BKV Related Hemorrhagic Cystitis—An Insight into Risk Factors and Later Complications—An Analysis on Behalf of Polish Adult Leukemia Group
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. BKV-DNA PCR Analysis
3.2. GvHD and Other HC Risk Factors
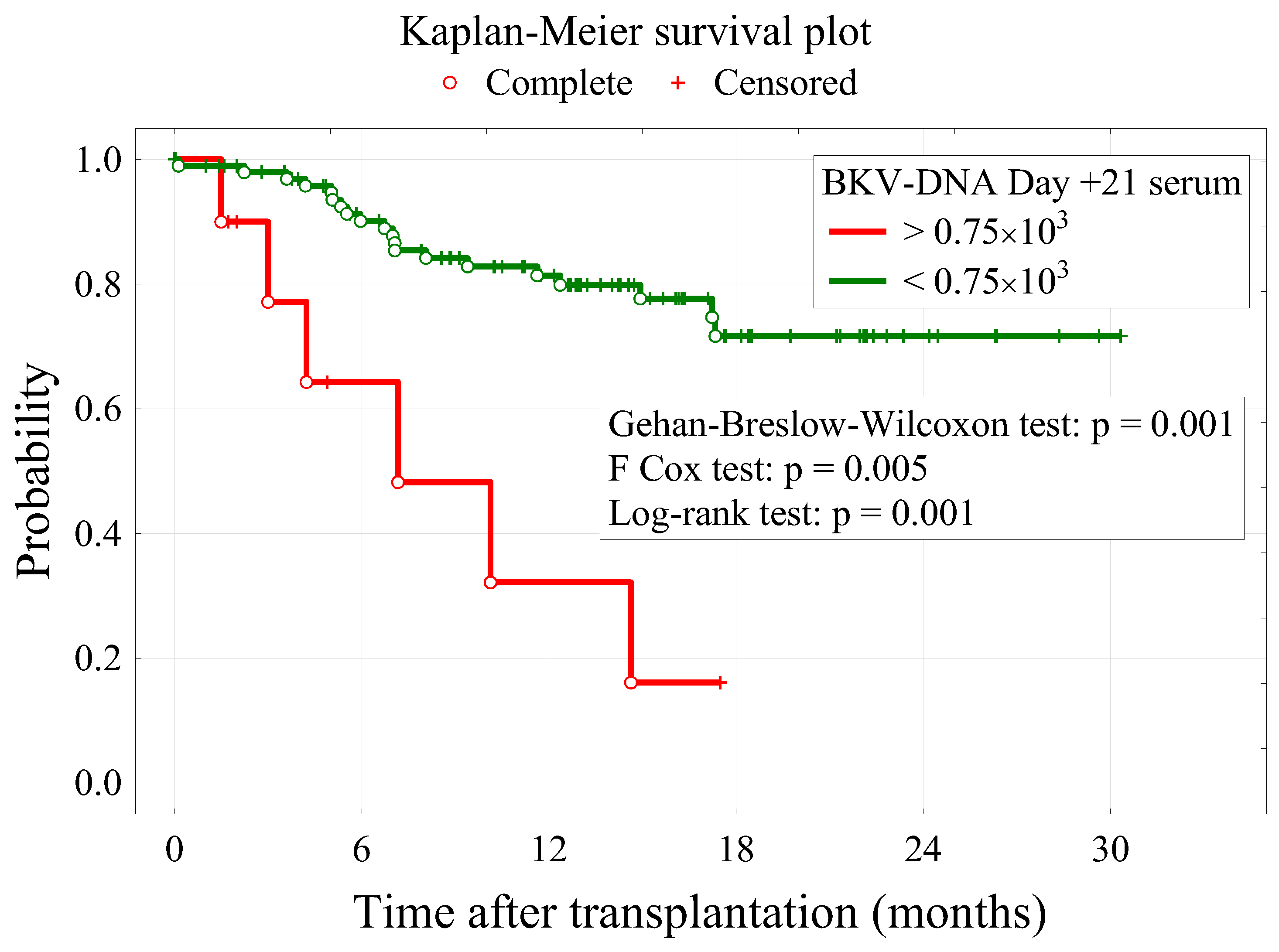
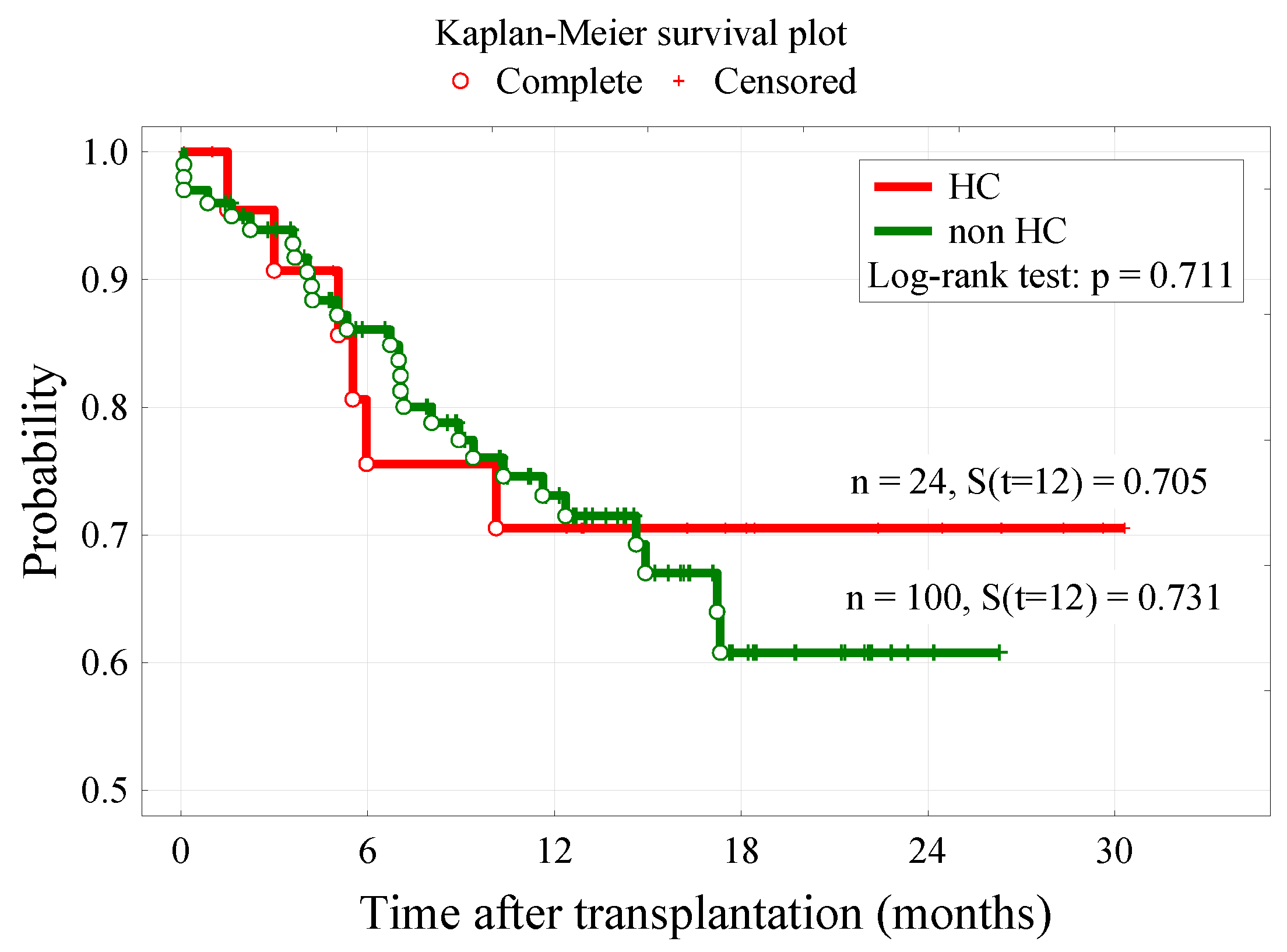
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jandial, A.; Mishra, K.; Sandal, R.; Kant Sahu, K. Management of BK virus-associated haemorrhagic cystitis in allogeneic stem cell transplant recipients. Ther. Adv. Infect. Dis. 2021, 8, 2049936121991377. [Google Scholar] [CrossRef] [PubMed]
- Rorije, N.M.G.; Shea, M.M.; Satyanarayana, G.; Hammond, S.P.; Ho, V.T.; Baden, L.R.; Antin, J.H.; Soiffer, R.J.; Marty, F.M. BK virus disease after allogeneic stem cell transplantation: A cohort analysis. Biol. Blood Marrow Transplant. 2014, 20, 564–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imlay, H.; Xie, H.; Leisenring, W.M.; Duke, E.R.; Kimball, L.E.; Huang, M.L.; Pergam, S.A.; Hill, J.A.; Jerome, K.R.; Milano, F.; et al. Presentation of BK polyomavirus-associated hemorrhagic cystitis after allogeneic hematopoietic cell transplantation. Blood Adv. 2020, 4, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarche, C.; Orio, J.; Collette, S.; Senécal, L.; Hébert, M.J.; Renoult, É.; Tibbles, L.A.; Delisle, J.S. BK polyomavirus and the transplanted kidney: Immunopathology and therapeutic approaches. Transplantation 2016, 100, 2276–2287. [Google Scholar] [CrossRef] [Green Version]
- Gouvêa, A.L.F.; Cosendey, R.I.J.; Nascimento, A.L.R.; Carvalho, F.R.; Silva, A.A.; De Moraes, H.P.; Rochael, M.C.; Varella, R.B.; Almeida, S.G.; Almeida, J.R.; et al. BK polyomavirus nephropathy in two kidney transplant patients with distinct diagnostic strategies for BK virus and similar clinical outcomes: Two case reports. J. Med. Case Rep. 2017, 11, 146. [Google Scholar] [CrossRef] [Green Version]
- Vanichanan, J.; Udomkarnjananun, S.; Avihingsanon, Y.; Jutivorakool, K. Common viral infections in kidney transplant recipients. Kidney Res. Clin. Pract. 2018, 37, 323–337. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.H.; Zhao, H.; Huang, Y.P.; Zhang, X.; Gao, H.N.; Yang, M.F.; Fan, J.; Ma, W.H. Opportunistic posttransplantation virus infections in renal transplant recipients. Transplant. Proc. 2011, 43, 3715–3719. [Google Scholar] [CrossRef]
