Patient Selection for Local Aggressive Treatment in Oligometastatic Non-Small Cell Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Definition and Staging of Oligometastatic Non-Small Cell Lung Cancer
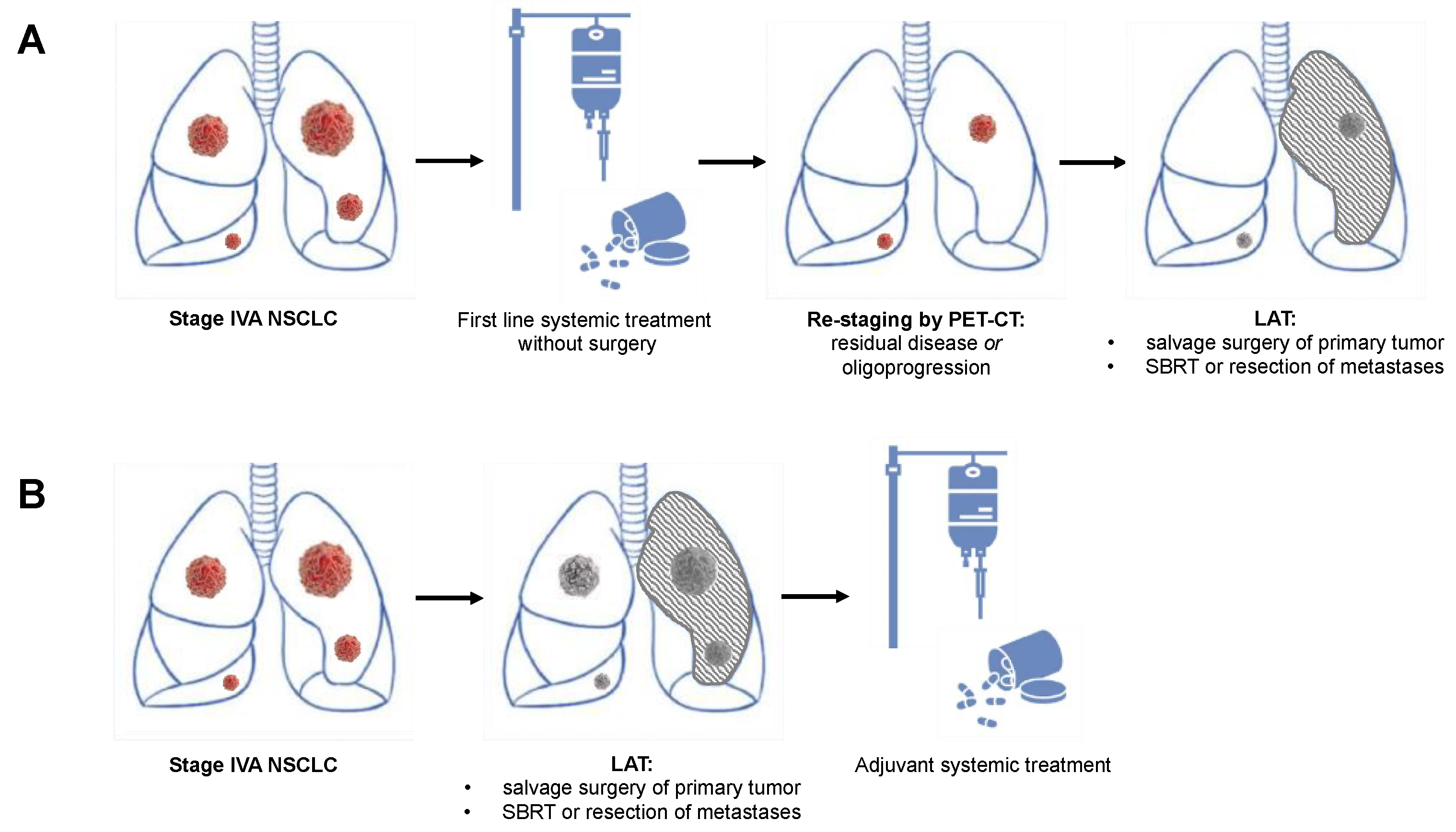
3. Evidence for Local Aggressive Therapy in Oligometastatic Non-Small Cell Lung Cancer
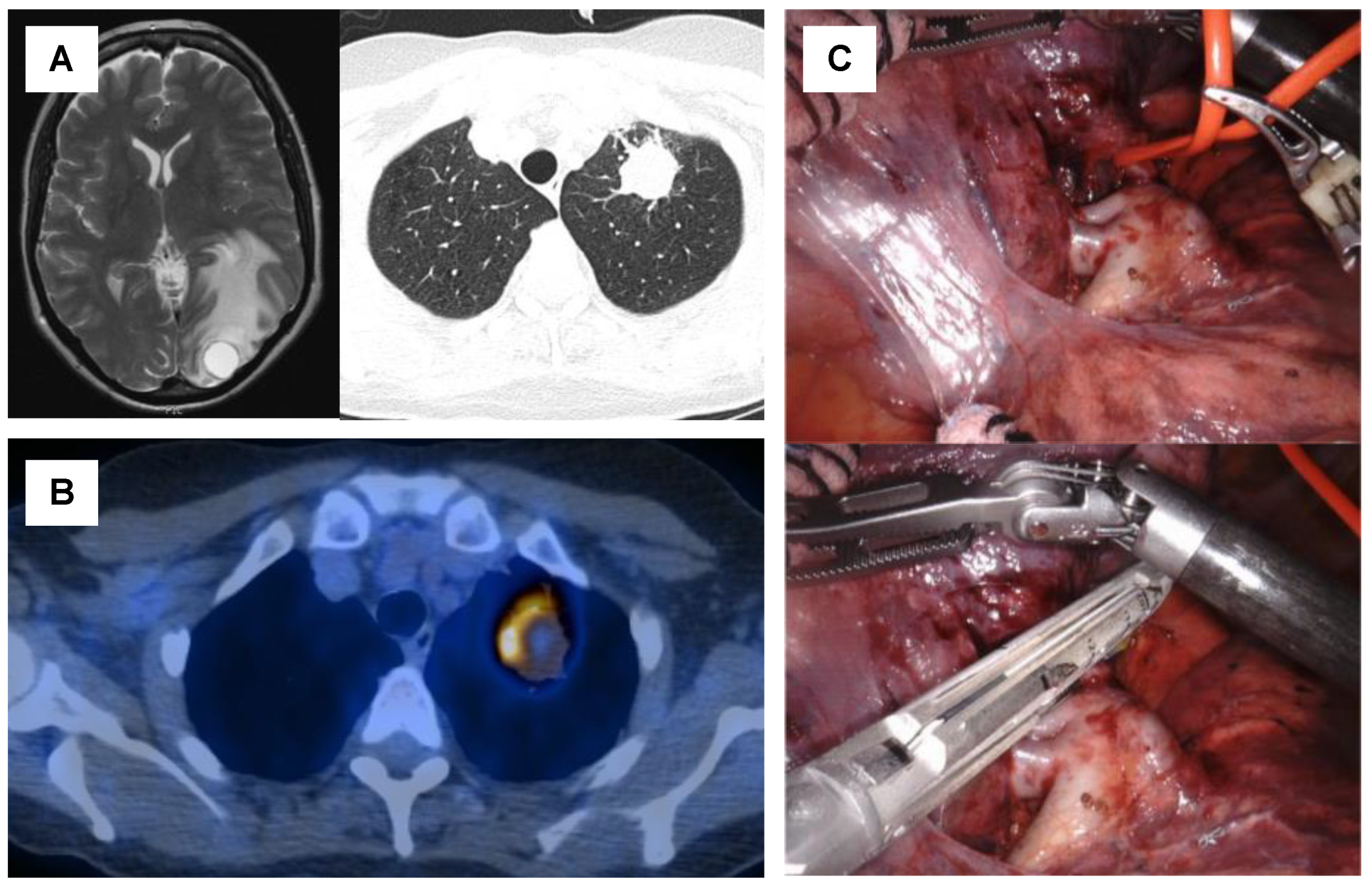
4. Surgical Treatment for Oligometastatic Non-Small Cell Lung Cancer
5. Radiation Therapy for Oligometastatic Non-Small Cell Lung Cancer
6. Patient Selection Criteria for Local Aggressive Therapy
6.1. Site of the Primary Tumor
6.2. Site of Metastases
6.3. Mediastinal Lymph Node Involvement
6.4. Synchronous and Metachronous Metastases
6.5. Performance Status
6.6. Response to Systemic Therapy and Oligoprogressive Disease
6.7. Histopathological and Molecular Markers
6.8. Quality of Life
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Wild, C.; Weiderpass, E.; Steward, B. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Besse, B.; Adjei, A.; Baas, P.; Meldgaard, P.; Nicolson, M.; Paz-Ares, L.; Reck, M.; Smit, E.F.; Syrigos, K.; Stahel, R.; et al. 2nd ESMO Consensus Conference on Lung Cancer: Non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann. Oncol. 2014, 25, 1475–1484. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghmans, T.; Dingemans, A.-M.; Hendriks, L.E.; Cadranel, J. Immunotherapy for nonsmall cell lung cancer: A new therapeutic algorithm. Eur. Respir. J. 2020, 55, 1901907. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; Wilson, G.; McGranahan, N.; Birkbak, N.J.; Watkins, T.B.K.; Veeriah, S.; Shafi, S.; Johnson, D.H.; Mitter, R.; Rosenthal, R.; et al. Tracking the Evolution of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2109–2121. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Berzenji, L.; Debaenst, S.; Hendriks, J.M.H.; Yogeswaran, S.K.; Lauwers, P.; Van Schil, P.E. The role of the surgeon in the management of oligometastatic non-small cell lung cancer: A literature review. Transl. Lung Cancer Res. 2021, 10, 3409–3419. [Google Scholar] [CrossRef] [PubMed]
- Giaj-Levra, N.; Giaj-Levra, M.; Durieux, V.; Novello, S.; Besse, B.; Hasan, B.; Hendriks, L.E.; Levy, A.; Dingemans, A.-M.C.; Berghmans, T. Defining Synchronous Oligometastatic Non–Small Cell Lung Cancer: A Systematic Review. J. Thorac. Oncol. 2019, 14, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.-M.C.; Hendriks, L.E.; Berghmans, T.; Levy, A.; Hasan, B.; Faivre-Finn, C.; Levra, M.G.; Giaj-Levra, N.; Girard, N.; Greillier, L.; et al. Definition of Synchronous Oligometastatic Non–Small Cell Lung Cancer—A Consensus Report. J. Thorac. Oncol. 2019, 14, 2109–2119. [Google Scholar] [CrossRef]
