Assessing the Acceptability of Home-Based HPV Self-Sampling: A Qualitative Study on Cervical Cancer Screening Conducted in Reunion Island Prior to the RESISTE Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
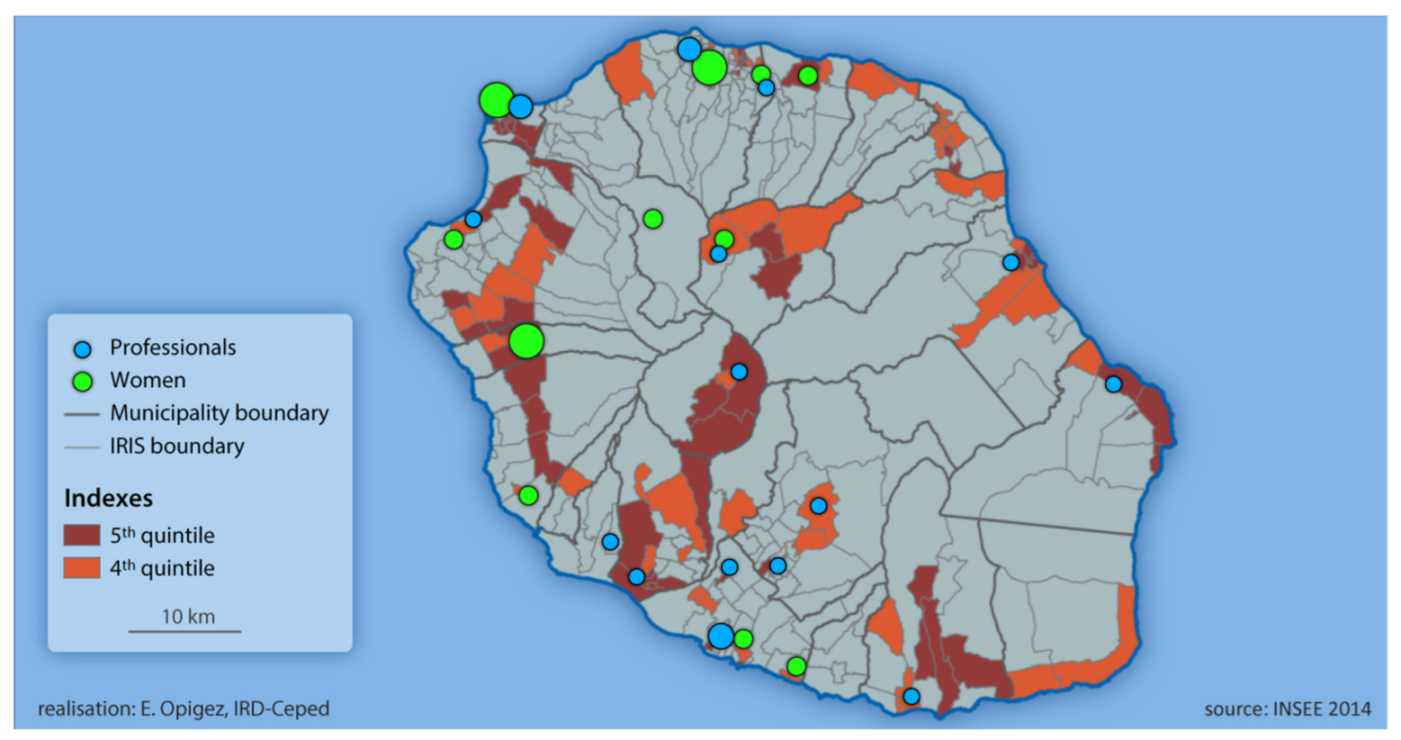
2.1. Site Description Section
2.2. Interview Guidelines
2.3. Target Populations
2.4. Interview Conduct and Analysis
2.5. Ethics
3. Results
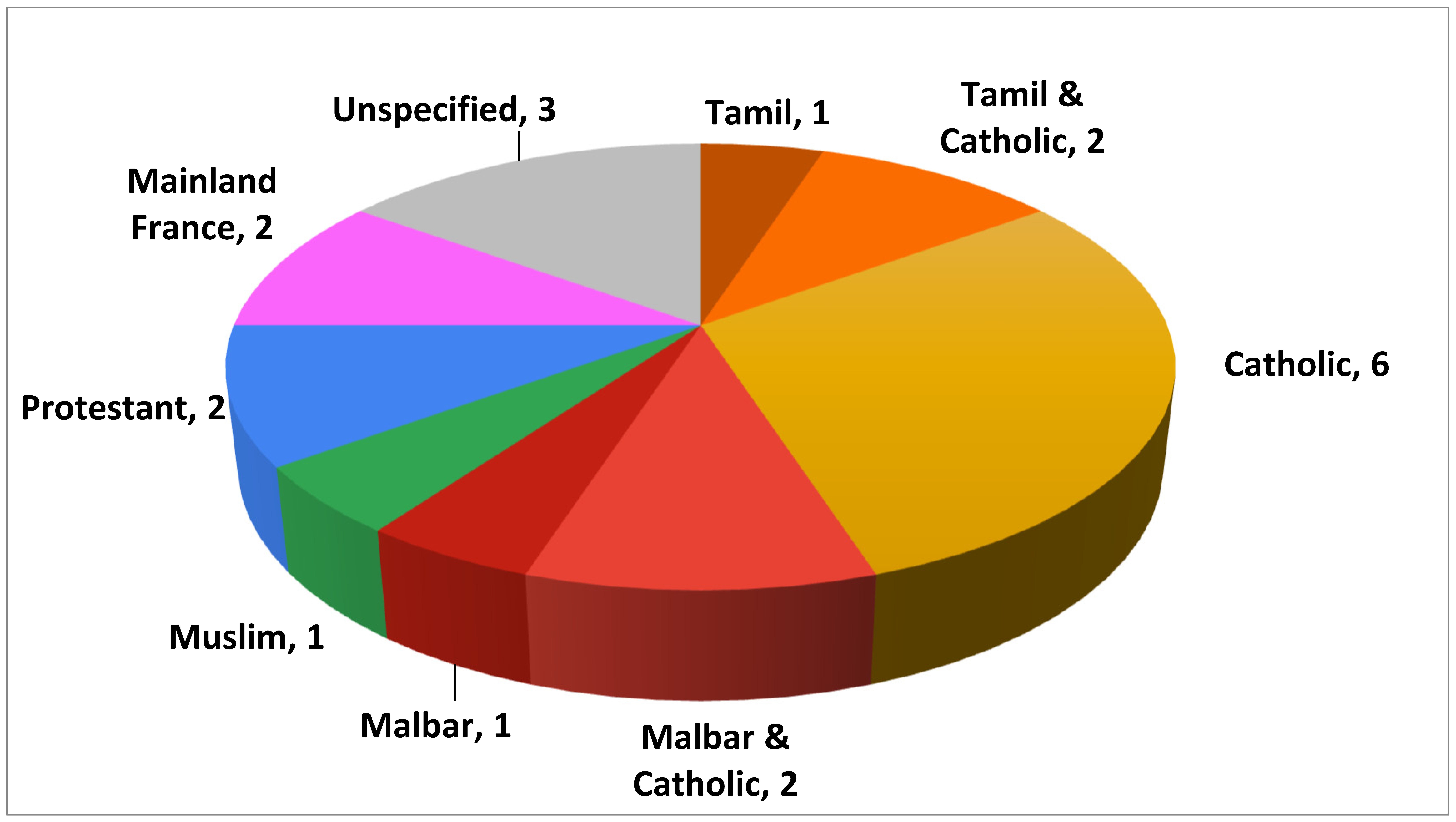
3.1. Study Participants
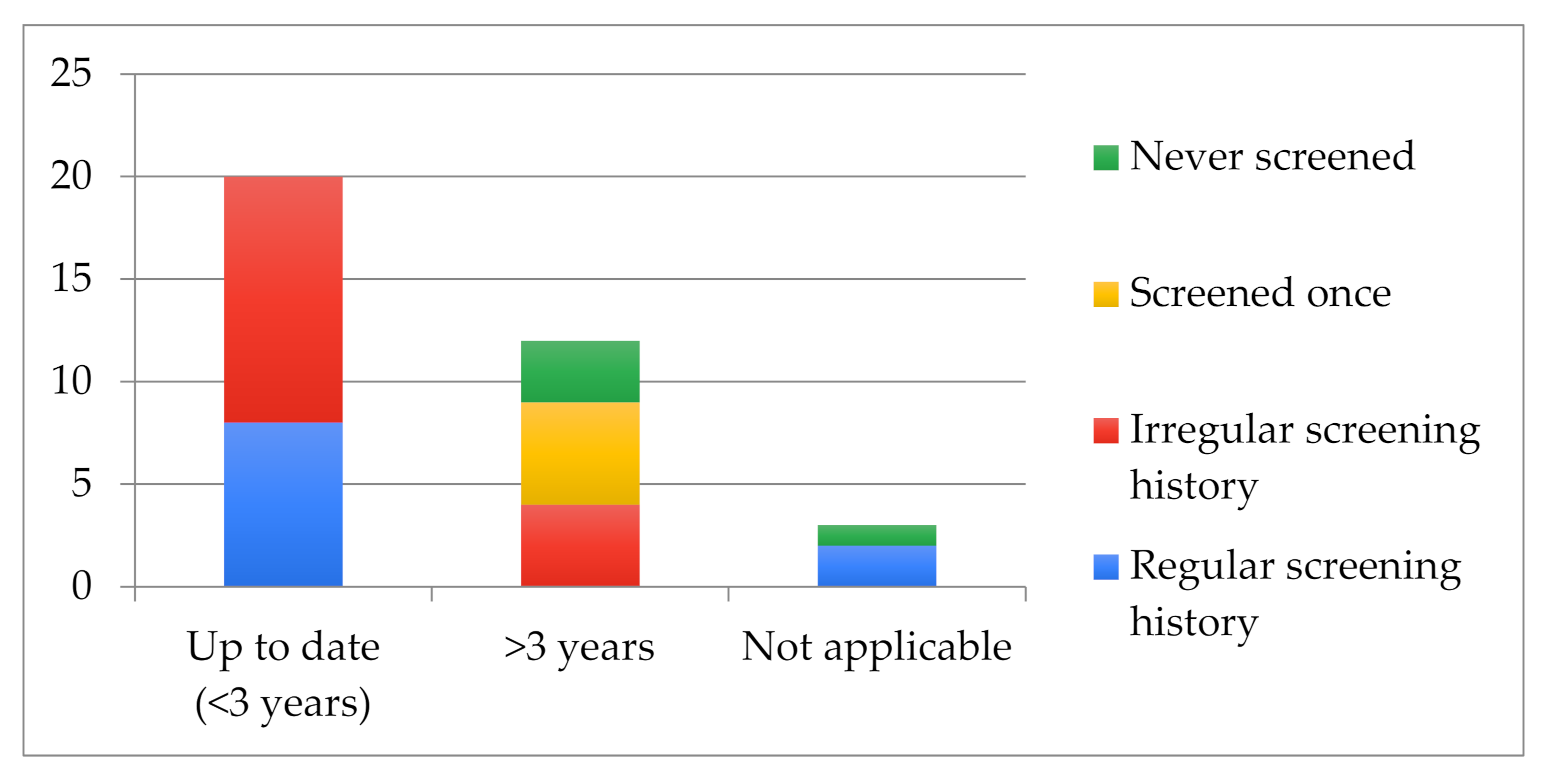
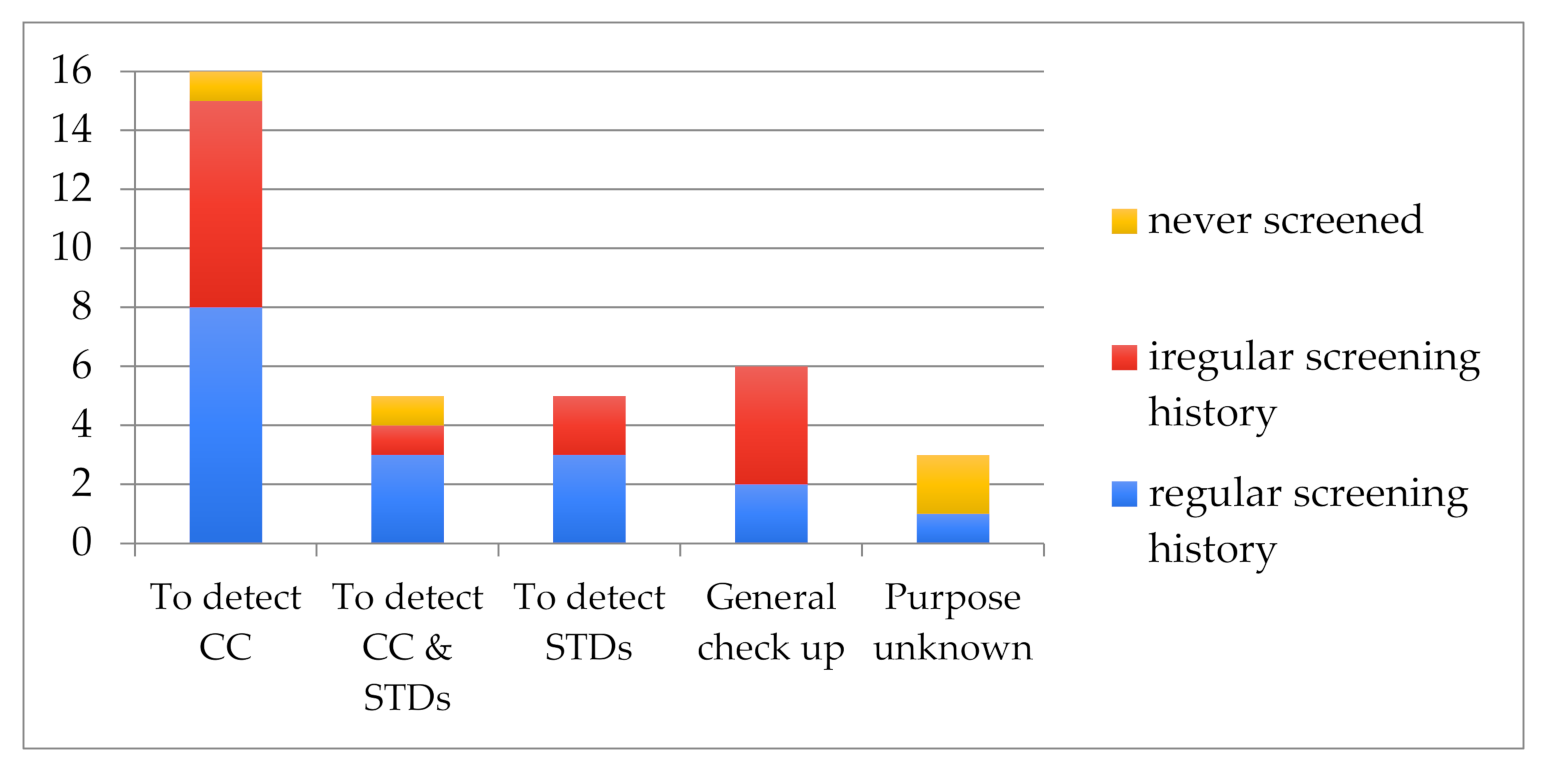
3.2. Screening Practices
3.3. Disease Knowledge Levels and Attitudes towards the Disease
3.3.1. Disease Awareness
3.3.2. Disease Origin Awareness
3.3.3. Disease Prevention Awareness
3.3.4. Perceptions of the Disease and of Vital Prognosis
3.3.5. Known Treatment Options
3.4. Screening Barriers and Triggers According to Women and Practitioners
3.5. The Impact of Social Disadvantage and Cultural Factors on Screening
3.6. Attitudes towards Home-Based Self-Sampling
4. Discussion
5. Limitations
6. Conclusions
- -
- Clearly and simply explain the importance of early diagnosis, the availability of non-invasive treatment and reassure regarding prognosis;
- -
- Present a simplified screening schedule;
- -
- Provide other screening options;
- -
- Reassure women on usage through simple instructions;
- -
- Reassure women on quality (results and sample preservation);
- -
- Train local health professionals so that they can better support women throughout the self-sampling process;
- -
- Provide accompaniment options: where to ask for advice in person and a toll-free phone number;
- -
- Provide additional support for women from categories the most at risk or more socially isolated: work with community organisms to reach women with language barriers and those with housing issues; work through the prison staff to reach incarcerated women;
- -
- Integrate HBSS into community health programs targeting socially isolated women.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Semi-Structured Interview Guide
- Women
- I-Socio-Demographic Characteristics and Living Conditions
- Please tell me a bit about yourself?:
- -
- Where do you live? How long have you lived there? Do you own or rent the house you live in?
