Simple Summary
As the number of patients with cancer continues to rise globally, developing methods to support these patients is important for providing high-quality care. The aim of our systematic review is to describe and examine the use of text-based communications in cancer supportive care. We identified 18 studies suitable for inclusion in our review. Overall, patients were satisfied with using text-based communication in their cancer care. Results were inconsistent within other outcome categories; however, specific interventions yielded some positive results such as the use of reminders for promoting medication adherence. While the results of this review should not be considered conclusive, it illustrates that further research on the use of text-based communications in cancer-supportive care is warranted.
Abstract
The global cancer disease burden is substantial, resulting in increased economic and clinical strain on our healthcare systems. A proposed solution is text-based communication, which can be used for cancer-supportive care. We conducted a systematic review to synthesize and describe the use of text-based communications for cancer-supportive care. Our population of interest included adult patients with cancer. A total of 18 studies were included in the review: 9 RCTs and 9 non-randomized interventional/observational studies. Patients were largely satisfied with text-based communication during their cancer care. Compared to controls, results for other outcomes including symptoms and quality of life were largely mixed; however, no harms were observed. Furthermore, positive outcomes were seen for specific interventions, such as text message medication reminders. These findings should be considered with caution due to the considerable heterogeneity observed between studies regarding their design and reported outcomes and the high risk of bias associated with 6/18 studies. Overall, this review suggests that text-based communication may be a complementary tool for cancer-supportive care; however, more research is needed to examine the feasibility of implementation and use.
1. Introduction
As the global cancer incidence continues to rise, so has the need for supportive services. Acting as an integral component of cancer care, supportive care aims to provide relief from side effects and aid in care management [1]. Patients with advanced cancer experience high symptom burden, which is associated with prolonged hospital stays and unplanned hospital readmissions [2]. Supportive care services for patients with cancer is a substantial healthcare cost [3]. The annual excess economic burden of cancer survivorship ranges from $4427 USD to $16,441 USD per survivor [4]. Given the considerable clinical and economic burden of caring for those with cancer, it is important to develop cost-effective and efficient supportive care strategies.
Digital health interventions facilitate independence and provide reassurance to patients with cancer [5]. Literature suggests that text-based interventions may be a promising alternative or addition to more traditional telehealth methods, such as phone call and internet-based interventions [6]. They have been shown to facilitate patient–provider communication, enhance adherence via medication reminders [7], educate and motivate patients in the self-management of their care, and simplify patient data collection [8,9]. Specifically, among patients with cancer, text-based communication has been found to improve patient communication and self-management of their care [10].
Text-based communication can complement other internet-based communication methods [11]. Employing internet-based communication as a sole method of communication may exacerbate quality of care inequities, as many patients with cancer have limited Internet access [12]. The World Internet Project, a collaborative research program that studies the impact of digital technology, reported wide disparities in Internet usage based on income levels in almost all countries [13].
The use of text-based communication is a relatively new mode of supporting cancer patients. While the literature suggests these interventions may be cost-effective and aid in patient-centered care, much of the available evidence is not disease-specific [9,14,15]. Previous reviews have examined text-based communication as a component of or under the umbrella of digital health or mHealth [8,16,17], or have examined text-based communication interventions in patients with chronic disease as a whole [15,18,19]. Reviews investigating the effects of text-message interventions specifically among patients with cancer are limited, and a full scope of patient-related outcomes has yet to be investigated. Therefore, we conducted a systematic review aimed at identifying and describing the use of text-based communications to provide supportive care for cancer patients.
2. Materials and Methods
We conducted a systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [20].
2.1. Eligibility Criteria
Our review included primary research studies (clinical trials and observational studies; all study designs) with an available full text and published in the English language. Conference abstracts (i.e., no available full text), reviews, case studies, case series, and commentaries/opinions were excluded. Our population of interest was adult patients (age ≥18 years) with diagnosed cancer. Our intervention of interest was use of text-based communication to assist in cancer-supportive care [1]. Studies that included text-based communication as part of a multi-modal intervention or those requiring internet were not included as we were unable to discern the individual effects of text-based communication. The primary outcome was cancer symptom control. Secondary outcomes included quality of life (defined as a multidimensional phenomenon made up of multiple domains including mental and physical health, physical activity, diet, etc., [21]), feasibility (e.g., adherence, implementation), patient satisfaction (e.g., patient opinions of the intervention including overall satisfaction, helpfulness, usefulness), and barriers to use. Both quantitative and qualitative outcomes were included. Table S1 contains detailed inclusion and exclusion criteria.
2.2. Search Strategy
Electronic searches were performed in MEDLINE, EMBASE, and CINAHL from January 2015 to November 2020. A previous study [22] surveyed literature up to 2015 on mobile technologies for cancer supportive care. Since the technology and utility of text-based communication has substantially changed in recent years, we chose to evaluate the studies published since 2015 making our results applicable to the current context. Table S2 contains the full search strategy and number of results corresponding to each database.
2.3. Data Extraction and Outcome Measures
The Covidence platform [23] was used to organize and screen all the studies identified by the search. Search results from all three databases were aggregated and duplicates were removed. Two independent reviewers (MB, IS) screened the studies at the title/abstract level for inclusion. The remaining studies were then assessed by the two reviewers for eligibility at the full-text level. Any studies deemed ineligible were excluded based on the following hierarchy: study design, population, exposure/intervention, and outcomes. Conflicts between the reviewers were resolved through mutual discussion and agreement, or, if no consensus was reached, by a third arbitrator (DTW/BG).
