The Impact of Healthy Lifestyles on Late Sequelae in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors. A Systematic Review by the Fondazione Italiana Linfomi
Abstract
Simple Summary
Abstract
1. Introduction
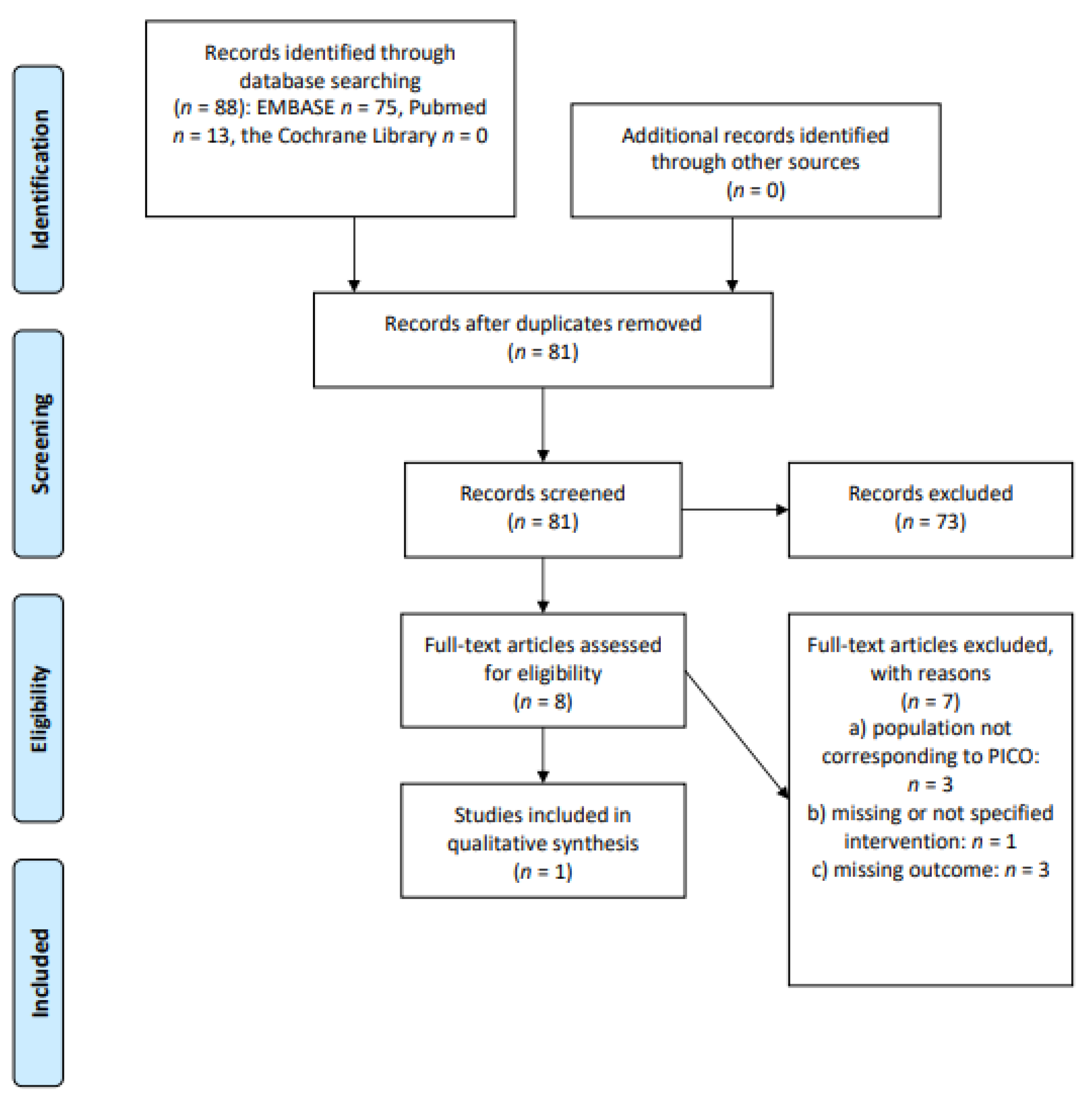
2. Materials and Methods
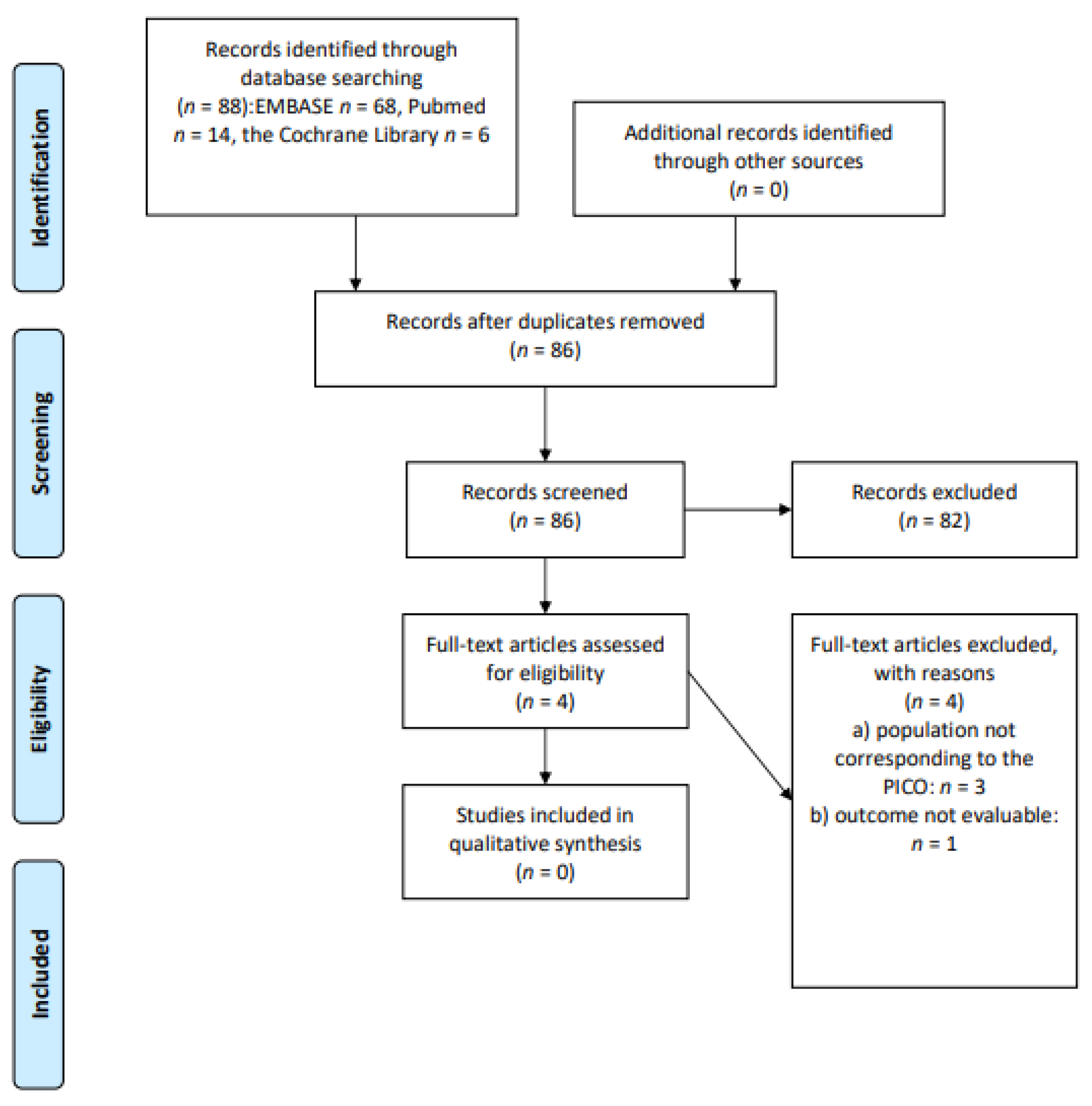
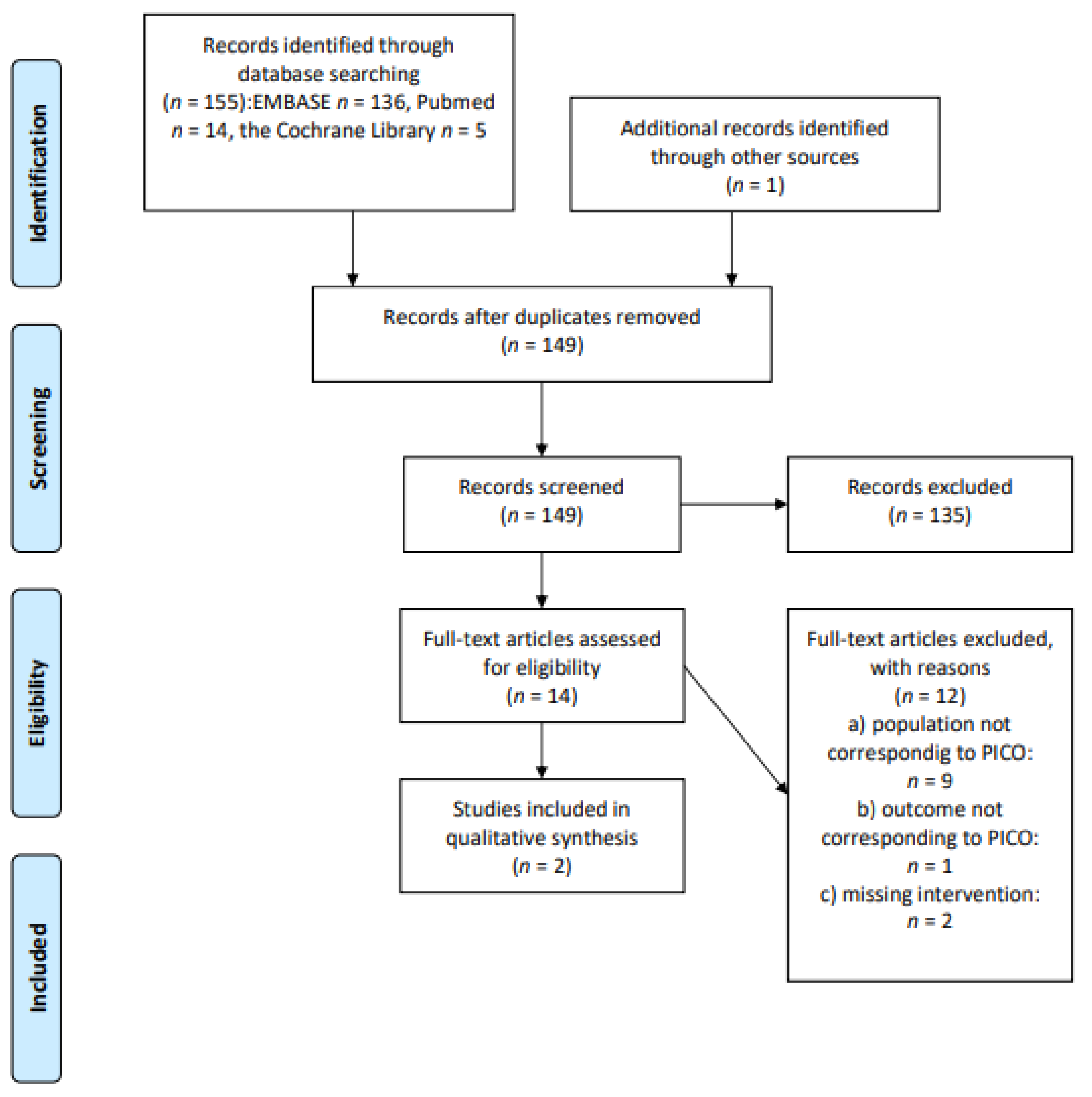
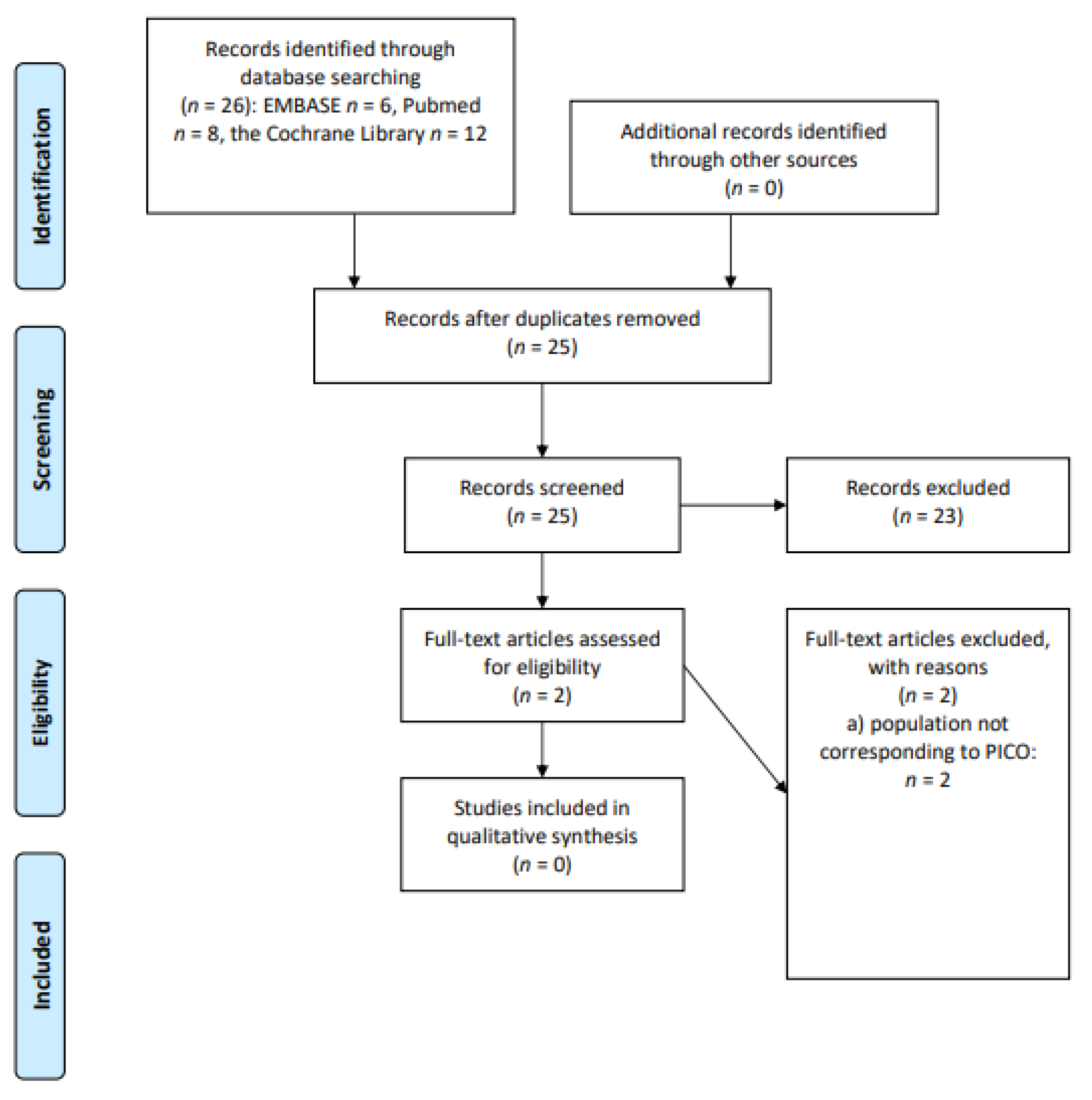