- -
- Are you married? Do you have someone in your life (if not married)? (If yes) For how long? (If not) Have you ever had a boyfriend? (we need to know if she has ever had sex)
- -
- What do you do for a living? How much do you earn approximately per month? Is it sufficient to support yourself? Do you manage to put a little aside? (If she is not able to support herself) How do you manage (support from brothers or sisters, parents, friends...)? (If married) What is your husband’s occupation?
- -
- Where were you born? How long have you lived in France/Reunion (if born elsewhere)? How did you get here? Where were your parents born?
- -
- Do you have social security coverage? Do you have a carte vitale, CMU (complete health coverage for those on benefits) or AME (partial health coverage)? Do you have a health insurance to cover all or part of your health expenses?
- -
- Have you been to school? If yes, what was the last class you attended?
- -
- Do you have children? How many? How old are they? Do they all live with you?
- II-Knowledge/Perceptions of CC and Risk Management
- (1) Knowledge and Perceptions
- Can you tell me what you know about the uterus and cervical cancer?
- -
- Do you know what the uterus is? Where is it located? What is its role in a woman’s body? The vagina?
- -
- Do you know of any diseases that can affect the uterus? What are these diseases?
- -
- Have you ever heard of cervical cancer? (If yes) Where, with whom and on what occasion? What information were you given?
- -
- Are there any words in your native language for CC? How does it manifest itself?
- -
- What causes cervical cancer?
- -
- How do you think a person can get this disease? Do you know what to do to avoid getting it? What do you do to avoid getting it? Are some people more at risk than others?
- -
- Do you know if this is a curable disease? (If so) what type of treatment is available? Where should you go? (If not) do you think it cannot be cured?
- -
- Do you know any women who have it (or any other cancer)? (If yes) What happened? How close were you to the person...?
- -
- Do you think that medicine can cure cancer? Under what circumstances?
- -
- Have you heard of HPV vaccination? Do you know people who have received it?
- Besides cervical cancer, do you know of any other diseases that only affect women?:
- -
- What are they? What part of the body do they affect?
- -
- What should be done to treat them? What can be done to prevent them?
- -
- Do you know the types of cancer that only affect women?
- -
- Who do you think gets these types of diseases?
- (2) Screening
- -
- How can you find out if you have cervical cancer or not?
- -
- Do you see any value in finding out early?
- -
- Have you even wanted to know if you had the disease? Why? What did you do?
- -
- Do you know what a smear test is?
- -
- What is the point of having a smear test?
- -
- Do you know what the word “screening” means?
- -
- Have you ever heard of a HPV test? What is its purpose?
- -
- Have you ever had a Pap smear? an HPV test?
- -
- At what point in life should women do it? Why should they do it?
- -
- (If you have already had a Pap smear) When? On what occasion did you do it? What prompted you to do it? Did someone encourage you to do it? What does this person mean to you? What was your reaction when that person suggested the test? What words did they use to encourage you to take the test? What words made you hesitate?
- -
- How did the test go? How did you experience the test?
- -
- Where did you go for the test? How did you find out about this facility?
- -
- How did you get your results? How long did it take to get the results? How did you experience the wait? Did you pay for the test? If yes, how much?
- -
- (If no, even though she knows about it) Why did you not want to go for the screening (fear of the result, cost, distance...)? Do you think you will do it one day? Under what conditions would you do it?
- -
- If you could do a test at home, how would you react, would you opt for this possibility? Why or why not?
- -
- Have you ever received a letter inviting you to get tested? What did you think? Who received it? Who read it? What did you do? Did you tell anyone about it? Role of men, spouse, others (e.g., if the woman cannot read...)
- -
- Do you prefer to read in Creole or in French? How would you react to a letter urging you to do a screening in French versus if it was in Creole? Or in both languages?
- -
- What happens when you receive mail: where does it arrive? who receives it? who reads it?
- (3) Practices around the genital area
- Can you tell me what you do to take care of your intimate health?
- -
- Intimate cleaning (after sexual relations...)
- -
- What did you do the last time you felt something was not right? Why or why not? Who do you talk to about it? Do you consult with anyone? (If not) Why? If you consult a health care provider, who do you go to? Why?
- -
- Do you pass on tips between women or between mother and daughter regarding intimate health? What are these tips? What habits were passed on to you? By who?
- -
- In what language are you most comfortable talking about this topic?
- -
- Do you ever consult a midwife or a gynecologist? How often? For what reasons in general? What do you think of the care provided? (If not) Why not?
- -
- Do you ever consult someone else (acupuncturist, osteopath, healer...) regarding your gynecological health? In which cases? What do you think of the care provided?
- In conclusion
- -
- Do you have any suggestions to encourage women around you to get screened?
- -
- Is there anything you would like to add?
Appendix B. Semi-Structured Interview Guide
- Health Providers
- General information
- -
- Age, gender
- -
- Status & role
- -
- Training & experience
- -
- How long have you been working at this facility? Have you changed position since you’re here?
- -
- Can you tell me more about your career so far?
- General information about the facility
- -
- Name and function of the facility
- -
- What does your work/function in the facility consist of (tasks, missions, roles, work structure)?
- -
- Who are the “users” of the structure? (if applicable)
- Perceptions/experiences related to the CC
- -
- What is your experience related to CC?
- -
- What is your involvement in preventing/treating this disease?
- -
- What difficulties have you encountered?
- For general practitioners:
- -
- What is the profile of your clientele?