Upon finalizing the included studies, the two reviewers (MB, IS) extracted relevant information using a predeveloped Microsoft Excel form. Detailed information was collected on the study design, location, population, baseline characteristics, intervention, control, and outcomes.
2.4. Bias Assessment
Bias assessments were performed on each study by two independent reviewers (MB, IS). The Cochrane Risk of Bias Tool [24], a 7-item tool that judges biases related to selection, performance, detection, attrition, reporting, and others, was used to assess the randomized trials. Each of the 7 items was scored on a 2-point scale, where a score of 2 represents low risk of bias, 1 represents an unclear risk of bias, and 0 represents high risk of bias. The individual item scores were then summed; a total score of 8 or higher was considered low risk of bias and a score below 8 was considered high risk of bias. The Risk Of Bias In Non-randomized Studies-of Interventions (ROBINS-I) tool [25] was used to assess the non-randomized interventional/observational studies. This is a 7-item tool that judges biases related to confounding, selection, intervention classification and adherence, missing data, and outcome measurement and reporting. Biases corresponding to each of the 7 items were rated as low, moderate, severe, critical, or no information and an overall score was also given.
3. Results
3.1. Overview
The electronic database search identified a total of 4449 studies, out of which 18 studies involving 5821 patients were included in the final qualitative synthesis (Figure 1). Of the included studies, 9 (50%) were randomized trials [26,27,28,29,30,31,32,33,34] and 9 (50%) were non-randomized interventional/observational studies [11,35,36,37,38,39,40,41,42] (Table 1, Table S3). Sixteen of 18 studies (89%) were conducted in high-income countries (HICs) [11,26,27,28,29,31,32,33,34,35,36,37,38,40,41,42], the majority (n = 11) in the United States [11,26,28,29,33,34,35,36,38,40,41]. Two studies (11%) were conducted in low- and middle-income countries (LMICs), both in Brazil [30,39].
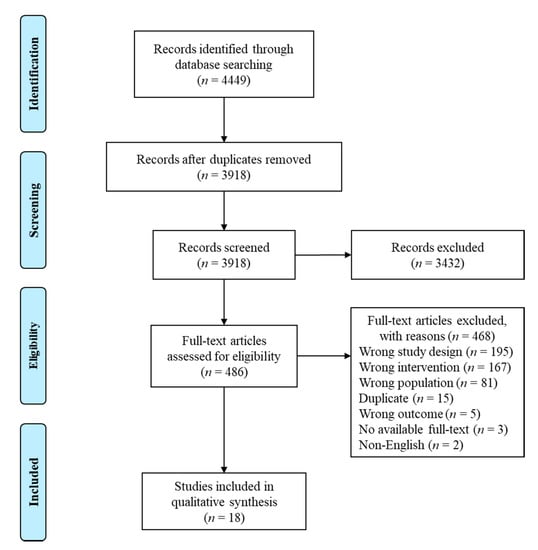
Figure 1.
PRISMA flow diagram.

Table 1.
Study characteristics (n = 18 studies).
Ten studies included patients with any cancer [26,27,30,32,33,34,36,39,41,42], while the remaining eight focused on specific cancer types (breast n = 4 [11,29,31,38], lung n = 2 [35,37], endometrial n = 1 [28], chronic myeloid leukemia n = 1 [40]) as seen in Table 1. One study included patients with late-stage cancer only (stage III–IV lung) [35] and three studies included patients with early stage cancer only [11,29,38], while the remainder did not use staging as part of their inclusion criteria. Further, 13 studies focused on patients in periods of active treatment [11,29,30,31,32,33,34,36,37,38,39,40,41], while 4 studies focused on patients during their survivorship phase [26,27,28,42] and 1 did not specify [35]. The total sample sizes at baseline ranged from 14 [39] to 3429 participants [41].
Sixteen studies assessed text-based communication interventions used solely for supportive care purposes, such as medication adherence, appointment reminders, weight loss, physical activity motivation, and self-management education [11,26,27,28,29,30,31,32,33,34,35,36,39,40,41,42]. The remaining two studies used text-based communication for supportive care as well as for symptom monitoring (i.e., tracking symptoms experienced by patients) [37,38]. The most common message frequency was daily (n = 7) [11,30,33,34,38,39,41], followed by weekly (n = 3) [31,32,36], multiple times per week (n = 3) [27,29,42], and multiple times per day (n = 3) [28,35,37]. Two studies had no set message frequency [26,40]. Text-based communication was initiated by the provider in 16 studies [11,27,28,29,30,31,32,33,34,35,36,38,39,40,41,42] and by the patient in two studies [26,37]. Thirty-nine percent (n = 7) of studies sent messages that were personalized to the individual patient [26,27,28,35,37,40,41], while the remainder used non-personalized messages [11,29,30,31,32,33,34,36,38,39,42]. Eleven studies used unidirectional text-based communication (provider to patient) [11,29,30,31,32,33,34,35,39,41,42], six used bidirectional communication (provider to patient and patient to provider) [26,27,28,37,38,40], and the remainder used text-based communication to initiate an additional form of communication (phone call, mobile app, patient portal, etc.,) [36]. Of the eight studies that reported the language used for text messaging, four [26,28,33,34] used English, two [30,39] used Portuguese, one [32] used French, and one [31] had the option of either English, Mandarin, or Malay. Study follow-up duration ranged from 1 month [35,36,37,39,42] to 3 years [29]. The following sections discuss study findings within five main outcome categories: patient satisfaction, barriers to use, symptoms, QoL, and feasibility.