- Ashworth, A.B.; Senan, S.; Palma, D.A.; Riquet, M.; Ahn, Y.C.; Ricardi, U.; Congedo, M.T.; Gomez, D.R.; Wright, G.; Melloni, G.M.; et al. An Individual Patient Data Metaanalysis of Outcomes and Prognostic Factors After Treatment of Oligometastatic Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2014, 15, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, G.H.; Toguri, D.; Dahele, M.; Warner, A.; de Haan, P.F.; Rodrigues, G.B.; Slotman, B.; Yaremko, B.P.; Senan, S.; Palma, D.A. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): Patient outcomes and prognostic factors. Lung Cancer 2013, 82, 95–102. [Google Scholar] [CrossRef]
- Opitz, I.; Patella, M.; Payrard, L.; Perentes, J.Y.; Inderbitzi, R.; Gelpke, H.; Schulte, S.; Diezi, M.; Gonzalez, M.; Krueger, T.; et al. Prognostic factors of oligometastatic non-small-cell lung cancer following radical therapy: A multicentre analysis. Eur. J. Cardio-Thorac. Surg. 2020, 57, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.M.; Mick, R.; Ciunci, C.; Aggarwal, C.; Davis, C.; Evans, T.; Deshpande, C.; Miller, L.; Patel, P.; Alley, E.; et al. Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer: A Phase 2 Trial. JAMA Oncol. 2019, 5, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Wanders, R.; van Baardwijk, A.; Dingemans, A.M.; Reymen, B.; Houben, R.; Bootsma, G.; Pitz, C.; van Eijsden, L.; Geraedts, W.; et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: Long-term results of a prospective phase II trial (Nct01282450). J. Thorac. Oncol. 2012, 7, 1547–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ruysscher, D.; Wanders, R.; Hendriks, L.E.; van Baardwijk, A.; Reymen, B.; Houben, R.; Bootsma, G.; Pitz, C.; van Eijsden, L.; Dingemans, A.M.C.; et al. Progression-Free Survival and Overall Survival Beyond 5 Years of NSCLC Patients With Synchronous Oligometastases Treated in a Prospective Phase II Trial (NCT 01282450). J. Thorac. Oncol. 2018, 13, 1958–1961. [Google Scholar] [CrossRef] [Green Version]
- Gomez, D.R.; Blumenschein, G.R., Jr.; Lee, J.J.; Hernández, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef] [Green Version]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non–Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN) Guidelines: Non-Small Cell Lung Cancer. Version 7. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 25 October 2021).