- -
- Do you conduct gynaecological examinations? Smear tests?
- -
- If not: does he/she encourage smear tests?
- -
- Does he encourage young girls to be vaccinated against HPV?
- CC Screening Services Provided
- -
- What types of services do you offer in relation to CC screening? Since when?
- -
- How many caregivers are trained to provide these services? Have you been trained? If so, since when?
- -
- How is CC screening organized? How does it fit into overall services?
- -
- What is the pathway for a woman looking to be CC screened? How does the screening offer work? Is it done at the request of women, the association that manages organized screening in your region, or at the suggestion of caregivers? (If screening is done at the suggestion of caregivers) What prompts you to offer screening (systematic, suspicion, etc.)? How do women react (refusal, request for time to reflect, immediate acceptance)? If you want to encourage a woman to undergo screening, what words do you use? Why do you think some women do not accept?
- -
- Who conducts the smear? How does the sampling go in general? How do women experience it? What difficulties have you encountered? What do you do/say?
- -
- Where do you send the sample? How long does it take to get the results? How are the results provided to the women (when it is positive? when it is negative?)
- -
- How often do you encounter requests related to CC screening services?
- -
- What other difficulties do you encounter in the prevention and/or management of CC (in relation to the organization of the offer? in relation to the beneficiaries)? What do you do to overcome such difficulties?
- HPV self-testing (knowledge, representations)
- -
- What do you think about HPV testing? and self-sampling? Do you think that home-based self-sampling for HPV can be an alternative to Pap smears? Which women should be offered HPV self-sampling?
- -
- Do you feel you have sufficient knowledge to encourage HPV self-sampling?
- -
- What do you think would be the barriers to implementing such a strategy?
- -
- Are there any potential levers to build on to implement primary HPV self-sampling?
- -
- What is the most appropriate way to provide self-sampling to women who need it? What do you think about sending a kit to women’s homes?
- -
- What is the most appropriate method for collecting self-samples? Should specimens be collected at home? Through health professionals? Pharmacists? What is your opinion on returning the sample to a provider versus returning it by post?
- -
- What do you think about a financial incentive to encourage women who do not participate in screening to self-sample at home?
- Experiences with women
- -
- Do you discuss this disease with patients? How? Systematically? When?
- -
- Do you talk about prevention? Screening? Management? Treatment? With those who are positive or with all patients?
- -
- What do you say to patients when they undergo screening?
References
- Binder-Foucard, F.; Belot, A.; Delafosse, P.; Remontet, L.; Woronoff, A.; Bossard, N. Estimation Nationale de l’incidence et de La Mortalité Par Cancer En France Entre 1980 et 2012. Etude à Partir Des Registres Des Cancers Du Réseau Francim—Partie 1: Tumeurs Solides; Institut de Veille Sanitaire: Saint-Maurice, France, 2013; p. 122.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bryere, J.; Dejardin, O.; Launay, L.; Colonna, M.; Grosclaude, P.; Launoy, G. Environnement socioéconomique et incidence des cancers en France. Bull. Epidémiol. Hebd. 2014, 4, 68–77. [Google Scholar]
- Singh, G.K.; Jemal, A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J. Environ. Public Health 2017, 2017, e2819372. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.L.; Morice, P.; Chirpaz, E.; Lazaro, G.; Boukerrou, M. Impact of Management on Mortality in Patients with Invasive Cervical Cancer in Reunion Island. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 215, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Institut National du Cancer Le Cancer Du Col de l’utérus En France, État Des Lieux; InCA: Paris, France, 2010.
- Touzani, R.; Bendiane, M.-K.; Bouhnik, A.-D.; Bruneau, L.; Mancini, J.; Chirpaz, E.; Huiart, L. Connaissances sur le dépistage et le cancer du col de l’utérus à la Réunion: Application d’une classification ascendante hiérarchique. Rev. D’épidémiol. St. Publique 2018, 66, S187–S188. [Google Scholar] [CrossRef]
- Sultana, F.; Mullins, R.; English, D.R.; Simpson, J.A.; Drennan, K.T.; Heley, S.; Wrede, C.D.; Brotherton, J.M.L.; Saville, M.; Gertig, D.M. Women’s Experience with Home-Based Self-Sampling for Human Papillomavirus Testing. BMC Cancer 2015, 15, 849. [Google Scholar] [CrossRef] [PubMed]
- Mariño, H.; Serra, E.; Gutiérrez, A. Self-Sampling Is as Much Effective as Gynecologist Samples for HPV Detection. Med. Balear 2015, 30, 16–20. [Google Scholar]
- Petignat, P.; Faltin, D.L.; Bruchim, I.; Tramèr, M.R.; Franco, E.L.; Coutlée, F. Are Self-Collected Samples Comparable to Physician-Collected Cervical Specimens for Human Papillomavirus DNA Testing? A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2007, 105, 530–535. [Google Scholar] [CrossRef]
- Schmeink, C.E.; Bekkers, R.L.M.; Massuger, L.F.A.G.; Melchers, W.J.G. The Potential Role of Self-Sampling for High-Risk Human Papillomavirus Detection in Cervical Cancer Screening. Rev. Med. Virol. 2011, 21, 139–153. [Google Scholar] [CrossRef]
- Available online: https://Clinicaltrials.Gov/Ct2/Show/NCT04312178?Cond=NCT04312178&draw=2&rank=1. (accessed on 2 March 2022).