3.2. Risk of Bias
Seven of the nine randomized trials were classified as low risk of bias (LRB), while the remaining two were high risk of bias (HRB) (Figure S1, Table S4). Of the nine non-randomized interventional/observational studies, six reported a serious risk of bias (some important problems exist), two reported not enough information to judge level of bias, and the final study reported moderate risk of bias (sound for a non-randomized study, but not comparable to a well-performed randomized trial) (Figure S2, Table S5). Overall, a large portion of the included studies were at a high risk of bias and should be considered cautiously. To provide context for readers interpreting our findings, we have denoted the level of bias as either low risk (LRB) or high risk (HRB) throughout the subsequent results sections.
3.3. Patient Satisfaction
Twelve studies (67%) reported outcomes related to patient satisfaction (Table 2). Overall, patient satisfaction with the text-based interventions was high. For example, Bade et al. [35] reported that 92% of patients found a twice-daily motivational text communication service helpful for improving their physical activity (HRB). In a pilot study of text messages for self-care and emotional support (sent using the cHEmotHErAPP), Rico et al. [39] reported that all patients felt they could better cope with their cancer treatment through feelings of increased confidence and knowledge (LRB). Similarly, a two-armed, prospective, single-center RCT of the same intervention reported a patient satisfaction rate of 100% in the intervention group, where 72% were very satisfied and 28% were satisfied (HRB) [30]. Krok-Schoen and colleagues [11] conducted a pre-post study of daily text message reminders to improve hormone therapy adherence among breast cancer patients and found that 97% had a positive experience (HRB).

Table 2.
Patient satisfaction (n = 12 studies) and barrier (n = 7 studies) outcomes.
3.4. Barriers to the Use of Text-Based Communication
Seven studies (39%) reported barriers to the use of text-based communication (Table 2). Three of those studies [11,27,38] reported barriers to text-based communication adherence, which included scheduling conflicts, forgetfulness, feeling unwell, not liking the language used in the messages, and not feeling well enough to continue without the messages [11,27]. The study by Mougalian and colleagues [38] was the only study to document cost-related outcomes, where over 70% of patients had no financial impact associated with the text-based communications (HRB). Three studies [33,34,37] identified technological barriers to completing the intervention, and overall reported few technological problems. For example, Spoelstra et al. [34] found that just 5.3% of patients encountered problems with the medication adherence text messaging system in their two-armed, prospective, multi-center RCT (LRB). Lastly, two studies [37,42] reported barriers to study recruitment, which included poor health status, lack of familiarity/confidence with the technology, and perceived lack of need for additional supportive interventions (HRB, HRB respectively).
3.5. Symptom Outcomes
Six studies (33%) examined symptoms (Table 3). Overall, results for composite symptom scores were mixed while symptom-specific results were not significantly different between study groups. In terms of composite scores, Rico et al. [30] found that the total number of side effects from cycle one of chemotherapy was significantly lower in the text-based communication group compared to those receiving standard care (number of patients experiencing 4–14 side effects in intervention (I) vs. control (C) groups: n = 28 vs. 42, p = 0.05, HRB), but this was not seen in the following two chemotherapy cycles (cycle 2: p = 0.4, cycle 3: p = 0.4). Conversely, Krok-Schoen et al. [11] found no significant difference in mean Breast Cancer Prevention Trial Symptom Checklist score post treatment with text-based communication vs. baseline (mean difference (MD) (post-baseline) 0.04, 95%CI -0.06 to 0.14, p = 0.412, HRB). Despite the heterogeneity between studies, positive results were found in specific settings such as Spoelstra et al. [33]. This study [33] was a two-armed, prospective, multi-center RCT examining a population of patients with various forms of cancer and newly prescribed oral anticancer medication. A significant reduction in mean total number of symptoms was reported among those receiving daily medication adherence text messages compared to those who did not (I vs. C mean 3.9 SD 0.5 vs. 5. SD 0.46, p = 0.04, LRB).

Table 3.
Symptom (n = 6 studies) and quality of life (n = 10 studies) outcomes.
Among symptom-specific results, Krok-Schoen et al. [11] found no significant change in mean Brief Pain Inventory Score (MD (I–C) 0.8, 95% CI −0.4 to 2.02, p = 0.2) or mean Fatigue Symptom Inventory (MD (I–C) −0.3, 95% CI −0.8 to 0.3, p = 0.3) from post-treatment vs. baseline (HRB).