- Ashworth, A.; Rodrigues, G.; Boldt, G.; Palma, D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer 2013, 82, 197–203. [Google Scholar] [CrossRef]
- Suzuki, S.; Goto, T. Role of Surgical Intervention in Unresectable Non-Small Cell Lung Cancer. J. Clin. Med. 2020, 9, 3881. [Google Scholar] [CrossRef]
- Zheng, Y.; Jaklitsch, M.T.; Bueno, R. Neoadjuvant Therapy in Non–Small Cell Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yang, C.; Gonzalez-Rivas, D.; Zhong, Y.; He, P.; Deng, H.; Liu, J.; Liang, W.; He, J.; Li, S. Sleeve lobectomy after neoadjuvant chemoimmunotherapy/chemotherapy for local advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-F.J.; McSherry, F.; Mayne, N.R.; Wang, X.; Berry, M.F.; Tong, B.; Harpole, D.H.; D’Amico, T.A.; Christensen, J.D.; Ready, N.E.; et al. Surgical Outcomes After Neoadjuvant Chemotherapy and Ipilimumab for Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2018, 105, 924–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weder, W.; Collaud, S.; Eberhardt, W.E.; Hillinger, S.; Welter, S.; Stahel, R.; Stamatis, G. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 139, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Beattie, R.; Furrer, K.; Dolan, D.P.; Curioni-Fontecedro, A.; Lee, D.N.; Frauenfelder, T.; Hoeller, S.; Weder, W.; Bueno, R.; Opitz, I.; et al. Two centres experience of lung cancer resection in patients with advanced non-small cell lung cancer upon treatment with immune checkpoint inhibitors: Safety and clinical outcomes. Eur. J. Cardio-Thorac. Surg. 2021, 60, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Dickhoff, C.; Otten, R.H.J.; Heymans, M.; Dahele, M. Salvage surgery for recurrent or persistent tumour after radical (chemo)radiotherapy for locally advanced non-small cell lung cancer: A systematic review. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaba, E.; Ozyurtkan, M.O.; Ayalp, K.; Cosgun, T.; AlOmari, M.R.; Toker, A. Salvage thoracic surgery in patients with lung cancer: Potential indications and benefits. J. Cardiothorac. Surg. 2018, 13, 13. [Google Scholar] [CrossRef] [Green Version]
- Romero-Vielva, L.; Viteri, S.; Moya-Horno, I.; Toscas, J.I.; Maestre-Alcácer, J.A.; Cajal, S.R.Y.; Rosell, R. Salvage surgery after definitive chemo-radiotherapy for patients with Non-Small Cell Lung Cancer. Lung Cancer 2019, 133, 117–122. [Google Scholar] [CrossRef]
