Using Low-Intensity Focused Ultrasound to Treat Depression and Anxiety Disorders: A Review of Current Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- (1)
- Research using depressive/anxious-like animal models where LIFU was applied to modulate the phenotypes and behaviors in preclinical studies.
- (2)
- Research recruiting healthy volunteers, where LIFU was used to modulate emotional processes, along with the collection of subjective/objective emotion evaluations or neurophysiology data.
- (3)
- Studies involving patients with depression (comorbid depression) or anxiety disorders, where LIFU was employed to treat these symptoms.
- (1)
- Studies utilizing high-intensity focused ultrasound (HIFU) for capsulotomy or thermal ablation surgery.
- (2)
- Research on the effects of exposure to unfocused ultrasound.
- (3)
- Studies using LIFU to facilitate drug delivery via the blood–brain barrier.
- (4)
- Research involving gene engineering, specifically sonogenetics.
- (5)
- Studies combining LIFU or unfocused ultrasound with microbubble injections for neuromodulation.
- (6)
- Research presented only in the form of conference abstracts.
- (7)
- Studies unrelated to the specified themes of this review.
3. Results
3.1. Search Results and Categories
3.2. LIFU Preclinical Studies of Depressive/Anxious-like Animal Models
3.3. Mood Effects of LIFU Among Healthy Participants
- (1)
- The ultrasound energy was delivered by a diagnostic ultrasound system in scan mode and resulted in an unfocused ultrasound beam that lacked precise targeting of brain regions, making it challenging to confirm the affected neural circuits.
- (2)
- Patients participating in the research were enrolled based on symptoms of chronic pain rather than mood disorders, making it difficult to determine whether mood improvements stemmed from direct emotional effects or reductions in pain sensation.
- (3)
- Quantitative data records to evaluate functional changes in the brain resulting from ultrasound stimulation were lacking.
3.4. Studies on Clinical Depression Treatment with LIFU
3.5. Studies on Clinical Anxiety Treatment with LIFU
4. Discussion
4.1. Discussion on Animal Studies with LIFU
4.2. Discussion on LIFU’s Mood Effects Among Healthy Volunteers
4.3. Discussion on LIFU’s Treatment Study Among Patients with Depression/Anxiety
4.4. Other Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | serotonin |
| ADAS | Alzheimer’s Disease Assessment Scale |
| ALB | anxious-like behavior |
| amPFC | anterior medial prefrontal cortex |
| ANT | anterior nucleus of the thalamus |
| BAI | Beck Anxiety Inventory |
| BDI | Beck Depression Inventory |
| BDNF | brain-derived neurotrophic factor |
| BNST | bed nucleus of the stria terminalis |
| CB | cingulum bundle |
| CERAD | Consortium to Establish a Registry for Alzheimer’s Disease |
| CRS | chronic restraint stress |
| CUS | chronic unpredictable stress |
| DA | dopamine |
| DLB | depressive-like behavior |
| dACC | dorsal anterior cingulate cortex |
| DBS | deep brain stimulation |
| DC | duty cycle |
| DMN | Default Mode Network |
| DRN | dorsal raphe nucleus |
| EFD | energy flux density |
| EPM | elevated plus maze |
| ERPs | event-related potentials |
| FC | functional connectivity |
| FF | foundation frequency |
| FM | forceps minor |
| FST | forced swimming test |
| GASE | General Assessment of Side Effects |
| GDS | Geriatric Depression Scale |
| HC | healthy control |
| HIFU | high-intensity focused ultrasound |
| HRSD/HRSD-6 | Hamilton Rating Scale for Depression/6-item short version |
| HRV | heart rate variability |
| ISI | inter-stimulus interval |
| ISPTA | spatial peak temporal average intensity |
| ISPPA | spatial peak pulse average intensity |
| K-POMS | Korean edition of the Profile of Mood States |