3.6. Quality of Life Outcomes
A total of ten studies (56%) examined the QoL outcomes (Table 3). Of these, six studies [11,27,28,32,35,37] examined the physical health outcomes including step counts, physical activity change, physical fatigue, and self-care change. Of the two studies that reported step counts one found no consistent change in steps in the text-based communication vs.no text communication groups over an 8-week period (HRB) [32], while the other found that mean daily steps significantly increased for both the text-based communication and weekly phone call groups over a 3 week period compared to controls (effect sizes 0.02 and 0.05, respectively, HRB) [35]. Physical activity change was reported by two studies and mixed results were found. In their single-center prospective RCT, Gomersall et al. [27] reported an increase in physical activity among those receiving text-based communication versus those undergoing a 4-week exercise rehabilitation without texts (mean time light stepping, change score MD (I–C) 6.9, 95% CI 0.8, 12.9, p = 0.03, LRB). Physical component scores from various questionnaires (Functional Assessment of Cancer Therapy-Lung (FACT-L), Short Form Health Survey (SF-8), Quality of Life Questionnaire (QLQ-30), Patient-Reported Outcomes Measurement Information System (PROMIS)) reported mixed results. Krok-Schoen et al. [11] found that physical health, as reported by the SF-8, was better at post-treatment vs.baseline, though this difference was not significant (MD (I–C) 0.95, 95% CI −1.71 to 3.61, p = 0.473, HRB). In their single-center RCT, Villaron et al. [32] reported significant results for the physical capacity of the QLQ-30, finding increased capacity among those receiving text-based communication vs.no text messages. However, their results were significant only within 7 weeks of follow-up (I vs. C: mean 88.3 SD 13.5 vs. 75.5 SD 18.9, p < 0.01, HRB).
Seven [11,32,33,34,36,37,42] studies looked at an array of mental health outcomes including depression, anxiety, motivation, and emotional well-being (Table 3). Depression was the most highly reported outcome (33% [11,33,34,36,37,42] studies), and results were varied. In their multi-center RCTs, Spoelstra et al. [34] and Spoelstra et al. [33] both found no significant difference in PROMIS depression scores among those receiving text-based communication vs.standard care (p = 0.8, p = 0.9, respectively, LRB). Krok-Schoen and colleagues [11] found those who received texts to prompt medication adherence had lower Center of Epidemiology Study-Depression (CESD-D) score compared to baseline scores (MD −1.2, 95% CI −3.5, 1.01, p = 0.3, HRB). Similar results were found with anxiety outcomes; Wells et al. [42] reported a trend for greater improvement in anxiety among those receiving text-based communication to support mindfulness-based cognitive therapy (MBCT) (p < 0.001, HRB), however this did not significantly differ from those receiving MBCT without the additional supportive texts.
Among patients undergoing outpatient chemotherapy or systemic treatment for various forms of cancer, Villaron et al. [32]’s RCT found significantly lower decline in motivation (I vs. C week 8 mean 7.88 SD 3.74 vs. 10.73 SD 3.87 p < 0.05), activity (I vs. C week 8 mean 8.24 SD 4.09 vs. 11.82 SD 4.16 p < 0.01), and both mental (I vs. C week 8 mean 6.94 SD 3.77 vs. 9.95 SD 4.19 p < 0.05), and physical functioning (I vs. C week 8 mean 9.76 SD 4.63 vs. 12.27 SD 4.63 p < 0.05) among those receiving text-based communication that provided motivation to encourage physical activity vs. those receiving no text messages (HRB).
3.7. Feasibility and Implementation Outcomes
Sixteen studies (89%) examined feasibility or implementation outcomes (Table 4). A variety of feasibility outcomes were reported, including adherence to the intervention and medications, number of text messages sent, text-message response rate, and odds of completing the intervention. Seven studies [11,29,31,34,38,40] reported adherence to prescribed medications or therapy, with mixed results. Three studies reported an adherence rate > 50% [31,38,40] (HRB, HRB, LRB, respectively) among those receiving text-based communication. Conversely, one study found adherence of <50% [29] among those receiving text-based communication (LRB). Those that examined the medication adherence between study groups found mixed results. One study [11] found control groups not receiving texts to have higher adherence scores compared to the text-message intervention group (p < 0.05, HRB) while two studies [31,40] found that the text-based communication groups had higher adherence levels (p < 0.05, HRB).

Table 4.
Feasibility and implementation outcomes (n = 16 studies).
On a systems level, number of text messages sent was the most ubiquitous feasibility outcome [27,30,33,38]. Number of text messages sent ranged from 52 messages over 3 cycles of chemotherapy (LRB) [30] to 21 messages sent per week (HRB) [38]. Outcomes such as text message response rates and number of alerts generated were reported by fewer studies. Text message response rate ranged from 40% (HRB) [40] to 86.1% (HRB) [38] among studies in which it was reported [27,38,40].
4. Discussion
In this systematic review of text-based communications in cancer-supportive care, we find that text-based communication tools are a potentially feasible intervention that tends to improve patient satisfaction, with heterogenous but no detrimental effect on symptom or QoL outcomes. However, the majority of studies included in this review were judged to be at a high risk of bias, suggesting that our results should be interpreted with caution. These findings help establish the role of text-based communication in cancer-supportive care and provide a benchmark for implementation in cancer-care facilities. They also highlight the need for future, well-designed studies.
Text-based communication is a pragmatic virtual care tool, enabling real-time bidirectional communication between provider and patient at a low cost and in an accessible format [43,44]. Amid heterogeneous results, we identified potential support for the use of text-based interventions within specific settings. For example, providing a specific call to action (e.g., for medication adherence or appointment attendance) via text-based communication may be successful in producing the desired behavior change [31,40,41,42], thereby potentially translating to improved clinical outcomes [33,34]. Similar text-driven behavioral changes have been seen among other chronic disease populations. A meta-analysis looking specifically at text-messaging for medication adherence in chronic diseases reported a significant improvement in medication adherence compared to those without text-messages [7]. Our findings also suggest that when interventions facilitate self-management, improvements in fatigue and QoL outcomes can occur. This may be due to the nature of self-management interventions, which usually include an information-based component aimed at building self-efficacy, known as a key causal mechanism for behavior change and improved health outcomes [45]. Notably, many of these positive outcomes were seen in the RCTs as opposed to the non-RCTs, indicating a higher quality of evidence in support of text-based communication feasibility.