- Bauman, J.E.; Mulligan, M.S.; Martins, R.G.; Kurland, B.; Eaton, K.D.; Wood, D.E. Salvage Lung Resection After Definitive Radiation (>59 Gy) for Non-Small Cell Lung Cancer: Surgical and Oncologic Outcomes. Ann. Thorac. Surg. 2008, 86, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Maisonneuve, P.; Piperno, G.; Bellini, R.; Brambilla, D.; Petrella, F.; De Marinis, F.; Spaggiari, L. Salvage Surgery After Definitive Chemoradiotherapy for Non–small Cell Lung Cancer. Semin. Thorac. Cardiovasc. Surg. 2017, 29, 233–241. [Google Scholar] [CrossRef]
- Sonobe, M.; Yutaka, Y.; Nakajima, D.; Hamaji, M.; Menju, T.; Ohsumi, A.; Chen-Yoshikawa, T.F.; Sato, T.; Date, H. Salvage Surgery After Chemotherapy or Chemoradiotherapy for Initially Unresectable Lung Carcinoma. Ann. Thorac. Surg. 2019, 108, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.K.; Horinouchi, H.; Nakayama, Y.; Ohe, Y.; Yotsukura, M.; Uchida, S.; Asakura, K.; Yoshida, Y.; Nakagawa, K.; Watanabe, S.-I. Salvage surgery after chemotherapy and/or radiotherapy including SBRT and proton therapy: A consecutive analysis of 38 patients. Lung Cancer 2020, 145, 105–110. [Google Scholar] [CrossRef]
- Ohtaki, Y.; Shimizu, K.; Suzuki, H.; Suzuki, K.; Tsuboi, M.; Mitsudomi, T.; Takao, M.; Murakawa, T.; Ito, H.; Yoshimura, K.; et al. Salvage surgery for non-small cell lung cancer after tyrosine kinase inhibitor treatment. Lung Cancer 2021, 153, 108–116. [Google Scholar] [CrossRef]
- Jones, G.; Lengel, H.; Hsu, M.; Tan, K.; Caso, R.; Ghanie, A.; Connolly, J.; Bains, M.; Rusch, V.; Huang, J.; et al. Management of Synchronous Extrathoracic Oligometastatic Non-Small Cell Lung Cancer. Cancers 2021, 13, 1893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, S.-G.; Xue, Q.; Guo, X.-T.; Wang, L.-X.; Yu, X.; Yang, Y.-K.; Mu, J.-W. Surgery of primary non-small cell lung cancer with oligometastasis: Analysis of 172 cases. J. Thorac. Dis. 2018, 10, 6540–6546. [Google Scholar] [CrossRef]
- Arrieta, O.; Escamilla-López, I.; Lyra-González, I.; Barrón, F.; Ramírez-Tirado, L.A.; Vergara, E.; Corona-Cruz, J.; Maldonado, F.; Jiménez-Fuentes, E. Radical aggressive treatment among non-small cell lung cancer patients with malignant pleural effusion without extra-thoracic disease. J. Thorac. Dis. 2019, 11, 595–601. [Google Scholar] [CrossRef]
- Amini, A.; Verma, V.; Simone, C.B., II; Chetty, I.J.; Chun, S.G.; Donington, J.; Edelman, M.J.; Higgins, K.A.; Kestin, L.L.; Movsas, B.; et al. American Radium Society Appropriate Use Criteria for Radiation Therapy in Oligometastatic or Oligoprogressive Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, in press. [Google Scholar] [CrossRef]