- Grangé, C. Le taux de Pauvreté Reste Stable en 2018 à La Réunion; Insee Flash Réunion: Paris, France, 2021; Volume 194, pp. 1–3. [Google Scholar]
- Benoist, J. Anthropologie Médicale En Société Créole; PUF: Paris, France, 1993. [Google Scholar]
- Desprès, C. Être Malade d’un Cancer à l’île de La Réunion: L’histoire Individuelle Au Cœur Du Sens de La Maladie et Des Parcours Thérapeutiques. In Territoires Contemporains de la Recherche Biographique; Delory-Momberger, C., Niewadomski, C., Eds.; Editions Teraedre: Paris, France, 2012; pp. 213–224. [Google Scholar]
- Desprès, C. Soigner par la nature à la Réunion: L’usage des plantes médicinales comme recours thérapeutique dans la prise en charge du cancer. Anthropol. Santé. Rev. Int. Francoph. D’anthropol. Santé 2011, 2. [Google Scholar] [CrossRef]
- Pourchez, L. La Réinterprétation Réunionnaise Des Apports de La Biomédecine Dans Le Domaine de La Naissance et de La Petite Enfance. In Santé, société et Cultures à La Réunion; Benoist, J., Ed.; Karthala: Paris, France, 2001; pp. 75–90. [Google Scholar]
- Pourchez, L.; Dupé, S. Les Grossesses Précoces Chez Les Mineures à La Réunion (Étude Anthropologique). Info Réunion-Publ. de l’ARS 2011, 21, 1–7. [Google Scholar]
- Widmer, I.; Pourette, D. Les Violences Envers les Femmes à l’île de la Réunion poids des Chiffres et Paroles de Victimes; Publications de l’Université de Provence: Aix-en-Provence, France, 2009; ISBN 978-2-85399-721-8. [Google Scholar]
- Olivier de Sardan, J.P. Anthropologie et Développement. Essai En Socio-Anthropologie Du Changement Social; Hommes et Sociétés; KARTHALA-APAD: Paris, France, 1995. [Google Scholar]
- Brunet, L.; Carpentier, S.; Laporte, A.; Le Méner, E.; Pourette, D.; Guillon, B. Féminité, Accès Aux Soins, Maternité et Risques Vécus Par Les Femmes En Grande Précarité. Une Contribution à l’amélioration de Leur Santé Gynécologique; INPES: Paris, France, 2005; p. 102. [Google Scholar]
- Razafimahatratra, M.J.J.; Pourette, D. Cancer Du Col de l’utérus à Madagascar: Des Facteurs Multiples de Retard Au Diagnostic et à La Prise En Charge. In Femmes, Enfants et Santé à Madagascar. Approches Anthropologiques Comparées; Pourette, D., Mattern, C., Bellas Cabane, C., Ravololomanga, B., Eds.; Anthropologies & Médecines: Paris, France, 2018; pp. 223–233. ISBN 978-2-343-14681-2. [Google Scholar]
- Dumont, A.; Bessières, N.; Benbassa, A.; Razafindrafara, G.; Rabearison, F.; Philippe, H.-J. Dépistage Du Cancer Du Col Utérin En Milieu Rural à Madagascar: Faisabilité, Couverture et Incidence. Rev. Méde. Périnatale 2017, 9, 25–31. [Google Scholar] [CrossRef]
- Mensah, K.; Kaboré, C.; Zeba, S.; Bouchon, M.; Duchesne, V.; Pourette, D.; DeBeaudrap, P.; Dumont, A. Implementation of HPV-Based Screening in Burkina Faso: Lessons Learned from the PARACAO Hybrid-Effectiveness Study. BMC Women’s Health 2021, 21, 251. [Google Scholar] [CrossRef]
- Pourette, D.; Duchesne, V.; Bouchon, M.; Zongo, S.; Mensah, K.; DeBeaudrap, P.; Dumont, A. Accès à La Prévention et Aux Soins Du Cancer Du Col de l’utérus à Ouagadougou (Burkina Faso). Etude Socio-Anthropologique; Médecins du Monde/Ceped: Paris, France, 2019; p. 72. [Google Scholar]
- Escobar, N.; Plugge, E. Prevalence of Human Papillomavirus Infection, Cervical Intraepithelial Neoplasia and Cervical Cancer in Imprisoned Women Worldwide: A Systematic Review and Meta-Analysis. J. Epidemiol. Community Health 2020, 74, 95–102. [Google Scholar] [CrossRef]
- Vorsters, A.; Cornelissen, T.; Leuridan, E.; Bogers, J.; Vanden Broeck, D.; Benoy, I.; Goossens, H.; Hens, N.; Van Damme, P. Prevalence of High-Risk Human Papillomavirus and Abnormal Pap Smears in Female Sex Workers Compared to the General Population in Antwerp, Belgium. BMC Public Health 2016, 16, 477. [Google Scholar] [CrossRef]