Our review suggests that text-based communication interventions were consistently associated with high levels of patient satisfaction and produced no harmful effects. The majority of studies reported positive patient satisfaction outcomes such as improved self-management skills, increased confidence and knowledge, and general feelings of positivity. Further, patients reported that text-based communications were helpful and easy-to-understand. These findings align with the existing research; a recent systematic review of telehealth interventions found that patient satisfaction outcomes were generally positive [46]. Notably, only one included study assessed intervention costs, showing no added cost to the patient with text-based communication [38]. This was unsurprising, given that the literature is sparse regarding cost-effectiveness of text-based interventions [44,47]. While more research is needed, high patient satisfaction suggests that text-based interventions are well-accepted and may help to reduce some of the patient-perceived barriers to care.
We observed an overall lack of evidence for understanding the real-world effectiveness of text-based communication. Although 89% of included studies reported on feasibility, the included outcomes were highly heterogeneous and few assessed the practicality of implementing this technology. Thus, future studies should utilize standardized methods for study design and outcome reporting, facilitating inter-study comparison. Researchers should consider the use of the Research, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework to identify meaningful study outcomes for determining the real-world success of text-based interventions [48]. When studying text-based communications, it is important to understand factors affecting their uptake. Therefore, we also propose the use of effectiveness-implementation hybrid study designs, which facilitate timely adoption of a given clinical intervention through the simultaneous study of both its effectiveness and uptake [49]. Moving forward, these tools will help guide researchers in identifying meaningful outcomes for studying text-based communications in cancer-supportive care.
Our review identified a clear lack of evidence from LMICs. Of the 18 included studies, only 2 (11%) took place in a single LMIC (Brazil, an upper-middle-income country). Similarly, in their review of mobile technologies in cancer care, Nasi and colleagues [22] identified a total of 106 studies, of which only 13 (12%) focused on LMICs. Comparable trends also exist in the broader cancer literature. A retrospective analysis of all phase 3 treatment studies published from 2014–2017 (n = 694 RCTs) found that just 8% were led by LMIC investigators [50]. LMICs experience high cancer incidence as well as disproportionately high mortality [51]. For example, in 2020 Asia reported 49.3% of global cancer cases and a disproportionate 58.3% of deaths [52]. As of 2015, approximately 8 in 10 individuals in developing countries owned a mobile phone [53]. Thus, future research efforts should focus on testing the feasibility, acceptability, and effectiveness of text-based communication in LMICs.
In the context of the pandemic, the use of telemedicine is gaining global popularity globally including in LMICs [54]. This study captured both high-quality RCTs and real-world observational studies. Although none of the studies included in our review were conducted in the context of the pandemic, this review will provide a benchmark for better integrating supportive care for cancer patients in HICs and LMICs using a text-based communication both during and after the pandemic.
Strengths and Limitations
Our review captured both interventional and observational study designs. While interventional studies provide a higher standard of evidence, observational studies are useful for understanding the long-term and real-world outcomes. Additionally, our review focused on studies published in the past 5 years making our results timely and topical. We also performed a bias assessment of the included studies using validated risk of bias tools [24,25]. This allowed us to identify those studies at a high risk of bias and interpret their findings accordingly. Lastly, the potential for selection bias was reduced due to a rigorous study screening process.
A limitation of this review was the substantial heterogeneity observed between studies regarding their population of interest, intervention design, outcome reporting, and follow-up duration. This heterogeneity may be due, in part, to the variety of phases in the healthcare process captured by this review. As demonstrated by Nasi et al. [22], text-based interventions can be employed at any phase of cancer care. Our review focused on the treatment and follow-up periods, two phases that tend to highlight different outcome types. Methodological standardization using tools such as the effectiveness-implementation study design and RE-AIM framework may help improve comparability of future works. In addition, several included studies were likely underpowered, with a limited ability to detect differences between treatment groups. Six non-randomized interventional studies were identified serious risk of bias and two RCTs were identified as high risk of bias. Including outcomes reported by these studies regarding the feasibility and effectiveness of text-based communications may have prevented accurate conclusions from being drawn. As most studies included had short follow-up periods, the findings of this review cannot speak for long-term effects of text-based communication. Additionally, the needs of patients with chronic diseases vary greatly, limiting to the generalizability of these results beyond patients with cancer. Lastly, for feasibility reasons our search was restricted to studies written in the English language and we did not conduct a search for grey literature. This may have limited our search and excluded relevant studies from inclusion.
5. Conclusions
In conclusion, text-based intervention in cancer-supportive care trends toward high levels of patient satisfaction, suggesting that this may be an acceptable tool for augmenting supportive care. Although findings were inconsistent regarding the effects of text-based communications on patient symptoms, QoL, and feasibility/implementation, we observed the potential success of text-based communication within specific settings. More research is needed to identify what is needed to achieve a successful text-based intervention. Given its low costs and more equitable coverage, text-based communication has the potential to improve global cancer care.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13143542/s1. Figure S1: Bias assessment of the randomized controlled trials (n = 9). Figure S2: Bias assessment of the non-randomized interventional/observational studies (n = 9). Table S1: Study inclusion and exclusion criteria. Table S2: Search strategy for included databases. Table S3: Detailed characteristics and outcomes of included studies (n = 18). Table S4: Cochrane Risk of Bias table for the randomized controlled trials (n = 9). A score of 0 represents high risk of bias, 1 represents unclear risk of bias, and 2 represents low risk of bias. Table S5: Risk of Bias in Non-randomized Studies-of Interventions (ROBINS-I) table for the non-randomized interventional/observational studies (n = 9).