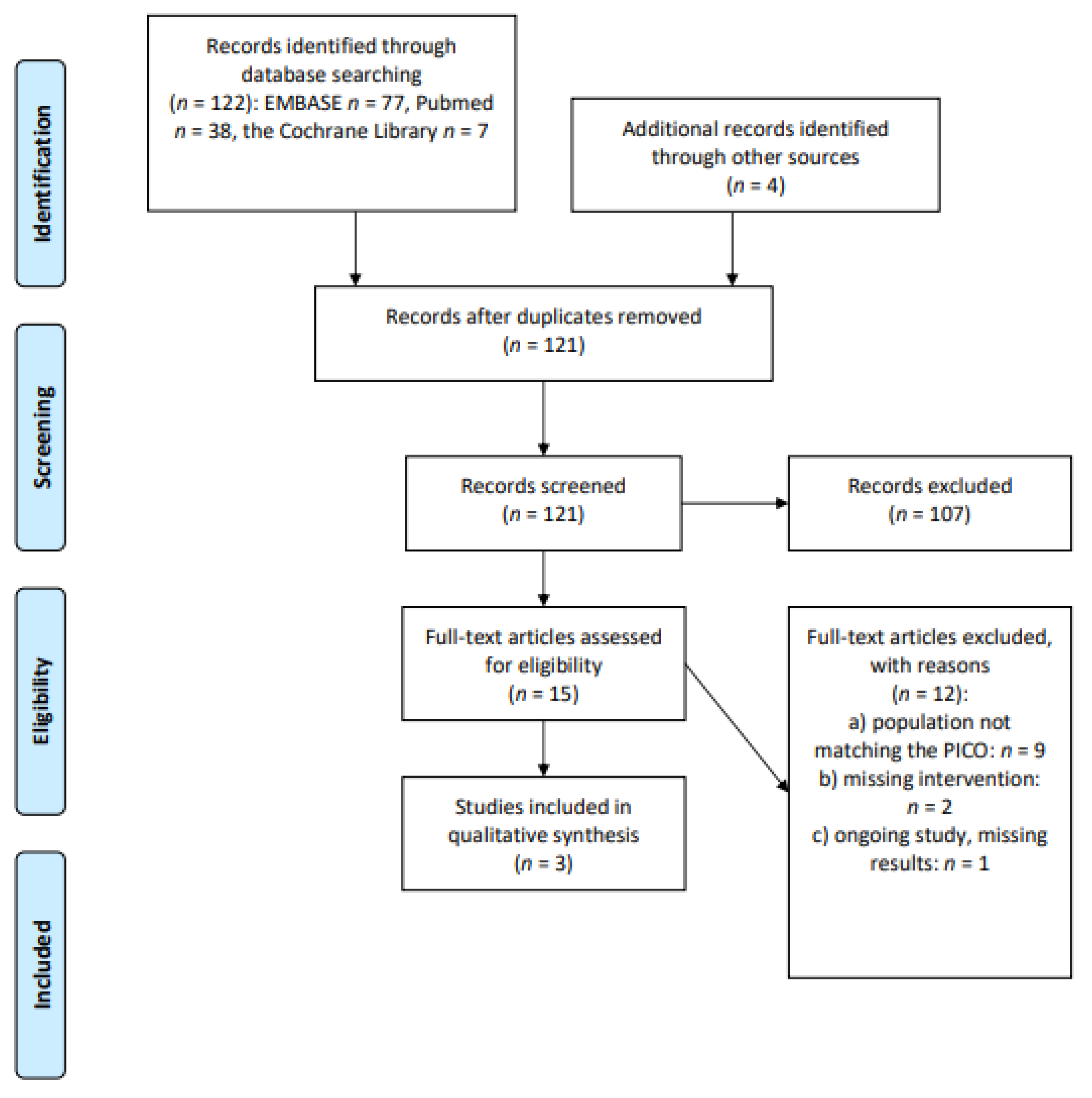
2.1. Study Identification
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Risk of Bias and Quality of Evidence Assessment
2.5. Data Synthesis
3. Results
3.1. Physical Activity/Exercise: Does Regular Physical Activity/Exercise (at Least 150 min/week of Moderate Physical Activity or 75 min/week of Intense Physical Activity) Determine a Clinical Benefit in Long-Term cHL or DLBCL Survivors?