- Asgary, R.; Alcabes, A.; Feldman, R.; Garland, V.; Naderi, R.; Ogedegbe, G.; Sckell, B. Cervical Cancer Screening Among Homeless Women of New York City Shelters. Matern. Child Health J. 2016, 20, 1143–1150. [Google Scholar] [CrossRef][Green Version]
- Iezzoni, L.I.; Rao, S.R.; Agaronnik, N.D.; El-Jawahri, A. Associations Between Disability and Breast or Cervical Cancers, Accounting for Screening Disparities. Med. Care 2021, 59, 139–147. [Google Scholar] [CrossRef]
- Soccio, J.; Brown, M.; Comino, E.; Friesen, E. Pap Smear Screening, Pap Smear Abnormalities and Psychosocial Risk Factors among Women in a Residential Alcohol and Drug Rehabilitation Facility. J. Adv. Nurs. 2015, 71, 2858–2866. [Google Scholar] [CrossRef]
- Morse, J.M. The Significance of Saturation. Qual. Health Res. 1995, 5, 147–149. [Google Scholar] [CrossRef]
- Olivier de Sardan, J.-P. La Rigueur Du Qualitatif. Les Contraintes Empiriques de l’interprétation Socio-Anthropologique; Anthropologie Prospective; Academia Bruylant: Louvain-la-Neuve, Belgium, 2008. [Google Scholar]
- Ahmed, S.; Shahid, R.K. Disparity in Cancer Care: A Canadian Perspective. Curr. Oncol. 2012, 19, e376–e382. [Google Scholar] [CrossRef]
- Binka, C.; Nyarko, S.H.; Awusabo-Asare, K.; Doku, D.T. Barriers to the Uptake of Cervical Cancer Screening and Treatment among Rural Women in Ghana. BioMed Res. Int. 2019, 2019, 6320938. [Google Scholar] [CrossRef]
- Osei, E.A.; Appiah, S.; Gaogli, J.E.; Oti-Boadi, E. Knowledge on Cervical Cancer Screening and Vaccination among Females at Oyibi Community. BMC Women’s Health 2021, 21, 148. [Google Scholar] [CrossRef]
- Gelberg, L.; Browner, C.H.; Lejano, E.; Arangua, L. Access to Women’s Health Care: A Qualitative Study of Barriers Perceived by Homeless Women. Women Health 2004, 40, 87–100. [Google Scholar] [CrossRef]
- Couderc, M. «Il Faut Me Dire La Vérité Si j’ai Un Cancer!» Perceptions et Expérience(s) Des Participantes à Une Étude Clinique de Prévention Du Cancer Du Col de l’utérus Chez Les Femmes Séropositives Au Sénégal: Analyse Anthropologique. In Regards Croisés sur la Santé et la Maladie. Recherches Anthropologiques, Recherches Cliniques; Desprès, C., Gottot, S., Mellerio, H., Teixeira, M., Eds.; Editions des Archives Contemporaines: Paris, France, 2016; pp. 197–212. [Google Scholar]
- Aranda Flores, C.E.; Gomez Gutierrez, G.; Ortiz Leon, J.M.; Cruz Rodriguez, D.; Sørbye, S.W. Self-Collected versus Clinician-Collected Cervical Samples for the Detection of HPV Infections by 14-Type DNA and 7-Type MRNA Tests. BMC Infect. Dis. 2021, 21, 504. [Google Scholar] [CrossRef]
- Mensah, K.; Assoumou, N.; Duchesne, V.; Pourette, D.; DeBeaudrap, P.; Dumont, A. Acceptability of HPV Screening among HIV-Infected Women Attending an HIV-Dedicated Clinic in Abidjan, Côte d’Ivoire. BMC Women’s Health 2020, 20, 155. [Google Scholar] [CrossRef]
- Rositch, A.F.; Gatuguta, A.; Choi, R.Y.; Guthrie, B.L.; Mackelprang, R.D.; Bosire, R.; Manyara, L.; Kiarie, J.N.; Smith, J.S.; Farquhar, C. Knowledge and Acceptability of Pap Smears, Self-Sampling and HPV Vaccination among Adult Women in Kenya. PLoS ONE 2012, 7, e40766. [Google Scholar] [CrossRef]
- Wong, J.P.H.; Vahabi, M.; Miholjcic, J.; Tan, V.; Owino, M.; Li, A.T.W.; Poon, M.K.L. Knowledge of HPV/Cervical Cancer and Acceptability of HPV Self-Sampling among Women Living with HIV: A Scoping Review. Curr. Oncol. 2018, 25, e73–e82. [Google Scholar] [CrossRef]
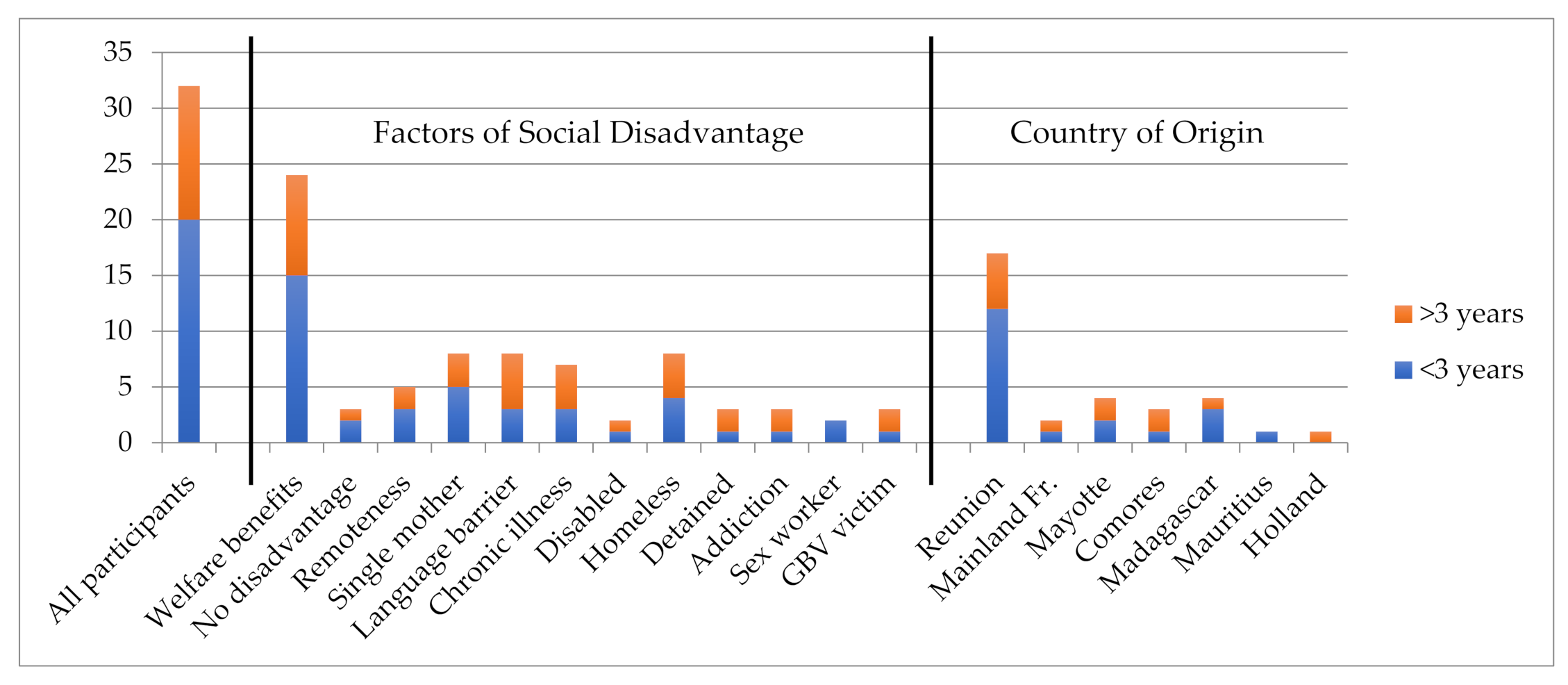