- Giraud, G.; Priftakis, P.; Bogdanovic, G.; Remberger, M.; Dubrulle, M.; Hau, A.; Gutmark, R.; Mattson, J.; Svahn, B.M.; Ringden, O.; et al. BK-viruria and haemorrhagic cystitis are more frequent in allogeneic haematopoietic stem cell transplant patients receiving full conditioning and unrelated-HLA-mismatched grafts. Bone Marrow Transplant. 2008, 41, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, S.; Azuma, E.; Hori, H.; Hirayama, M.; Kobayashi, M.; Komada, Y.; Nishimori, H.; Miyahara, M.; Nishide, Y. BK virus-associated fatal renal failure following late-onset hemorrhagic cystitis in an unrelated bone marrow transplantation. Pediatr. Hematol. Oncol. 2002, 19, 255–261. [Google Scholar] [CrossRef]
- Cesaro, S.; Dalianis, T.; Rinaldo, C.H.; Koskenvuo, M.; Pegoraro, A.; Einsele, H.; Cordonnier, C.; Hirsch, H.H.; Akova, M.; Aljurf, M.; et al. ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J. Antimicrob. Chemother. 2018, 73, 12–21. [Google Scholar] [CrossRef]
- Wang, X.; Patel, S.A.; Haddadin, M.; Cerny, J. Post-allogeneic hematopoietic stem cell transplantation viral reactivations and viremias: A focused review on human herpesvirus-6, BK virus and adenovirus. Ther. Adv. Infect. Dis. 2021, 8, 20499361211018027. [Google Scholar] [CrossRef] [PubMed]
- Laskin, B.L.; Denburg, M.R.; Furth, S.L.; Moatz, T.; Altrich, M.; Kleiboeker, S.; Lutzko, C.; Zhu, X.; Blackard, J.T.; Jodele, S.; et al. The natural history of BK polyomavirus and the host immune response after stem cell transplantation. Clin. Infect. Dis. 2020, 71, 3044–3054. [Google Scholar] [CrossRef] [PubMed]
- Han, T.T.; Xu, L.P.; Liu, D.H.; Liu, K.Y.; Fu, H.X.; Zhao, X.Y.; Zhao, X.S.; Huang, X.J. Cytomegalovirus is a potential risk factor for late-onset hemorrhagic cystitis following allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2014, 89, 55–61. [Google Scholar] [CrossRef]
- Arai, Y.; Maeda, T.; Sugiura, H.; Matsui, H.; Jo, T.; Ueda, T.; Okada, K.; Kawata, T.; Onishi, T.; Mizutani, C.; et al. Risk factors for and prognosis of hemorrhagic cystitis after allogeneic stem cell transplantation: Retrospective analysis in a single institution. Hematology 2012, 17, 207–214. [Google Scholar] [CrossRef]