- Theelen, W.S.M.E.; Chen, D.; Verma, V.; Hobbs, B.P.; Peulen, H.M.U.; Aerts, J.G.J.V.; Bahce, I.; Niemeijer, A.L.N.; Chang, J.Y.; de Groot, P.M.; et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir. Med. 2021, 9, 467–475. [Google Scholar] [CrossRef]
- Congedo, M.T.; Cesario, A.; Lococo, F.; De Waure, C.; Apolone, G.; Meacci, E.; Cavuto, S.; Granone, P. Surgery for oligometastatic non–small cell lung cancer: Long-term results from a single center experience. J. Thorac. Cardiovasc. Surg. 2012, 144, 444–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collen, C.; Christian, N.; Schallier, D.; Meysman, M.; Duchateau, M.; Storme, G.; De Ridder, M. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann. Oncol. 2014, 25, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A Randomized Trial of Surgery in the Treatment of Single Metastases to the Brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef]
- Coster, J.N.; Groth, S.S. Surgery for Locally Advanced and Oligometastatic Non–Small Cell Lung Cancer. Surg. Oncol. Clin. N. Am. 2020, 29, 543–554. [Google Scholar] [CrossRef]
- Raz, D.J.; Lanuti, M.; Gaissert, H.C.; Wright, C.D.; Mathisen, D.J.; Wain, J.C. Outcomes of Patients With Isolated Adrenal Metastasis From Non-Small Cell Lung Carcinoma. Ann. Thorac. Surg. 2011, 92, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Bertolaccini, L.; Sedda, G.; Petrella, F.; Galetta, D.; Guarize, J.; Maisonneuve, P.; De Marinis, F.; Spaggiari, L. Lung cancer surgery in oligometastatic patients: Outcome and survival. Eur. J. Cardio-Thorac. Surg. 2020, 57, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Salah, S.; Tanvetyanon, T.; Abbasi, S. Metastatectomy for extra-cranial extra-adrenal non-small cell lung cancer solitary metastases: Systematic review and analysis of reported cases. Lung Cancer 2012, 75, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Billing, P.S.; Miller, D.L.; Allen, M.S.; Deschamps, C.; Trastek, V.F.; Pairolero, P.C. Surgical treatment of primary lung cancer with synchronous brain metastases. J. Thorac. Cardiovasc. Surg. 2001, 122, 548–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curioni-Fontecedro, A.; Ickenberg, C.; Franzen, D.; Rogler, G.; Burger, I.A.; Broek, M.V.D. Diffuse pseudoprogression in a patient with metastatic non-small-cell lung cancer treated with Nivolumab. Ann. Oncol. 2017, 28, 2040–2041. [Google Scholar] [CrossRef] [PubMed]
- Isbell, J.M.; Li, B.T.; Gomez, D.R. The emerging role of local therapy in oligometastatic non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2021. [Google Scholar] [CrossRef]