| l-amygdala | left amygdala |
| l-Forb | left frontal orbital cortex |
| l-PLC | left prelimbic cortex |
| l-vlPFC | left ventrolateral prefrontal cortex |
| LIFU | low-intensity focused ultrasound |
| LPS | lipopolysaccharide |
| MADRS | Montgomery–Åsberg Depression Rating Scale |
| MASQ-GD | Mood and Anxiety Symptom Questionnaire–General Distress |
| MATRDs | mood, anxiety, and trauma-related disorders |
| MFT | midfrontal theta activity |
| MMSE | Mini-Mental Status Examination |
| MI | mechanical index |
| MoCA | Montreal Cognitive Assessment |
| mPFC | medial prefrontal cortex |
| mTUS | metalens-based transcranial ultrasound stimulation |
| NE | norepinephrine |
| NFT | novelty-suppressed feeding test |
| NOPs | number of pulses |
| NRSs | Numeric Rating Scales |
| OASIS | Overall Anxiety Severity and Impairment Scale |
| OFT | open field test |
| PANAS/PANAS-X | Positive and Negative Affect Scale/Expanded version |
| PCC | posterior cingulate cortex |
| PGI-I | Patient Global Impression–Improvement |
| PL | pulse length |
| PLV | phase locking value |
| PRF | pulse repetition frequency |
| PRP | pulse repetition period |
| PSQI | Pittsburgh Sleep Quality Index |
| PSWQ | Penn State Worry Questionnaire |
| PTQ | Perseverative Thinking Questionnaire |
| QIDS-SR/IDS-SR | Quick/Inventory of Depressive Symptomatology, Self Report |
| r-AINS | right anterior insula |
| r-DLPFC | right dorsolateral prefrontal cortex |
| r-IFG | right inferior frontal gyrus |
| r-MFG | right middle frontal gyrus |
| r-PFC | right prefrontal cortex |
| RS | restraint stress |
| r-VLPFC | right ventrolateral prefrontal cortex |
| RRS | Ruminative Responses Scale |
| rsFC | resting-state functional connectivity |
| SAM | Self-Assessment Manikin |
| SCC | subcallosal cingulate cortex |
| SD | sonication duration |
| sgACC | subgenual anterior cingulate cortex |
| SGC | subgenual cingulate cortex |
| SIT | social interaction test |
| SN | salience network |
| SPI | sucrose preference index |
| SPT | sucrose preference test |
| SSI | Scale for Suicide Ideation |
| STAI | State-Trait Anxiety Inventory |
| TBD | tone burst duration |
| tDCS | direct current stimulation |
| tFUS | transcranial focused ultrasound stimulation |
| TMS | transcranial magnetic stimulation |
| TPS | transcranial pulse stimulation |
| TRD | treatment-resistant depression |
| trGAD | treatment-refractory Generalized Anxiety Disorder |
| TST | tail suspension test |
| TUS | transcranial ultrasound stimulation |
| UF | uncinate fasciculus |
| VAS/VAMS | visual analog scales/visual analog mood scales |
| VC | ventral capsule |
| VMN | ventromedial network |
| vmPFC | ventromedial prefrontal cortex |
| VTA | ventral tegmental area |
References
- World Health Organization (WHO). Depression Fact Sheet. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 1 October 2025).
- Rosenblat, J.D.; Cha, D.S.; Mansur, R.B.; McIntyre, R.S. Inflamed moods: A review of the interactions between inflammation and mood disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 53, 23–34. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Jorm, A.F.; Patten, S.B.; Brugha, T.S.; Mojtabai, R. Has increased provision of treatment reduced the prevalence of common mental disorders? Review of the evidence from four countries. World Psychiatry 2017, 16, 90–99. [Google Scholar] [CrossRef]
