Cell Carcinoma of the Oral Cavity after Nasopharyngeal Carcinoma. Cancer Res. Treat. 2020, 52, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yang, R.; Wu, K.; Lou, C.; Xiao, M.; Guo, W.; Ren, G. Second Primary Oral Squamous Cell Carcinoma after Radiotherapy: A Retrospective Cohort Study. Transl. Cancer Res. 2021, 10, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.P.; Jomaa, M.; Pollard, C.; Berends, J.; Ayoub, Z.; Mohamed, A.S.R.; Gunn, G.B.; Frank, S.J.; Garden, A.S.; Rosenthal, D.I.; et al. Second Primary Malignancies in Head and Neck Cancer Patients Treated with Definitive Radiotherapy. Int. J. Radiat. Oncol. 2017, 99, S122. [Google Scholar] [CrossRef]
- Zhao, W.; Lei, H.; Zhu, X.; Li, L.; Qu, S.; Liang, X.; Wang, X. The Clinical Characteristics of Secondary Primary Tumors in Patients with Nasopharyngeal Carcinoma after Intensity-Modulated Radiotherapy A Retrospective Analysis. Medicine 2016, 95, e5364. [Google Scholar] [CrossRef]
- Liu, C.; Liao, L.; Wu, G.; Yan, H.; Chen, X.; Wang, C.; Zheng, X.; Zeng, Z.; Zhao, Z.; Wu, D.; et al. Radiation-Induced Second Primary Squamous Cell Carcinoma of the Oral Cavity after Radiotherapy for Nasopharyngeal Carcinoma. Oral Oncol. 2020, 109, 104863. [Google Scholar] [CrossRef]
- Hashibe, M.; Ritz, B.; Le, A.D.; Li, G.; Sankaranarayanan, R.; Zhang, Z.F. Radiotherapy for Oral Cancer as a Risk Factor for Second Primary Cancers. Cancer Lett. 2005, 220, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Louie, A.D.; Bhatia, R.; O’Donnell, M.R.; Fung, H.; Kashyap, A.; Krishnan, A.; Molina, A.; Nademanee, A.; Niland, J.C.; et al. Solid Cancers After Bone Marrow Transplantation. J. Clin. Oncol. 2001, 2, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jawanda, M.K. Oral Lichen Planus: An Update on Etiology, Pathogenesis, Clinical Presentation, Diagnosis and Management. Indian J. Dermatol. 2015, 60, 222–229. [Google Scholar] [CrossRef]
- Halonen, P. Cancer Risk of Lichen Planus: A Cohort Study of 13,100 Women in Finland. Int. J. Cancer 2018, 142, 18–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsushima, F.; Sakurai, J.; Uesugi, A.; Oikawa, Y.; Ohsako, T.; Mochizuki, Y.; Hirai, H.; Kayamori, K.; Harada, H. Malignant Transformation of Oral Lichen Planus: A Retrospective Study of 565 Japanese Patients. BMC Oral Health 2021, 21, 298. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; de Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy versus Transoral Robotic Surgery and Neck Dissection for Oropharyngeal Squamous Cell Carcinoma (ORATOR): An Open-Label, Phase 2, Randomised Trial. Lancet Oncol. 2019, 20, 1349–1359. [Google Scholar] [CrossRef]
- Weinstein, G.S.; Quon, H.; O’Malley, B.W.; Kim, G.G.; Cohen, M.A. Selective Neck Dissection and Deintensified Postoperative Radiation and Chemotherapy for Oropharyngeal Cancer: A Subset Analysis of the University of Pennsylvania Transoral Robotic Surgery Trial. Laryngoscope 2010, 120, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, G.; Maniaci, A.; Bianchi, G.; Cammaroto, G.; Iannella, G.; Catalano, A.; Sgarzani, R.; De Vito, A.; Capaccio, P.; Pelucchi, S.; et al. Neck Dissection and Trans Oral Robotic Surgery for Oropharyngeal Squamous Cell Carcinoma. Auris Nasus Larynx 2022, 49, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.E.; Rizvi, K.; Grunfeld, E.A.; McGurk, M. Pilot Study to Estimate the Accuracy of Mouth Self-Examination in an at-Risk Group. Head Neck 2010, 32, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Ramadas, K.; Thomas, G.; Muwonge, R.; Thara, S.; Mathew, B.; Rajan, B. Trivandrum Oral Cancer Screening Study Group Effect of Screening on Oral Cancer Mortality in Kerala, India: A Cluster-Randomised Controlled Trial. Lancet 2005, 365, 1927–1933. [Google Scholar] [CrossRef]