- Tanvetyanon, T.; Robinson, L.A.; Schell, M.J.; Strong, V.E.; Kapoor, R.; Coit, D.G.; Bepler, G. Outcomes of Adrenalectomy for Isolated Synchronous Versus Metachronous Adrenal Metastases in Non–Small-Cell Lung Cancer: A Systematic Review and Pooled Analysis. J. Clin. Oncol. 2008, 26, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Ampil, F.; Caldito, G.; Milligan, S.; Mills, G.; Nanda, A. The elderly with synchronous non-small cell lung cancer and solitary brain metastasis: Does palliative thoracic radiotherapy have a useful role? Lung Cancer 2007, 57, 60–65. [Google Scholar] [CrossRef]
- Frost, N.; Tessmer, A.; Schmittel, A.; van Laak, V.; Raspe, M.; Ruwwe-Glösenkamp, C.; Brunn, M.; Senger, C.; Böhmer, D.; Ochsenreither, S.; et al. Local ablative treatment for synchronous single organ oligometastatic lung cancer—A propensity score analysis of 180 patients. Lung Cancer 2018, 125, 164–173. [Google Scholar] [CrossRef] [PubMed]
- West, H. Management of Oligometastatic Disease in Advanced Non–Small Cell Lung Cancer. Clin. Chest Med. 2020, 41, 249–258. [Google Scholar] [CrossRef]
- Weickhardt, A.J.; Scheier, B.; Burke, J.M.; Gan, G.; Lu, X.; Bunn, P.A.; Aisner, D.L.; Gaspar, L.E.; Kavanagh, B.D.; Doebele, R.C.; et al. Local Ablative Therapy of Oligoprogressive Disease Prolongs Disease Control by Tyrosine Kinase Inhibitors in Oncogene-Addicted Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2012, 7, 1807–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.A.; Sima, C.S.; Huang, J.; Solomon, S.B.; Rimner, A.; Paik, P.; Pietanza, M.C.; Azzoli, C.G.; Rizvi, N.A.; Krug, L.M.; et al. Local Therapy with Continued EGFR Tyrosine Kinase Inhibitor Therapy as a Treatment Strategy in EGFR-Mutant Advanced Lung Cancers That Have Developed Acquired Resistance to EGFR Tyrosine Kinase Inhibitors. J. Thorac. Oncol. 2013, 8, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Belluomini, L.; Dodi, A.; Caldart, A.; Kadrija, D.; Sposito, M.; Casali, M.; Sartori, G.; Ferrara, M.G.; Avancini, A.; Bria, E.; et al. A narrative review on tumor microenvironment in oligometastatic and oligoprogressive non-small cell lung cancer: A lot remains to be done. Transl. Lung Cancer Res. 2021, 10, 3369–3384. [Google Scholar] [CrossRef]
- Bertolaccini, L.; Casiraghi, M.; Sedda, G.; de Marinis, F.; Spaggiari, L. Clinical prognostic factors in surgically treated oligometastatic non-small cell lung cancer: A systematic review. Transl. Lung Cancer Res. 2021, 10, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Lussier, Y.A.; Xing, H.R.; Salama, J.K.; Khodarev, N.N.; Huang, Y.; Zhang, Q.; Khan, S.A.; Yang, X.; Hasselle, M.D.; Darga, T.E.; et al. MicroRNA expression characterizes oligometastasis(es). PLoS ONE 2011, 6, e28650. [Google Scholar] [CrossRef]