3.2. Mediterranean Diet/Nutritional Intervention: Does a Controlled Diet (Mediterranean Diet or Nutritional Plan/Intervention) Determine a Clinical Benefit in Long-Term cHL or DLBCL Survivors?
3.3. Bodyweight and BMI: Does a Controlled Bodyweight or Adequate BMI Determine a Clinical Benefit in Long-Term cHL or DLBCL Survivors?
3.4. Dietary Supplements: Does the Use of Dietary Supplements Determine a Clinical Benefit in Long-Term cHL or DLBCL Survivors?
3.5. Chronic Fatigue/Cognitive Impairment: Does the Use of Non-Pharmacological Preventive Interventions (Physical and Mental Exercise) Reduce the Incidence of Chronic Fatigue and Cognitive Impairment in Long-Term cHL or DLBCL Survivors?
3.6. Survivorship Care Plans (SCP): Does the Use of SCP Determine a Clinical Benefit in Long-Term cHL or DLBCL Survivors?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shapiro, C.L. Cancer Survivorship. N. Engl. J. Med. 2018, 379, 2438–2450. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Networ Guidelines Version 2.2020 “Survivorship”. Available online: https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf (accessed on 15 March 2020).
- Haematological Malignancy Research Network (HMRN). Available online: https://www.hmrn.org/statistics/survival (accessed on 15 January 2021).
- European Commission: A Cancer Plan for Europe. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/promoting-our-european-way-life/european-health-union/cancer-plan-europe_en (accessed on 5 June 2021).
- Conquering Cancer: Mission Impossible. Report of the Mission Board for Cancer. European Commission. Available online: https://op.europa.eu/en/web/eu-law-and-publications/publication-detail/-/publication/d0235612-b68a-11ea-bb7a-01aa75ed71a1 (accessed on 5 June 2021).
- Puccini, B.; Nassi, L.; Minoia, C.; Volpetti, S.; Ciancia, R.; Riccomagno, P.C.; Di Rocco, A.; Mulè, A.; Toldo, C.; Sassone, M.C.; et al. Fondazione Italiana Linfomi Postgraduate Master course. Role of bone marrow biopsy in staging of patients with classical Hodgkin’s lymphoma undergoing positron emission tomography/computed tomography. Ann. Hematol. 2017, 96, 1147–1153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferrari, C.; Minoia, C.; Asabella, A.N.; Nicoletti, A.; Altini, C.; Antonica, F.; Ficco, M.; Guarini, A.; Maggialetti, N.; Rubini, G. Whole body magnetic resonance with diffusion weighted sequence with body signal suppression compared to (18)F-FDG PET/CT in newly diagnosed lymphoma. Hell. J. Nucl. Med. 2014, 17 (Suppl. S1), 40–49. [Google Scholar]
- Eichenauer, D.A.; Aleman, B.M.P.; André, M.; Federico, M.; Hutchings, M.; Illidge, T.; Engert, A.; Ladetto, M. ESMO Guidelines Committee. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv19–iv29. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/statfacts/html/hodg.html (accessed on 5 June 2021).
- National Comprehensive Cancer Networ Guidelines Version 4.2021 “Hodgkin Lymphoma”. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf (accessed on 5 June 2021).
- Tilly, H.; Gomes da Silva, M.; Vitolo, U.; Jack, A.; Meignan, M.; Lopez-Guillermo, A.; Walewski, J.; André, M.; Johnson, P.W.; Pfreundschuh, M.; et al. ESMO Guidelines Committee. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v116–v125. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Networ Guidelines Version 4.2021 “B-Cell lymphomas”. Available online: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf (accessed on 5 June 2021).