| Interviewee Characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Place of birth | Reunion | Mayotte | Comoros | Madagascar | Mauritius | Mainland France | Holland | |||||
| 20 | 4 | 3 | 4 | 1 | 2 | 1 | ||||||
| Socio-economic criteria | Unemployed | Employed | Retired | Welfare benefits | Single mother | Remote area | ||||||
| 24 | 6 | 5 | 24 | 8 | 5 | |||||||
| Factors of sever social disadvantage and isolation | Migrant | Language Barriers | Illiterate | Homeless | Sheltered Accommodation | In Prison | ||||||
| 15 | 8 | 3 | 3 | 3 | 3 | |||||||
| Other Criteria | Family exclusion | Disability | Chronic Illness | Gender Based Violence | Sex Worker | Alcoholism | ||||||
| 6 | 3 | 8 | 3 | 2 | 3 | |||||||
| Profession | Men | Women | Details |
|---|---|---|---|
| Medical Doctors | 4 | 2 | 1 prison doctor and 5 general practitioners: 2 at MCPCs, 1 at a screening unit, 2 private sector practitioners |
| Gynaecologists | 2 | 2 | 2 obstetricians working in hospitals, 1 gynaecologist–sexologist working in a screening centre, 1 private sector |
| Midwives | 1 | 5 | 1 at a MCPC, 5 private sector practitioners |
| Pharmacists | 2 | 2 private sector practitioners | |
| Laboratory Practitioners | 1 | 1 | 1 biologist at a sampling centre, 1 pathologist at an analysis centre |
| Factors of Influence | Barriers | Triggers/Encouraging Factors | |
|---|---|---|---|
| Knowledge | CC | Not knowing about CC Believing CC is incurable Believing the treatments offered are difficult to bear Language barriers | Becoming aware of the disease Believing CC is curable if detected sufficiently early Understanding treatment options Being informed by a practitioner |
| Screening | Screening schedule unknown | ||
| Attitudes | CC | Not feeling concerned by CC An invisible disease | Feeling concerned by CC |
| Screening | Not feeling at ease with the practitioner or the act itself An additional burden in terms of time and obligations Fear of the results | Feeling at ease with the practitioner and the act itself Part of taking care of oneself Not being able to “see inside” oneself Wanting to know | |
| Practices and past experience | Screening | No longer receiving an invitation letter A negative screening experience | Reminders in person or by letter Gynaecological monitoring (contraception, pregnancy…) Integrated into a health routine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pourette, D.; Cripps, A.; Guerrien, M.; Desprès, C.; Opigez, E.; Bardou, M.; Dumont, A. Assessing the Acceptability of Home-Based HPV Self-Sampling: A Qualitative Study on Cervical Cancer Screening Conducted in Reunion Island Prior to the RESISTE Trial. Cancers 2022, 14, 1380. https://doi.org/10.3390/cancers14061380
Pourette D, Cripps A, Guerrien M, Desprès C, Opigez E, Bardou M, Dumont A. Assessing the Acceptability of Home-Based HPV Self-Sampling: A Qualitative Study on Cervical Cancer Screening Conducted in Reunion Island Prior to the RESISTE Trial. Cancers. 2022; 14(6):1380. https://doi.org/10.3390/cancers14061380
Chicago/Turabian StylePourette, Dolorès, Amber Cripps, Margaux Guerrien, Caroline Desprès, Eric Opigez, Marc Bardou, and Alexandre Dumont. 2022. "Assessing the Acceptability of Home-Based HPV Self-Sampling: A Qualitative Study on Cervical Cancer Screening Conducted in Reunion Island Prior to the RESISTE Trial" Cancers 14, no. 6: 1380. https://doi.org/10.3390/cancers14061380
APA StylePourette, D., Cripps, A., Guerrien, M., Desprès, C., Opigez, E., Bardou, M., & Dumont, A. (2022). Assessing the Acceptability of Home-Based HPV Self-Sampling: A Qualitative Study on Cervical Cancer Screening Conducted in Reunion Island Prior to the RESISTE Trial. Cancers, 14(6), 1380. https://doi.org/10.3390/cancers14061380