- Lussier, Y.A.; Khodarev, N.N.; Regan, K.; Corbin, K.; Li, H.; Ganai, S.; Khan, S.A.; Gnerlich, J.; Darga, T.E.; Fan, H.; et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS ONE 2012, 7, e50141. [Google Scholar] [CrossRef] [Green Version]
- Uppal, A.; Ferguson, M.K.; Posner, M.C.; Hellman, S.; Khodarev, N.N.; Weichselbaum, R.R. Towards a molecular basis of oligometastatic disease: Potential role of micro-RNAs. Clin. Exp. Metastasis 2014, 31, 735–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleijnen, S.; Alves, T.L.; Meijboom, K.; Lipska, I.; De Boer, A.; Leufkens, H.G.; Goettsch, W.G. The impact of quality-of-life data in relative effectiveness assessments of new anti-cancer drugs in European countries. Qual. Life Res. 2017, 26, 2479–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.; Hurst, N.; Movsas, B. The State of the Science in Patient-Reported Outcomes for Patients with Lung Cancer. Semin. Respir. Crit. Care Med. 2020, 41, 377–385. [Google Scholar] [CrossRef]
- Ben Bouazza, Y.; Chiairi, I.; El Kharbouchi, O.; De Backer, L.; Vanhoutte, G.; Janssens, A.; van Meerbeeck, J. Patient-reported outcome measures (PROMs) in the management of lung cancer: A systematic review. Lung Cancer 2017, 113, 140–151. [Google Scholar] [CrossRef]
- EuroQol, G. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy (Amsterdam, Netherlands) 1990, 16, 199–208. [Google Scholar]
- Pompili, C.; Koller, M.; Velikova, G. Choosing the right survey: The lung cancer surgery. J. Thorac. Dis. 2020, 12, 6892–6901. [Google Scholar] [CrossRef]
- Giesinger, J.M.; Efficace, F.; Aaronson, N.; Calvert, M.; Kyte, D.; Cottone, F.; Cella, D.; Gamper, E.-M. Past and Current Practice of Patient-Reported Outcome Measurement in Randomized Cancer Clinical Trials: A Systematic Review. Value Health 2021, 24, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Moloczij, N.; Gough, K.; Solomon, B.; Ball, D.; Mileshkin, L.; Duffy, M.; Krishnasamy, M. Development of a hospital-based patient-reported outcome framework for lung cancer patients: A study protocol. Health Qual. Life Outcomes 2018, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Sui, X.; Song, F.; Li, Y.; Li, K.; Chen, Z.; Yang, F.; Chen, X.; Zhang, Y.; Wang, X.; et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat. Commun. 2021, 12, 2581. [Google Scholar] [CrossRef] [PubMed]