- Lin, H.; Chen, H.; Weng, L.; Shao, J.; Lin, J. Automatic Detection of Oral Cancer in Smartphone-Based Images Using Deep Learning for Early Diagnosis. J. Biomed. Opt. 2021, 26, 086007. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Chen, Y.; Li, Z.; Jing, Q.; Hu, C.; Liu, H.; Bao, J.; Hong, Y.; Shi, T.; Li, K.; et al. A Deep Learning Algorithm for Detection of Oral Cavity Squamous Cell Carcinoma from Photographic Images: A Retrospective Study. EClinicalMedicine 2020, 27, 100558. [Google Scholar] [CrossRef]
- Mazur, M.; Ndokaj, A.; Venugopal, D.C.; Roberto, M.; Tomao, S.; Vozza, I.; Trybek, G.; Ottolenghi, L.; Albu, C.; Jedli, M. In Vivo Imaging-Based Techniques for Early Diagnosis of Oral Potentially Malignant Disorders—Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 11775. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| History of HCT | Case report/case series article |
| Diagnosis of SOC | Fanconi anemia |
| Article | Total Patients (n) | Patients with cGVHD (n) | HCT Patients and GVHD (%) | Patients with SOC (n) | Patients with SOC and GVHD (n (%)) | Most Common SOC Site | GVHD Severity (n) | Newcastle–Ottawa Score (n)/AMSTAR-2 Criteria 1 |
|---|---|---|---|---|---|---|---|---|
| Atsuta et al. [15] | 17,545 | NR | NR | 64 | 39 (60.10%) | NR | Limited (10), Extensive (29) | 8 |
| Douglas et al. [9] | NR | NR | NR | 23 | 18 (83%) | Tongue (13), buccal (3), alveolus (3), palate (3), lower lip (1) | Not specified | 7 |
| Hanna et al. [12] | NR | NR | NA | 31 | 20 (65%) | Tongue (14), buccal (5), retromolar trigone (4), alveolar (3), palate (3), floor of the mouth (2) | NIH 1 (12), NIH 2 (4), NIH 3 (4) | 7 |
| Kruse et al. [8] | NR | NR | NR | 53 | 42 (79.20%) | Tongue (16), lip (6), buccal (3) Not specified (30) | NR | Moderate |
| Leuci et al. [13] | NR | NR | NR | 7 | 7 (100%) | Tongue (2), buccal (2), alveolar ridge (1), gingiva (1), lip (1) | NR | 8 |
| Mawardi et al. [14] | NR | NR | NR | 18 | NR | Tongue (10), buccal (7), gingiva (4), lower lip (3), alveolar (2), palate (1) | NR | 7 |
| Santarone et al. [16] | 908 | 767 | 84,5% | 12 | 9 (75%) | Tongue (6), buccal (3), lip (1), palate (1), gingiva (1) | Extensive (7), Limited (2) | 9 |
| Oral cGVHD Coexistence | N (%) |
|---|---|
| Yes | 136 (65.38) |
| No | 54 (25.96) |
| Not specified | 18 (8.65) |
| SOC Localization | N (%) 1 |
|---|---|
| Tongue | 61 (28.24) |
| Buccal mucosa | 23 (10.64) |
| Lip | 12 (5.56) |
| Alveolus | 9 (4.17) |
| Gingiva | 6 (2.78) |
| Hard palate | 5 (2.31) |
| Retromolar trigone | 4 (1.85) |
| Floor of the mouth | 2 (0.93) |
| Not specified | 102 (43.52) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janowiak-Majeranowska, A.; Osowski, J.; Mikaszewski, B.; Majeranowski, A. Secondary Oral Cancer after Systemic Treatment of Hematological Malignancies and Oral GVHD: A Systematic Review. Cancers 2022, 14, 2175. https://doi.org/10.3390/cancers14092175
Janowiak-Majeranowska A, Osowski J, Mikaszewski B, Majeranowski A. Secondary Oral Cancer after Systemic Treatment of Hematological Malignancies and Oral GVHD: A Systematic Review. Cancers. 2022; 14(9):2175. https://doi.org/10.3390/cancers14092175
Chicago/Turabian StyleJanowiak-Majeranowska, Aleksandra, Jakub Osowski, Bogusław Mikaszewski, and Alan Majeranowski. 2022. "Secondary Oral Cancer after Systemic Treatment of Hematological Malignancies and Oral GVHD: A Systematic Review" Cancers 14, no. 9: 2175. https://doi.org/10.3390/cancers14092175