- Laskin, B.L.; Denburg, M.; Furth, S.; Diorio, D.; Goebel, J.; Davies, S.M.; Jodele, S. BK viremia precedes hemorrhagic cystitis in children undergoing allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2013, 19, 1175–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaziev, J.; Paba, P.; Miano, R.; Germani, S.; Sodani, P.; Bove, P.; Perno, C.F.; Marziali, M.; Gallucci, C.; Isgrò, A.; et al. Late-onset hemorrhagic cystitis in children after hematopoietic stem cell transplantation for thalassemia and sickle cell anemia: A prospective evaluation of polyoma (BK) virus infection and treatment with Cidofovir. Biol. Blood Marrow Transplant. 2010, 16, 662–671. [Google Scholar] [CrossRef]
- Bogdanovic, G.; Priftakis, P.; Giraud, G.; Kuzniar, M.; Ferraldeschi, R.; Kokhaei, P.; Mellstedt, H.; Remberger, M.; Ljungman, P.; Winiarski, J.; et al. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. J. Clin. Microbiol. 2004, 42, 5394–5396. [Google Scholar] [CrossRef] [Green Version]
- Hayden, R.T.; Gu, Z.; Liu, W.; Lovins, R.; Kasow, K.; Woodard, P.; Srivastava, K.; Leung, W. Risk factors for hemorrhagic cystitis in pediatric allogeneic hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2015, 17, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erard, V.; Kim, H.W.; Corey, L.; Limaye, A.; Huang, M.L.; Myerson, D.; Davis, C.; Boeckh, M. BK DNA viral load in plasma: Evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood 2005, 106, 1130–1132. [Google Scholar] [CrossRef]
- Megged, O.; Stein, J.; Ben-Meir, D.; Shulman, L.M.; Yaniv, I.; Shalit, I.; Levy, I. BK-virus-associated hemorrhagic cystitis in children after hematopoietic stem cell transplantation. J. Pediatr. Hematol. Oncol. 2011, 33, 190–193. [Google Scholar] [CrossRef]
- Bedi, A.; Miller, C.B.; Hanson, J.L.; Goodman, S.; Ambinder, R.F.; Charache, P.; Arthur, R.R.; Jones, R.J. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J. Clin. Oncol. 1995, 13, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Randhawa, P.S. BK polyomavirus in solid organ transplantation—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, 13528. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, S.; Facchin, C.; Tridello, G.; Messina, C.; Calore, E.; Biasolo, M.A.; Pillon, M.; Varotto, S.; Brugiolo, A.; Mengoli, C.; et al. A prospective study of BK-virus-associated haemorrhagic cystitis in paediatric patients undergoing allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2008, 41, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.Y.H.; Suen, C.K.M.; Lie, A.K.W.; Liang, R.H.S.; Yuen, K.Y.; Kwong, Y.L. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood 2001, 98, 1971–1978. [Google Scholar] [CrossRef] [Green Version]
- Cesaro, S.; Tridello, G.; Pillon, M.; Calore, E.; Abate, D.; Tumino, M.; Carucci, N.; Varotto, S.; Cannata, E.; Pegoraro, A.; et al. A prospective study on the predictive value of plasma BK virus-DNA load for hemorrhagic cystitis in pediatric patients after stem cell transplantation. J. Pediatr. Infect. Dis. Soc. 2015, 4, 134–142. [Google Scholar] [CrossRef]
- Ghosh, A.; Tan, T.T.; Linn, Y.C.; Gopalakrishnan, S.; Goh, Y.T.; Hwang, W.; Tan, B.H.; Ho, A.; Phipps, C. What we learned from plasma BK-virus monitoring in allogeneic hematopoietic transplant recipients. Transplantation 2016, 100, e17–e18. [Google Scholar] [CrossRef]

| Factor | Cut-Off | Sens. | Spec. | Acc. | LR(+) | AUC |
|---|---|---|---|---|---|---|
| BKV-DNA Day +14 serum | ≥0.098 × 103 | 22.7% | 93.8% | 80.5% | 3.64 | 0.580 (0.437–0.722) |
| BKV-DNA Day +14 urine | ≥0.036 × 109 | 65.0% | 85.9% | 82.1% | 4.60 | 0.752 (0.623–0.881) |
| BKV-DNA Day +21 serum | ≥0.36 × 103 | 41.7% | 93.3% | 82.5% | 6.25 | 0.673 (0.536–0.809) |
| BKV-DNA Day +21 urine | ≥0.001 × 109 | 70.8% | 73.3% | 72.8% | 2.65 | 0.728 (0.609–0.848) |
| BKV-DNA Day +28 serum | ≥0.098 × 103 | 28.6% | 87.5% | 75.2% | 2.29 | 0.568 (0.426–0.709) |
| BKV-DNA Day +28 urine | ≥0.03 × 109 | 57.9% | 86.5% | 80.6% | 4.28 | 0.707 (0.566–0.848) |
| Variable | HC | p-Value | |
|---|---|---|---|
| Yes n = 24 | No n = 100 | ||
| Female, n (%) | 7 (29.2%) | 45 (45.9%) | 0.237 |
| Median age at transplant (years), Me (Q1; Q3) | 39 (31; 52) | 46 (30; 58) | 0.304 |
| Time from diagnosis to transplantation (months) [Q1:Q3] | 7 (6; 20) | 11 (6; 21) | 0.473 |
| Time from HC manifestation following HSCT (days) | 22.5 (14; 30.25) | - | - |
| Conditioning MAC | 16 (66.7%) | 65 (65.0%) | 0.932 |
| TBI | 8 (33.3%) | 32 (32.0%) | 0.906 |