Author Contributions
Conceptualization, D.T.W., M.B., I.S., S.S. and B.G.; methodology, D.T.W., M.J., M.B. and I.S.; validation, D.T.W., M.B., I.S. and B.G.; investigation, D.T.W., M.B., I.S. and B.G.; resources, D.T.W. and S.S.; data curation, M.B. and I.S.; writing—original draft preparation, M.B. and I.S.; writing—review and editing, D.T.W., S.S., M.J. and B.G.; visualization, M.B., I.S.; supervision, D.T.W. and B.G.; funding acquisition, D.T.W. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by a Southeastern Ontario Academic Medical Organization (SEAMO) Innovation Grant (Don Thiwanka Wijeratne, Siddhartha Srivastava). Bishal Gyawali gratefully acknowledges salary support from Ontario Institute of Cancer Research through funding provided by the Government of Ontario. Additionally, Don Thiwanka Wijeratne received funding from Ontario Drug Policy Research Network and Canadian Institute of Health Research, Cardiovascular Network.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Society, C.C. Supportive Care. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/brain-spinal-childhood/supportive-care/?region=on (accessed on 3 May 2021).
- Nipp, R.D.; El-Jawahri, A.; Moran, S.M.; D’Arpino, S.M.; Johnson, P.C.; Lage, D.E.; Wong, R.L.; Pirl, W.F.; Traeger, L.; Lennes, I.T. The relationship between physical and psychological symptoms and health care utilization in hospitalized patients with advanced cancer. Cancer 2017, 123, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Lentz, R.; Benson, A.B., III; Kircher, S. Financial toxicity in cancer care: Prevalence, causes, consequences, and reduction strategies. J. Surg. Oncol. 2019, 120, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P., Jr.; Ekwueme, D.U.; Yabroff, K.R.; Dowling, E.C.; Li, C.; Rodriguez, J.L.; de Moor, J.S.; Virgo, K.S. Economic burden of cancer survivorship among adults in the United States. J. Clin. Oncol. 2013, 31, 3749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, A.; Lucas, G.; Marcu, A.; Piano, M.; Grosvenor, W.; Mold, F.; Maguire, R.; Ream, E. Cancer Survivors’ Experience With Telehealth: A Systematic Review and Thematic Synthesis. J. Med. Internet Res. 2017, 19, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kuerbis, A.; Mulliken, A.; Muench, F.; Moore, A.A.; Gardner, D. Older adults and mobile technology: Factors that enhance and inhibit utilization in the context of behavioral health. Ment. Health Addict. Res. 2017, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, J.; Kurup, R.; Laba, T.-L.; Santo, K.; Thiagalingam, A.; Rodgers, A.; Woodward, M.; Redfern, J.; Chow, C.K. Mobile telephone text messaging for medication adherence in chronic disease: A meta-analysis. JAMA Intern. Med. 2016, 176, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Hamine, S.; Gerth-Guyette, E.; Faulx, D.; Green, B.B.; Ginsburg, A.S. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: A systematic review. J. Med. Internet Res. 2015, 17, e52. [Google Scholar] [CrossRef] [Green Version]
- Beratarrechea, A.; Lee, A.G.; Willner, J.M.; Jahangir, E.; Ciapponi, A.; Rubinstein, A. The impact of mobile health interventions on chronic disease outcomes in developing countries: A systematic review. Telemed. E Health 2014, 20, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Koopmeiners, J.S.; Rhee, T.G.; Raveis, V.H.; Ahluwalia, J.S. Mobile phone text messaging intervention for cervical cancer screening: Changes in knowledge and behavior pre-post intervention. J. Med. Internet Res. 2014, 16, e196. [Google Scholar] [CrossRef]
- Krok-Schoen, J.L.; Naughton, M.J.; Young, G.S.; Moon, J.; Poi, M.; Melin, S.A.; Wood, M.E.; Hopkins, J.O.; Paskett, E.D.; Post, D.M. Increasing Adherence to Adjuvant Hormone Therapy Among Patients With Breast Cancer: A Smart Phone App-Based Pilot Study. Cancer Control 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Telemedicine: Opportunities and Developments in Member States: Report on the Second Global Survey on E Health 2009; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Dunahee, M.; Lebo, H. The World Internet Project International Report. 2016. Available online: http://www.digitalcenter.org/wp-content/uploads/2013/06/2015-World-Internet-Report.pdf (accessed on 20 January 2021).