- Ciavarella, S.; Minoia, C.; Quinto, A.M.; Oliva, S.; Carbonara, S.; Cormio, C.; Cox, M.C.; Bravo, E.; Santoro, F.; Napolitano, M.; et al. Improving Provision of Care for Long-term Survivors of Lymphoma. Clin. Lymphoma Myeloma Leuk. 2017, 17, e1–e9. [Google Scholar] [CrossRef]
- Silvestris, E.; Cormio, G.; Skrypets, T.; Dellino, M.; Paradiso, A.V.; Guarini, A.; Minoia, C. Novel aspects on gonadotoxicity and fertility preservation in lymphoproliferative neoplasms. Crit. Rev. Oncol. Hematol. 2020, 151, 102981. [Google Scholar] [CrossRef] [PubMed]
- Damlaj, M.; El Fakih, R.; Hashmi, S.K. Evolution of survivorship in lymphoma, myeloma and leukemia: Metamorphosis of the field into long term follow-up care. Blood Rev. 2019, 33, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Majhail, N.S. Long-term complications after hematopoietic cell transplantation. Hematol. Oncol. Stem. Cell Ther. 2017, 10, 220–227. [Google Scholar] [CrossRef]
- Krolak, D.; Collins, B.; Weiss, L.; Harris, C.; Van der Jagt, R. Cognitive function and its relationship to other psychosocial factors in lymphoma survivors. Support. Care Cancer 2017, 25, 905–913. [Google Scholar] [CrossRef]
- Minoia, C.; Ciavarella, S.; Lerario, G.; Daniele, A.; De Summa, S.; Napolitano, M.; Guarini, A. Improvable Lifestyle Factors in Lymphoma Survivors. Acta Haematol. 2018, 139, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Karavasiloglou, N.; Pestoni, G.; Wanner, M.; Faeh, D.; Rohrmann, S. Healthy lifestyle is inversely associated with mortality in cancer survivors: Results from the Third National Health and Nutrition Examination Survey (NHANES III). PLoS ONE 2019, 14, e0218048. [Google Scholar] [CrossRef]
- Ruiz-Vozmediano, J.; Löhnchen, S.; Jurado, L.; Recio, R.; Rodríguez-Carrillo, A.; López, M.; Mustieles, V.; Expósito, M.; Arroyo-Morales, M.; Fernández, M.F. Influence of a Multidisciplinary Program of Diet, Exercise, and Mindfulness on the Quality of Life of Stage IIA-IIB Breast Cancer Survivors. Integr. Cancer Ther. 2020, 19, 1534735420924757. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Scherer, R.W.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O.; Gotay, C.C.; Snyder, C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev. 2012, CD007566. [Google Scholar] [CrossRef]
- Minoia, C.; Bari, A.; Nassi, L.; Banzi, R.; Gerardi, C.; Lenti, V.; Calabrese, M.; Spina, M.; Guarini, A. Management of lymphoma survivor patients in Italy: An evaluation by Fondazione Italiana Linfomi. Tumori 2021, 107, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Wolin, K.Y.; Schwartz, A.L.; Matthews, C.E.; Courneya, K.S.; Schmitz, K.H. Implementing the exercise guidelines for cancer survivors. J. Support. Oncol. 2012, 10, 171–177. [Google Scholar] [CrossRef]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef]
- Bellizzi, K.M.; Rowland, J.H.; Arora, N.K.; Hamilton, A.S.; Miller, M.F.; Aziz, N.M. Physical activity and quality of life in adult survivors of non-Hodgkin’s lymphoma. J. Clin. Oncol. 2009, 27, 960–966. [Google Scholar] [CrossRef]
- Spector, D.J.; Noonan, D.; Mayer, D.K.; Benecha, H.; Zimmerman, S.; Smith, S.K. Are lifestyle behavioral factors associated with health-related quality of life in long-term survivors of non-Hodgkin lymphoma? Cancer 2015, 121, 3343–3351. [Google Scholar] [CrossRef]
- Bersvendsen, H.S.; Haugnes, H.S.; Fagerli, U.M.; Fluge, Ø.; Holte, H.; Smeland, K.B.; Wilsgaard, T.; Kiserud, C.E. Lifestyle behavior among lymphoma survivors after high-dose therapy with autologous hematopoietic stem cell transplantation, assessed by patient-reported outcomes. Acta Oncol. 2019, 58, 690–699. [Google Scholar] [CrossRef]
- Fischetti, F.; Greco, G.; Cataldi, S.; Minoia, C.; Loseto, G.; Guarini, A. Effects of Physical Exercise Intervention on Psychological and Physical Fitness in Lymphoma Patients. Medicina 2019, 55, 379. [Google Scholar] [CrossRef]
- Chang, D.K.; Park, J.; Wan, Y.; Fraser, A.; Rowe, K.; Snyder, J.; Deshmukh, V.; Newman, M.; Herget, K.; Smith, K.; et al. Increased risk of diabetes mellitus among adult survivors of B-cell non-hodgkin lymphoma. Blood 2018, 132 (Suppl. S1), 4769. [Google Scholar] [CrossRef]
- Van Nimwegen, F.A.; Schaapveld, M.; Cutter, D.J.; Janus, C.P.; Krol, A.D.; Hauptmann, M.; Kooijman, K.; Roesink, J.; van der Maazen, R.; Darby, S.C.; et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J. Clin. Oncol. 2016, 34, 235–243. [Google Scholar] [CrossRef]