| CD34 [Q1:Q3] | 5.8 (4.7; 6.7) | 6.0 (5.1; 7.5) | 0.227 |
| PBSC | 20 (83,3%) | 92 (92%) | 0.591 |
| Donor MUD | 18 (75.0%) | 74 (74.0%) | 0.874 |
| Age, donor (Q1:Q3) | 25 (6; 35) | 9 (4; 35) | 0.273 |
| Corticosteroids | 16 (67%) | 36 (36%) | 0.027 |
| BKV-DNA Day +14 serum (×103) (Q1:Q3) | 0.00 (0.00; 0.00) | 0.00 (0.00; 0.00) | 0.027 |
| BKV-DNA Day +14 urine (×109) (Q1:Q3) | 0,14 (0.00; 4,48) | 0.00 (0.00; 0.00) | <0.001 |
| BKV-DNA Day +21 serum (×103) (Q1:Q3) | 0.00 (0.00; 1,07) | 0.00 (0.00; 0.00) | <0.001 |
| BKV-DNA Day +21 urine (×109) (Q1:Q3) | 0.42 (0.00; 1,68) | 0.00 (0.00; 0.00) | <0.001 |
| BKV-DNA Day +28 serum (×103) (Q1:Q3) | 0.00 (0.00; 0,10) | 0.00 (0.00; 0.00) | 0.153 |
| BKV-DNA Day +28 urine (×109) (Q1:Q3) | 0.07 (0.00; 5.40) | 0.00 (0.00; 0.00) | <0.001 |
| Acute GvHD | 16 (66.7%) | 38 (38.0%) | 0.021 |
| Chronic GvHD | 11 (45.8%) | 30 (30.0%) | 0.139 |
| CMV | 17 (71%) | 42 (46.7%) | 0.126 |
| CMV, donor | 9 (38%) | 48 (48%) | 0.322 |
| Dead | 6 (25.0%) | 28 (28.0%) | 0.967 |
| Survival (months) (Q1:Q3) | 12.9 (5.0; 20.4) | 11.2 (5.5; 16.3) | 0.374 |
| HC Risk Factor | b | SEb | p | OR (95% CI) |
|---|---|---|---|---|
| BKV-DNA Day +14 serum | 3.2 × 10−4 | 2.4 × 10−4 | 0.181 | - |
| BKV-DNA Day +14 urine | −1.6 × 10−12 | 5.8 × 10−12 | 0.780 | - |
| BKV-DNA Day +21 serum | 4.0 × 10−4 | 1.5 × 10−4 | 0.007 | 1.0004 (1.0001–1.0007) |
| BKV-DNA Day +21 urine | 7.5 × 10−11 | 6.4 × 10−11 | 0.237 | - |
| BKV-DNA Day +28 urine | −8.0 × 10−12 | 1.5 × 10−12 | 0.584 | - |
| Acute GvHD | 1.332 | 0.533 | 0.012 | 3.787 (1.332–10.765) |
| Variable | Status | p-Value | |
|---|---|---|---|
| Dead n = 34 | Alive n = 90 | ||
| Female, n (%) | 11 (32.4%) | 41 (45.6%) | 0.184 |
| Age (years), Me [Q1; Q3] | 48 [34; 58] | 43 [30; 55] | 0.190 |
| Time from diagnosis to transplantation (months) [Q1; Q3] | 18 [8; 44] | 9 [6; 18] | 0.017 |
| Conditioning MAC | 17 (50.0%) | 64 (71.1%) | 0.028 |
| TBI | 11 (32.4%) | 29 (32.2%) | 0.989 |
| CD34 [Q1; Q3] | 6.2 [4.9; 7.3] | 6.0 [5.2; 7.3] | 0.606 |
| PBSC | 30 (88.2%) | 82 (91.1%) | 0.523 |
| Donor MUD | 27 (79.4%) | 65 (72.2%) | 0.558 |
| Age, donor [Q1; Q3] | 14 [4; 36] | 9 [4; 34] | 0.573 |
| Female, donor | 12 (35.3%) | 24 (26.7%) | 0.345 |
| HLA | 29 (85.3%) | 83 (92.2%) | 0.410 |