- Déglise, C.; Suggs, L.S.; Odermatt, P. SMS for disease control in developing countries: A systematic review of mobile health applications. J. Telemed. Telecare 2012, 18, 273–281. [Google Scholar] [CrossRef]
- De Jongh, T.; Gurol-Urganci, I.; Vodopivec-Jamsek, V.; Car, J.; Atun, R. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst. Rev. 2012, 12. [Google Scholar] [CrossRef]
- Hasvold, P.E.; Wootton, R. Use of telephone and SMS reminders to improve attendance at hospital appointments: A systematic review. J. Telemed. Telecare 2011, 17, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Dario, A.B.; Cabral, A.M.; Almeida, L.; Ferreira, M.L.; Refshauge, K.; Simic, M.; Pappas, E.; Ferreira, P.H. Effectiveness of telehealth-based interventions in the management of non-specific low back pain: A systematic review with meta-analysis. Spine J. 2017, 17, 1342–1351. [Google Scholar] [CrossRef]
- Yasmin, F.; Banu, B.; Zakir, S.M.; Sauerborn, R.; Ali, L.; Souares, A. Positive influence of short message service and voice call interventions on adherence and health outcomes in case of chronic disease care: A systematic review. BMC Med. Inform. Decis. Mak. 2016, 16, 46. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.R.; Lekhak, N.; Kaewluang, N. Using mobile phones and short message service to deliver self-management interventions for chronic conditions: A meta-review. Worldviews Evid. Based Nurs. 2014, 11, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Daundasekara, S.S.; Arlinghaus, K.R.; Johnston, C.A. Quality of Life: The Primary Goal of Lifestyle Intervention. Am. J. Lifestyle Med. 2020, 14, 267–270. [Google Scholar] [CrossRef]
- Nasi, G.; Cucciniello, M.; Guerrazzi, C. The role of mobile technologies in health care processes: The case of cancer supportive care. J. Med. Internet Res. 2015, 17, e26. [Google Scholar] [CrossRef] [PubMed]
- Veritas Health Innovation. Covidence. Available online: https://www.covidence.org/ (accessed on 15 January 2021).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355. [Google Scholar] [CrossRef] [Green Version]
- Casillas, J.N.; Schwartz, L.F.; Crespi, C.M.; Ganz, P.A.; Kahn, K.L.; Stuber, M.L.; Bastani, R.; Alquaddomi, F.; Estrin, D.L. The use of mobile technology and peer navigation to promote adolescent and young adult (AYA) cancer survivorship care: Results of a randomized controlled trial. J. Cancer Surviv. 2019, 13, 580–592. [Google Scholar] [CrossRef]
- Gomersall, S.R.; Skinner, T.L.; Winkler, E.; Healy, G.N.; Eakin, E.; Fjeldsoe, B. Feasibility, acceptability and efficacy of a text message-enhanced clinical exercise rehabilitation intervention for increasing ‘whole-of-day’ activity in people living with and beyond cancer. BMC Public Health 2019, 19, 542. [Google Scholar] [CrossRef]
- Haggerty, A.F.; Hagemann, A.; Barnett, M.; Thornquist, M.; Neuhouser, M.L.; Horowitz, N.; Colditz, G.A.; Sarwer, D.B.; Ko, E.M.; Allison, K.C. A Randomized, Controlled, Multicenter Study of Technology-Based Weight Loss Interventions among Endometrial Cancer Survivors. Obesity 2017, 25, S102–S108. [Google Scholar] [CrossRef] [Green Version]
- Hershman, D.L.; Unger, J.M.; Hillyer, G.C.; Moseley, A.; Arnold, K.B.; Dakhil, S.R.; Esparaz, B.T.; Kuan, M.C.; Graham, M.L.; Lackowski, D.M.; et al. Randomized Trial of Text Messaging to Reduce Early Discontinuation of Adjuvant Aromatase Inhibitor Therapy in Women with Early-Stage Breast Cancer: SWOG S1105. J. Clin. Oncol. 2020, 38, 2122–2129. [Google Scholar] [CrossRef]
- Rico, T.M.; dos Santos Machado, K.; Fernandes, V.P.; Madruga, S.W.; Santin, M.M.; Petrarca, C.R.; Dumith, S.C. Use of Text Messaging (SMS) for the Management of Side Effects in Cancer Patients Undergoing Chemotherapy Treatment: A Randomized Controlled Trial. J. Med. Syst. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Tan, E.H.; Wong, A.L.A.; Tan, C.C.; Wong, P.; Tan, S.H.; Ang, L.E.Y.; Lim, S.E.; Chong, W.Q.; Ho, J.; Lee, S.C.; et al. Improving medication adherence with adjuvant aromatase inhibitor in women with breast cancer: A randomised controlled trial to evaluate the effect of short message service (SMS) reminder. Breast 2020, 53, 77–84. [Google Scholar] [CrossRef]
- Villaron, C.; Cury, F.; Eisinger, F.; Cappiello, M.A.; Marqueste, T. Telehealth applied to physical activity during cancer treatment: A feasibility, acceptability, and randomized pilot study. Support. Care Cancer 2018, 26, 3413–3421. [Google Scholar] [CrossRef]