- McAllister-Williams, R.H.; Arango, C.; Blier, P.; Demyttenaere, K.; Falkai, P.; Gorwood, P.; Hopwood, M.; Javed, A.; Kasper, S.; Malhi, G.S.; et al. The identification, assessment and management of difficult-to-treat depression: An international consensus statement. J. Affect. Disord. 2020, 267, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Nunez, N.A.; Joseph, B.; Pahwa, M.; Kumar, R.; Resendez, M.G.; Prokop, L.J.; Veldic, M.; Seshadri, A.; Biernacka, J.M.; Frye, M.A.; et al. Augmentation strategies for treatment resistant major depression: A systematic review and network meta-analysis. J. Affect. Disord. 2022, 302, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Rosson, S.; de Filippis, R.; Croatto, G.; Collantoni, E.; Pallottino, S.; Guinart, D.; Brunoni, A.R.; Dell’Osso, B.; Pigato, G.; Hyde, J.; et al. Brain stimulation and other biological non-pharmacological interventions in mental disorders: An umbrella review. Neurosci. Biobehav. Rev. 2022, 139, 104743. [Google Scholar] [CrossRef]
- Herrington, T.M.; Cheng, J.J.; Eskandar, E.N. Mechanisms of deep brain stimulation. J. Neurophysiol. 2016, 115, 19–38. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 2013, 77, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of deep brain stimulation: Current status and future directions. Nat. Rev. Neurol. 2021, 17, 75–87. [Google Scholar] [CrossRef]
- Herrera-Melendez, A.L.; Bajbouj, M.; Aust, S. Application of Transcranial Direct Current Stimulation in Psychiatry. Neuropsychobiology 2020, 79, 372–383. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipovic, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Cortes, R.A.; Holzman, D.D.; Green, A.E. Neuromodulation to Enhance Creative Cognition: A Review of New and Emerging Approaches. J. Cogn. Enhanc. 2023, 7, 1–18. [Google Scholar] [CrossRef]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041.e16. [Google Scholar] [CrossRef] [PubMed]
- Darmani, G.; Bergmann, T.O.; Butts Pauly, K.; Caskey, C.F.; de Lecea, L.; Fomenko, A.; Fouragnan, E.; Legon, W.; Murphy, K.R.; Nandi, T.; et al. Non-invasive transcranial ultrasound stimulation for neuromodulation. Clin. Neurophysiol. 2022, 135, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, D.G.; Razansky, D.; Gotz, J. Ultrasound as a versatile tool for short- and long-term improvement and monitoring of brain function. Neuron 2023, 111, 1174–1190. [Google Scholar] [CrossRef] [PubMed]
- Sarica, C.; Nankoo, J.F.; Fomenko, A.; Grippe, T.C.; Yamamoto, K.; Samuel, N.; Milano, V.; Vetkas, A.; Darmani, G.; Cizmeci, M.N.; et al. Human Studies of Transcranial Ultrasound neuromodulation: A systematic review of effectiveness and safety. Brain Stimul. 2022, 15, 737–746. [Google Scholar] [CrossRef]
- Qin, P.P.; Jin, M.; Xia, A.W.; Li, A.S.; Lin, T.T.; Liu, Y.; Kan, R.L.; Zhang, B.B.; Kranz, G.S. The effectiveness and safety of low-intensity transcranial ultrasound stimulation: A systematic review of human and animal studies. Neurosci. Biobehav. Rev. 2024, 156, 105501. [Google Scholar] [CrossRef]
- Osada, T.; Konishi, S. Noninvasive intervention by transcranial ultrasound stimulation: Modulation of neural circuits and its clinical perspectives. Psychiatry Clin. Neurosci. 2024, 78, 273–281. [Google Scholar] [CrossRef]
- Kamimura, H.A.; Wang, S.; Chen, H.; Wang, Q.; Aurup, C.; Acosta, C.; Carneiro, A.A.; Konofagou, E.E. Focused ultrasound neuromodulation of cortical and subcortical brain structures using 1.9 MHz. Med. Phys. 2016, 43, 5730. [Google Scholar] [CrossRef] [PubMed]
- Legon, W.; Ai, L.; Bansal, P.; Mueller, J.K. Neuromodulation with single-element transcranial focused ultrasound in human thalamus. Hum. Brain Mapp. 2018, 39, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Cain, J.A.; Spivak, N.M.; Coetzee, J.P.; Crone, J.S.; Johnson, M.A.; Lutkenhoff, E.S.; Real, C.; Buitrago-Blanco, M.; Vespa, P.M.; Schnakers, C.; et al. Ultrasonic thalamic stimulation in chronic disorders of consciousness. Brain Stimul. 2021, 14, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Folloni, D.; Verhagen, L.; Mars, R.B.; Fouragnan, E.; Constans, C.; Aubry, J.F.; Rushworth, M.F.S.; Sallet, J. Manipulation of Subcortical and Deep Cortical Activity in the Primate Brain Using Transcranial Focused Ultrasound Stimulation. Neuron 2019, 101, 1109–1116.e5. [Google Scholar] [CrossRef] [PubMed]