| BKV-DNA Day +14 serum (×103) [Q1; Q3] | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.00] | 0.525 |
| BKV-DNA Day +14 urine (×109) [Q1; Q3] | 0.00 [0.00; 0,11] | 0.00 [0.00; 0,02] | 0.547 |
| BKV-DNA Day +21 serum (×103) [Q1; Q3] | 0.00 [0.00; 0.36] | 0.00 [0.00; 0.00] | 0.049 |
| BKV-DNA Day +21 urine (×109) [Q1; Q3] | 0.00 [0.00; 0,11] | 0.00 [0.00; 0.50] | 0.991 |
| BKV-DNA Day +28 serum (×103) | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.00] | 0.848 |
| BKV-DNA Day +28 urine (×109) [Q1; Q3] | 0.00 [0.00; 0.16] | 0.00 [0.00; 0.00] | 0.556 |
| Acute GvHD | 14 (41.2%) | 40 (44.4%) | 0.743 |
| Chronic GvHD | 8 (23.5%) | 33 (36.7%) | 0.241 |
| CMV | 17 (50%) | 42 (46.7%) | 0.319 |
| CMV, donor | 16 (47%) | 41 (45.6%) | 0.422 |
| HC | 6 (17.6%) | 18 (20.0%) | 0.967 |
| Death Risk Factors | b | SEb | p | OR (95% CI) |
|---|---|---|---|---|
| Time from diagnosis to transplantation (months) | 0.007 | 0.005 | 0.198 | 1.00 (0.996–1.018) |
| Conditioning MAC | −0.885 | 0.415 | 0.033 | 0.415 (0.183–0.930) |
| BKV-DNA Day +21 serum | 1.8 × 10−4 | 1.3 × 10−4 | 0.167 | 1.00 (0.9999–1.0004) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dybko, J.; Piekarska, A.; Agrawal, S.; Makuch, S.; Urbaniak-Kujda, D.; Biernat, M.; Rybka, B.; Dutka, M.; Sadowska-Klasa, A.; Giebel, S.; et al. BKV Related Hemorrhagic Cystitis—An Insight into Risk Factors and Later Complications—An Analysis on Behalf of Polish Adult Leukemia Group. Cancers 2022, 14, 764. https://doi.org/10.3390/cancers14030764
Dybko J, Piekarska A, Agrawal S, Makuch S, Urbaniak-Kujda D, Biernat M, Rybka B, Dutka M, Sadowska-Klasa A, Giebel S, et al. BKV Related Hemorrhagic Cystitis—An Insight into Risk Factors and Later Complications—An Analysis on Behalf of Polish Adult Leukemia Group. Cancers. 2022; 14(3):764. https://doi.org/10.3390/cancers14030764
Chicago/Turabian StyleDybko, Jarosław, Agnieszka Piekarska, Siddarth Agrawal, Sebastian Makuch, Donata Urbaniak-Kujda, Monika Biernat, Blanka Rybka, Magdalena Dutka, Alicja Sadowska-Klasa, Sebastian Giebel, and et al. 2022. "BKV Related Hemorrhagic Cystitis—An Insight into Risk Factors and Later Complications—An Analysis on Behalf of Polish Adult Leukemia Group" Cancers 14, no. 3: 764. https://doi.org/10.3390/cancers14030764