- Oldervoll, L.M.; Loge, J.H.; Kaasa, S.; Lydersen, S.; Hjermstad, M.J.; Thorsen, L.; Holte, H., Jr.; Jacobsen, A.B.; Fosså, S.D. Physical activity in Hodgkin’s lymphoma survivors with and without chronic fatigue compared with the general population—A cross-sectional study. BMC Cancer 2007, 7, 210. [Google Scholar] [CrossRef]
- Daniëls, L.A.; Oerlemans, S.; Krol, A.D.; Creutzberg, C.L.; van de Poll-Franse, L.V. Chronic fatigue in Hodgkin lymphoma survivors and associations with anxiety, depression and comorbidity. Br. J. Cancer 2014, 110, 868–874. [Google Scholar] [CrossRef]
- Husson, O.; Oerlemans, S.; Mols, F.; Schep, G.; Van De Poll-Franse, L.V. High levels of physical activity are associated with lower levels of fatigue among lymphoma patients: Results from the longitudinal PROFILES registry. Acta Oncol. 2015, 54, 678–684. [Google Scholar] [CrossRef]
- Williams, A.M.; Zent, C.S.; Janelsins, M.C. What is known and unknown about chemotherapy-related cognitive impairment in patients with haematological malignancies and areas of needed research. Br. J. Haematol. 2016, 174, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.E.; Gormally, J.F.; Butow, P.; Boyle, F.M.; Spillane, A.J. Survivorship care plans in cancer: A systematic review of care plan outcomes. Br. J. Cancer 2014, 111, 1899–1908. [Google Scholar] [CrossRef]
- Majhail, N.S.; Murphy, E.; Laud, P.; Preussler, J.M.; Denzen, E.M.; Abetti, B.; Adams, A.; Besser, R.; Burns, L.J.; Cerny, J.; et al. Randomized controlled trial of individualized treatment summary and survivorship care plans for hematopoietic cell transplantation survivors. Haematologica 2019, 104, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, F.; Van’t Veer, M.; Aleman, B.M.P.; Dekker, N.; Raemaekers, J.M.M. A dutch nationwide survivorship care program for hodgkin lymphoma survivors. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G. The Cochrane Collaboration’s tool for assessing risk of bias. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P., Green, S., Eds.; John Wiley and Sons: New York, NY, USA, 2010; pp. 194–202. [Google Scholar]
- Wells Ga, S.B.; O’connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses; Ottawa Health Research Institute: Ottawa, ON, Canada, 1999; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 March 2020).
- Ng, A.K.; Li, S.; Recklitis, C.; Neuberg, D.; Chakrabarti, S.; Silver, B.; Diller, L. A comparison between long-term survivors of Hodgkin’s disease and their siblings on fatigue level and factors predicting for increased fatigue. Ann. Oncol. 2005, 16, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Stenehjem, J.S.; Smeland, K.B.; Murbraech, K.; Holte, H.; Kvaløy, S.; Thorsen, L.; Arbo, I.; Jones, L.W.; Aakhus, S.; Lund, M.B.; et al. Cardiorespiratory fitness in long-term lymphoma survivors after high-dose chemotherapy with autologous stem cell transplantation. Br. J. Cancer 2016, 115, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Oldervoll, L.M.; Kaasa, S.; Knobel, H.; Loge, J.H. Exercise reduces fatigue in chronic fatigued Hodgkins disease survivors—Results from a pilot study. Eur. J. Cancer 2003, 39, 57–63. [Google Scholar] [CrossRef]
- Maraldo, M.V.; Giusti, F.; Van Der Kaaij, M.; Henry-Amar, M.; Aleman, B.; Raemaekers, J.; Meijnders, P.J.; Moser, E.C.; Kluin-Nelemans, H.; Spina, M.; et al. Cardiac disease and lifestyle risk factors following hodgkin lymphoma: An eortc lymphoma group and gela follow-up study. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99 (Suppl. S1), S139–S140. [Google Scholar] [CrossRef]
- Gates, P.R.; Seymour, J.F.; Krishnasamy, M. Development of a nurse-led survivorship intervention for long-term survivors of hodgkin lymphoma. Asia-Pac. J. Clin. Oncol. 2013, 9 (Suppl. S3), 138. [Google Scholar]
- Pekmezi, D.W.; Demark-Wahnefried, W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011, 50, 167–178. [Google Scholar] [CrossRef]
- Doyle, C.; Kushi, L.H.; Byers, T.; Courneya, K.S.; Demark-Wahnefried, W.; Grant, B.; McTiernan, A.; Rock, C.L.; Thompson, C.; Gansler, T.; et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J. Clin. 2006, 56, 323–353. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- ASCO Survivorship Care Planning Tools. Available online: https://www.asco.org/practice-policy/cancer-care-initiatives/prevention-survivorship/survivorship-compendium (accessed on 15 March 2020).