- Badran, B.W.; Caulfield, K.A.; Stomberg-Firestein, S.; Summers, P.M.; Dowdle, L.T.; Savoca, M.; Li, X.; Austelle, C.W.; Short, E.B.; Borckardt, J.J.; et al. Sonication of the anterior thalamus with MRI-Guided transcranial focused ultrasound (tFUS) alters pain thresholds in healthy adults: A double-blind, sham-controlled study. Brain Stimul. 2020, 13, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Yaakub, S.N.; White, T.A.; Roberts, J.; Martin, E.; Verhagen, L.; Stagg, C.J.; Hall, S.; Fouragnan, E.F. Transcranial focused ultrasound-mediated neurochemical and functional connectivity changes in deep cortical regions in humans. Nat. Commun. 2023, 14, 5318. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, P.; Song, B.; Li, Y. Numerical investigation of the energy distribution of Low-intensity transcranial focused ultrasound neuromodulation for hippocampus. Ultrasonics 2022, 124, 106724. [Google Scholar] [CrossRef]
- Kong, C.; Ahn, J.W.; Kim, S.; Park, J.Y.; Na, Y.C.; Chang, J.W.; Chung, S.; Chang, W.S. Long-lasting restoration of memory function and hippocampal synaptic plasticity by focused ultrasound in Alzheimer’s disease. Brain Stimul. 2023, 16, 857–866. [Google Scholar] [CrossRef]
- Kuhn, T.; Spivak, N.M.; Dang, B.H.; Becerra, S.; Halavi, S.E.; Rotstein, N.; Rosenberg, B.M.; Hiller, S.; Swenson, A.; Cvijanovic, L.; et al. Transcranial focused ultrasound selectively increases perfusion and modulates functional connectivity of deep brain regions in humans. Front. Neural Circuits 2023, 17, 1120410. [Google Scholar] [CrossRef] [PubMed]
- Harvey, E.N. The Effect of High Frequency Sound Waves on Heart Muscle and Other Irritable Tissues. Am. J. Physiol.-Leg. Content 1929, 91, 284–290. [Google Scholar] [CrossRef]
- Hameroff, S.; Trakas, M.; Duffield, C.; Annabi, E.; Gerace, M.B.; Boyle, P.; Lucas, A.; Amos, Q.; Buadu, A.; Badal, J.J. Transcranial Ultrasound (TUS) Effects on Mental States: A Pilot Study. Brain Stimul. 2013, 6, 409–415. [Google Scholar] [CrossRef]
- Tufail, Y.; Matyushov, A.; Baldwin, N.; Tauchmann, M.L.; Georges, J.; Yoshihiro, A.; Tillery, S.I.; Tyler, W.J. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 2010, 66, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, T.; Jordao, J.F.; O’Reilly, M.A.; Ellens, N.; Hynynen, K.; Aubert, I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014, 7, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-J. Transcranial focused ultrasound as a possible treatment for major depression. Med. Hypotheses 2015, 84, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Vlaicu, A.; Bustuchina Vlaicu, M. New neuromodulation techniques for treatment resistant depression. Int. J. Psychiatry Clin. Pract. 2020, 24, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Arulpragasam, A.R.; van ‘t Wout-Frank, M.; Barredo, J.; Faucher, C.R.; Greenberg, B.D.; Philip, N.S. Low Intensity Focused Ultrasound for Non-invasive and Reversible Deep Brain Neuromodulation-A Paradigm Shift in Psychiatric Research. Front. Psychiatry 2022, 13, 825802. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; Yang, G.; Liang, X.S.; Ding, X.S.; Xu, D.E.; Li, Z.; Ma, Q.H.; Chen, R.; Sun, Y.Y. Transcranial low-intensity ultrasound stimulation for treating central nervous system disorders: A promising therapeutic application. Front. Neurol. 2023, 14, 1117188. [Google Scholar] [CrossRef] [PubMed]