| Study Abbreviation | ClinicalTrails.gov Identifier | Phase | Setting | Type of Systemic Treatment | Type of LAT | Timing of LAT | n | No. of Metastases | Primary End Points | Planned Completion |
|---|---|---|---|---|---|---|---|---|---|---|
| 14-18 CHESS | NCT03965468 | II | Synchronous oligometastatic NSCLC | Durvalumab, Carboplatin, Paclitaxel | Primary: Surgery or radical radiotherapyMetastases: SBRT | Neoadjuvant systemic treatment | 47 | Max. 3 | PFS | 12/2021 |
| OMEGA | NCT03827577 | III | Oligometastatic NSCLC | Standard medical therapy | Surgery, Radiotherapy, RFA | Neoadjuvant systemic treatment or primary LAT | 195 | Max. 3 | OS | 09/2022 |
| n/a | NCT02759835 | II | EGFR-mutated OPD NSCLC | Osimertinib | Surgery, SBRT, radiofrequency ablation | LAT after oligoprogression under first-lineOsimertinib | 37 | n/a | PFS | 09/2022 |
| n/a | NCT02316002 | II | Oligometastatic NSCLC | Adjuvant Pembrolizumab | Completed first-line treatment (surgery, SBRT, radiotherapy, chemotherapy) | Any first-line treatment followed by adjuvant pembrolizumab | 51 | n/a | PFS | 09/2022 |
| LONESTAR | NCT03391869 | III | Stage IV NSCLC (incl. OMD subgroup) | Nivolumab and ipilimumab | Surgery, radiotherapy | Combined neoadjuvant and adjuvant immunotherapy | 270 | n/a | OS | 12/2022 |
| NORTHSTAR | NCT03410043 | II | EGFR-mutatedStage IIIB or IV NSCLC (incl. OMD subgroup) | Osimertinib | Surgery, radiotherapy | Combined neoadjuvant and adjuvant Osimertinib | 143 | n/a | PFS | 01/2023 |
| LAT-FLOSI | NCT04216121 | IIb | EGFR-mutated OPD NSCLC | Osimertinib | Surgery, SBRT | LAT after oligoprogression under first-lineOsimertinib | 39 | Max. 3 | PFS | 08/2023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, R.S.; Opitz, I. Patient Selection for Local Aggressive Treatment in Oligometastatic Non-Small Cell Lung Cancer. Cancers 2021, 13, 6374. https://doi.org/10.3390/cancers13246374
Werner RS, Opitz I. Patient Selection for Local Aggressive Treatment in Oligometastatic Non-Small Cell Lung Cancer. Cancers. 2021; 13(24):6374. https://doi.org/10.3390/cancers13246374
Chicago/Turabian StyleWerner, Raphael S., and Isabelle Opitz. 2021. "Patient Selection for Local Aggressive Treatment in Oligometastatic Non-Small Cell Lung Cancer" Cancers 13, no. 24: 6374. https://doi.org/10.3390/cancers13246374
APA StyleWerner, R. S., & Opitz, I. (2021). Patient Selection for Local Aggressive Treatment in Oligometastatic Non-Small Cell Lung Cancer. Cancers, 13(24), 6374. https://doi.org/10.3390/cancers13246374