| Clinical Question | PICOS |
|---|---|
| Does a regular physical activity/exercise (at least 150 min/week of moderate physical activity or 75 min/week of intense physical activity) determine clinical benefit in long-term cHL or DLBCL survivors? | P: long-term cHL or DLBCL survivors (≥5 years disease- or treatment-free), adults (≥18-year-old at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: physical activity/exercise (at least 150 min/week of moderate physical activity or 75 min/week of intense physical activity) C: no physical activity/exercise or physical activity O: reduced incidence of diabetes/metabolic syndrome/sarcopenia/osteoporosis; reduced incidence of late cardiovascular toxicity; reduced incidence of chronic fatigue/neuropathy/cognitive impairment; reduced incidence of secondary cancers; amelioration of QoL and OS |
| Does a controlled diet (Mediterranean diet or nutritional plan/intervention) determine a clinical benefit in long-term cHL or DLBCL survivors? | P: long-term cHL or DLBCL survivors (≥5 years disease- or treatment-free), adults (≥18-year-old at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: controlled diet (Mediterranean diet or nutritional plan/intervention) C: no controlled diet O: reduced incidence of diabetes/metabolic syndrome/sarcopenia/osteoporosis; reduced incidence of late cardiovascular toxicity; reduced incidence of chronic fatigue, neuropathy, cognitive impairment; reduced incidence of secondary cancers; amelioration of QoL and OS |
| Does a controlled bodyweight or adequate BMI determine a clinical benefit in long-term cHL or DLBCL survivors? | P: long-term cHL or DLBCL survivors (≥5 years disease- or treatment-free), adults (≥18-year-old at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: controlled bodyweight or adequate BMI C: BMI ≥ 25 O: reduced incidence of diabetes/metabolic syndrome/sarcopenia/osteoporosis; reduced incidence of late cardiovascular toxicity; reduced incidence of chronic fatigue, neuropathy, cognitive impairment; reduced incidence of secondary cancers; amelioration of QoL and OS |
| Does the use of dietary supplements determine a clinical benefit in long-term cHL or DLBCL survivors? | P: long-term cHL or DLBCL survivors (≥5 years disease- or treatment-free), adults (≥18-year-old at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: use of dietary supplements C: no dietary supplements O: reduced incidence of diabetes/metabolic syndrome/sarcopenia/osteoporosis; reduced incidence of late cardiovascular toxicity; reduced incidence of chronic fatigue, neuropathy, cognitive impairment; reduced incidence of secondary cancer; amelioration of QoL and OS |
| Does the use of non-pharmacological preventive interventions (physical and mental exercise) reduce the incidence of chronic fatigue and cognitive impairment in long-term cHL or DLBCL survivors? | P: long-term cHL or DLBCL survivors (≥5 years disease- or treatment-free), adults (≥18-year-old at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: non-pharmacological preventive interventions (physical and mental exercise) C: no non-pharmacological preventive interventions (physical and mental exercise) O: reduced incidence of chronic fatigue, cognitive impairment |
| Does the use of SCP determine a clinical benefit in long-term cHL or DLBCL survivors? | P: long-term cHL or DLBCL survivors (≥5 years disease- or treatment-free), adults (≥18-year-old at diagnosis) treated with first-line therapy or second-line therapy including ASCT I: use of personalized SCP C: no use of SCP O: reduced incidence of late toxicities; ameliorated compliance with screening programs for secondary cancers; amelioration of QoL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minoia, C.; Gerardi, C.; Allocati, E.; Daniele, A.; De Sanctis, V.; Bari, A.; Guarini, A. The Impact of Healthy Lifestyles on Late Sequelae in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors. A Systematic Review by the Fondazione Italiana Linfomi. Cancers 2021, 13, 3135. https://doi.org/10.3390/cancers13133135
Minoia C, Gerardi C, Allocati E, Daniele A, De Sanctis V, Bari A, Guarini A. The Impact of Healthy Lifestyles on Late Sequelae in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors. A Systematic Review by the Fondazione Italiana Linfomi. Cancers. 2021; 13(13):3135. https://doi.org/10.3390/cancers13133135
Chicago/Turabian StyleMinoia, Carla, Chiara Gerardi, Eleonora Allocati, Antonella Daniele, Vitaliana De Sanctis, Alessia Bari, and Attilio Guarini. 2021. "The Impact of Healthy Lifestyles on Late Sequelae in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors. A Systematic Review by the Fondazione Italiana Linfomi" Cancers 13, no. 13: 3135. https://doi.org/10.3390/cancers13133135
APA StyleMinoia, C., Gerardi, C., Allocati, E., Daniele, A., De Sanctis, V., Bari, A., & Guarini, A. (2021). The Impact of Healthy Lifestyles on Late Sequelae in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma Survivors. A Systematic Review by the Fondazione Italiana Linfomi. Cancers, 13(13), 3135. https://doi.org/10.3390/cancers13133135