- Spoelstra, S.L.; Given, C.W.; Sikorskii, A.; Coursaris, C.K.; Majumder, A.; DeKoekkoek, T.; Schueller, M.; Given, B.A. Feasibility of a text messaging intervention to promote self-management for patients prescribed oral anticancer agents. Oncol. Nurs. Forum 2015, 42, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra, S.L.; Given, C.W.; Sikorskii, A.; Coursaris, C.K.; Majumder, A.; DeKoekkoek, T.; Schueller, M.; Given, B.A. Proof of concept of a mobile health short message service text message intervention that promotes adherence to oral anticancer agent medications: A randomized controlled trial. Telemed. E Health 2016, 22, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Bade, B.C.; Hyer, J.M.; Bevill, B.T.; Pastis, A.; Rojewski, A.M.; Toll, B.A.; Silvestri, G.A. A Patient-Centered Activity Regimen Improves Participation in Physical Activity Interventions in Advanced-Stage Lung Cancer. Integr. Cancer Ther. 2018, 17, 921–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, P.I.; Drago, F.; Kennedy, E.M.; Chambers, N.; Sheffield, C.; Cohn, W.F. Examining the feasibility, acceptability, and potential utility of mobile distress screening in adult cancer patients. Psycho Oncol. 2019, 28, 1887–1893. [Google Scholar] [CrossRef]
- Maguire, R.; Ream, E.; Richardson, A.; Connaghan, J.; Johnston, B.; Kotronoulas, G.; Pedersen, V.; McPhelim, J.; Pattison, N.; Smith, A.; et al. Development of a novel remote patient monitoring system: The advanced symptom management system for radiotherapy to improve the symptom experience of patients with lung cancer receiving radiotherapy. Cancer Nurs. 2015, 1, E37–E47. [Google Scholar] [CrossRef] [Green Version]
- Mougalian, S.S.; Epstein, L.N.; Jhaveri, A.P.; Han, G.; Abu-Khalaf, M.; Hofstatter, E.W.; DiGiovanna, M.P.; Silber, A.L.; Adelson, K.; Pusztai, L. Bidirectional text messaging to monitor endocrine therapy adherence and patient-reported outcomes in breast Cancer. JCO Clin. Cancer Inform. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Rico, T.; Santos Machado, K.; Fernandes, V.; Madruga, S.; Noguez, P.c.; Barcelos, C.; Santin, M.; Petrarca, C.; Dumith, S. Text Messaging (SMS) Helping Cancer Care in Patients Undergoing Chemotherapy Treatment: A Pilot Study. J. Med. Syst. 2017, 41, 1–8. [Google Scholar] [CrossRef]
- Sawicki, C.; Friend, K.E.; Patel, R.; Polinski, J.M.; Singh, S. Two-Way Clinical Messaging in a CML Specialty Pharmacy Service Model. J. Manag. Care Spec. Pharm. 2019, 25, 1290–1296. [Google Scholar] [CrossRef]
- Tan, J.; Christie, A.; Montalvo, S.K.; Wallace, C.; Yan, Y.; Folkerts, M.; Yingling, A.; Sher, D.; Choy, H.; Jiang, S. Automated text message reminders improve radiation therapy compliance. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1045–1052. [Google Scholar] [CrossRef]
- Wells, C.; Malins, S.; Clarke, S.; Skorodzien, I.; Biswas, S.; Sweeney, T.; Moghaddam, N.; Levene, J. Using smart-messaging to enhance mindfulness-based cognitive therapy for cancer patients: A mixed methods proof of concept evaluation. Psycho Oncol. 2020, 29, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Klasnja, P.; Pratt, W. Healthcare in the pocket: Mapping the space of mobile-phone health interventions. J. Biomed. Inform. 2012, 45, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.K.; Cole-Lewis, H.; Bernhardt, J.M. Mobile text messaging for health: A systematic review of reviews. Annu. Rev. Public Health 2015, 36, 393–415. [Google Scholar] [CrossRef] [Green Version]
- Lorig, K.R.; Holman, H.R. Self-management education: History, definition, outcomes, and mechanisms. Ann. Behav. Med. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Kruse, C.S.; Krowski, N.; Rodriguez, B.; Tran, L.; Vela, J.; Brooks, M. Telehealth and patient satisfaction: A systematic review and narrative analysis. BMJ Open 2017, 7, e016242. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.M.; Kuhns, L.M. Economic Evaluation of Text-Messaging and Smartphone-Based Interventions to Improve Medication Adherence in Adolescents with Chronic Health Conditions: A Systematic Review. JMIR Mhealth Uhealth 2016, 4, e121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaglio, B.; Shoup, J.A.; Glasgow, R.E. The RE-AIM framework: A systematic review of use over time. Am. J. Public Health 2013, 103, e38–e46. [Google Scholar] [CrossRef] [PubMed]
- Curran, G.M.; Bauer, M.; Mittman, B.; Pyne, J.M.; Stetler, C. Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012, 50, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, J.C.; Sharma, S.; Del Paggio, J.C.; Hopman, W.M.; Gyawali, B.; Mukherji, D.; Hammad, N.; Pramesh, C.; Aggarwal, A.; Sullivan, R. An Analysis of Contemporary Oncology Randomized Clinical Trials From Low/Middle-Income vs High-Income Countries. JAMA Oncol. 2021, 7, 379–385. [Google Scholar] [CrossRef]
- Wilson, B.E.; Jacob, S.; Yap, M.L.; Ferlay, J.; Bray, F.; Barton, M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population-based study. Lancet Oncol. 2019, 20, 769–780. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- WorldBank. World Development Report 2016: Digital Dividends; World Bank Publications: Washington, DC, USA, 2016; pp. 5–9. [Google Scholar]
- Chávarri-Guerra, Y.; Ramos-López, W.A.; Covarrubias-Gómez, A.; Sánchez-Román, S.; Quiroz-Friedman, P.; Alcocer-Castillejos, N.; del Pilar Milke-García, M.; Carrillo-Soto, M.; Morales-Alfaro, A.; Medina-Palma, M. Providing Supportive and Palliative Care Using Telemedicine for Patients with Advanced Cancer During the COVID-19 Pandemic in Mexico. Oncologist 2021, 26, e512–e515. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).