- Asher, R.; Hyun, I.; Head, M.; Cosgrove, G.R.; Silbersweig, D. Neuroethical implications of focused ultrasound for neuropsychiatric illness. Brain Stimul. 2023, 16, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Guinjoan, S.M. Personalized definition of surgical targets in major depression and obsessive-compulsive disorder: A potential role for low-intensity focused ultrasound? Pers. Med. Psychiatry 2023, 37–38, 100100. [Google Scholar] [CrossRef]
- Cox, S.S.; Connolly, D.J.; Peng, X.; Badran, B.W. A Comprehensive Review of Low-Intensity Focused Ultrasound Parameters and Applications in Neurologic and Psychiatric Disorders. Neuromodulation 2024, 28, 1–15. [Google Scholar] [CrossRef]
- Henn, M.C.; Smith, H.D.; Lopez Ramos, C.G.; Shafie, B.; Abaricia, J.; Stevens, I.; Rockhill, A.P.; Cleary, D.R.; Raslan, A.M. A systematic review of focused ultrasound for psychiatric disorders: Current applications, opportunities, and challenges. Neurosurg. Focus 2024, 57, E8. [Google Scholar] [CrossRef] [PubMed]
- Keihani, A.; Sanguineti, C.; Chaichian, O.; Huston, C.A.; Moore, C.; Cheng, C.; Janssen, S.A.; Donati, F.L.; Mayeli, A.; Moussawi, K.; et al. Transcranial Focused Ultrasound Neuromodulation in Psychiatry: Main Characteristics, Current Evidence, and Future Directions. Brain Sci. 2024, 14, 1095. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Sun, J.; Hu, W.; Jin, W.; Li, S.; Tong, S. Antidepressant-Like Effect of Low-Intensity Transcranial Ultrasound Stimulation. IEEE Trans. Biomed. Eng. 2019, 66, 411–420. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.; Yang, J.; Jia, J.; Niu, L.; Sun, Z.; Shi, D.; Meng, L.; Qiu, W.; Wang, X.; et al. Low-intensity pulsed ultrasound ameliorates depression-like behaviors in a rat model of chronic unpredictable stress. CNS Neurosci. Ther. 2020, 27, 233–243. [Google Scholar] [CrossRef]
- Yi, S.-s.; Zou, J.-j.; Meng, L.; Chen, H.-m.; Hong, Z.-q.; Liu, X.-f.; Farooq, U.; Chen, M.-x.; Lin, Z.-r.; Zhou, W.; et al. Ultrasound Stimulation of Prefrontal Cortex Improves Lipopolysaccharide-Induced Depressive-Like Behaviors in Mice. Front. Psychiatry 2022, 13, 864481. [Google Scholar] [CrossRef]
- Zhu, Y.; He, J.; Wu, C.; Wu, J.; Cheng, Z.; Chen, Y.; Yuan, M.; Zeng, L.; Ji, X. Transcranial ultrasound stimulation relieves depression in mice with chronic restraint stress. J. Neural Eng. 2023, 20, 036011. [Google Scholar] [CrossRef]
- Zhu, Y.; He, J.; Wu, C.; Wu, J.; Cheng, Z.; Chen, Y.; Yuan, M.; Zeng, L.; Ji, X. Multi-Target Ultrasound Neuromodulation in the Treatment of Freely Moving Depression Mice. In Proceedings of the 2022 IEEE International Ultrasonics Symposium (IUS), Venice, Italy, 10–13 October 2022; pp. 1–3. [Google Scholar]
- Wu, C.; He, J.; Zhu, Y.; Wu, J.; Chen, Y.; Yuan, M.; Cheng, Z.; Zeng, L.; Ji, X. Ultrasound neuromodulation ameliorates chronic corticosterone-induced depression- and anxiety-like behaviors in mice. J. Neural Eng. 2023, 20, 036037. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chiu, A.; Lee, S.D.; Fischer, K.; Yoo, S.S. Focused ultrasound-mediated non-invasive brain stimulation: Examination of sonication parameters. Brain Stimul. 2014, 7, 748–756. [Google Scholar] [CrossRef] [PubMed]
- King, R.L.; Brown, J.R.; Newsome, W.T.; Pauly, K.B. Effective parameters for ultrasound-induced in vivo neurostimulation. Ultrasound Med. Biol. 2013, 39, 312–331. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yan, J.; Ma, Z.; Li, X. Effect of noninvasive focused ultrasound stimulation on gamma oscillations in rat hippocampus. Neuroreport 2016, 27, 508–515. [Google Scholar] [CrossRef]
- Yu, K.; Niu, X.; Krook-Magnuson, E.; He, B. Intrinsic functional neuron-type selectivity of transcranial focused ultrasound neuromodulation. Nat. Commun. 2021, 12, 2519. [Google Scholar] [CrossRef] [PubMed]
- Dell’Italia, J.; Sanguinetti, J.L.; Monti, M.M.; Bystritsky, A.; Reggente, N. Current State of Potential Mechanisms Supporting Low Intensity Focused Ultrasound for Neuromodulation. Front. Hum. Neurosci. 2022, 16, 872639. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.F.; Phipps, M.A.; Newton, A.T.; Jonathan, S.; Manuel, T.J.; Gore, J.C.; Grissom, W.A.; Caskey, C.F.; Chen, L.M. Differential dose responses of transcranial focused ultrasound at brain regions indicate causal interactions. Brain Stimul. 2022, 15, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Li, Z.; Xia, X.; Wang, Z.; Darmani, G.; Li, X.; Chen, R. Effects of different sonication parameters of theta burst transcranial ultrasound stimulation on human motor cortex. Brain Stimul. 2024, 17, 258–268. [Google Scholar] [CrossRef]
- Tufail, Y.; Yoshihiro, A.; Pati, S.; Li, M.M.; Tyler, W.J. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc. 2011, 6, 1453–1470. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, Q.; Ju, R.; Wang, S.; Liu, L.; Pan, M.; Sun, N.; Wang, X.; Wang, L.; Yang, J.; et al. Low-intensity focused ultrasound ameliorates depression-like behaviors associated with improving the synaptic plasticity in the vCA1-mPFC pathway. Cereb. Cortex 2023, 33, 8024–8034. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Mo, W.; Li, Y.; Yang, Q.; Tian, Y.; Zheng, C.; Yang, J.; Ming, D. Ultrasound Stimulation Attenuates CRS-Induced Depressive Behavior by Modulating Dopamine Release in the Prefrontal Cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 1314–1323. [Google Scholar] [CrossRef]
- Sanguinetti, J.L.; Hameroff, S.; Smith, E.E.; Sato, T.; Daft, C.M.W.; Tyler, W.J.; Allen, J.J.B. Transcranial Focused Ultrasound to the Right Prefrontal Cortex Improves Mood and Alters Functional Connectivity in Humans. Front. Hum. Neurosci. 2020, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Renner, F.; Siep, N.; Arntz, A.; van de Ven, V.; Peeters, F.; Quaedflieg, C.; Huibers, M.J.H. Negative mood-induction modulates default mode network resting-state functional connectivity in chronic depression. J. Affect. Disord. 2017, 208, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Tyler, W.J. Transcranial Focused Ultrasound Alters Conflict and Emotional Processing, Physiology, and Performance I: Dorsal Anterior Cingulate Cortex Targeting. medRxiv 2020. [Google Scholar] [CrossRef]
- Fini, M.; Tyler, W.J. Transcranial Focused Ultrasound Alters Conflict and Emotional Processing, Physiology, and Performance II: Right Anterior Insula/Frontal Operculum Targeting. PsyArxiv 2020. [Google Scholar] [CrossRef]
- Forster, A.; Rodrigues, J.; Ziebell, P.; Sanguinetti, J.L.; Allen, J.J.B.; Hewig, J. Transcranial focused ultrasound modulates the emergence of learned helplessness via midline theta modification. J. Affect. Disord. 2023, 329, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Ziebell, P.; Rodrigues, J.; Forster, A.; Sanguinetti, J.L.; Allen, J.J.; Hewig, J. Inhibition of midfrontal theta with transcranial ultrasound explains greater approach versus withdrawal behavior in humans. Brain Stimul. 2023, 16, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.; Deckersbach, T.; Guerin, B.; Sretavan Wong, K.; Borron, B.M.; Kanabar, A.; Hayden, A.N.; Long, M.P.; Daneshzand, M.; Pace-Schott, E.F.; et al. Transcranial focused ultrasound of the amygdala modulates fear network activation and connectivity. Brain Stimul. 2024, 17, 312–320. [Google Scholar] [CrossRef]
- Reznik, S.J.; Sanguinetti, J.L.; Tyler, W.J.; Daft, C.; Allen, J.J.B. A double-blind pilot study of transcranial ultrasound (TUS) as a five-day intervention: TUS mitigates worry among depressed participants. Neurol. Psychiatry Brain Res. 2020, 37, 60–66. [Google Scholar] [CrossRef]
- Li, X.; Weng, F.; Sun, W.; Liu, R.; Xu, H. Preliminary Research on Depression Treatment: Combination of Transcranial Magnetic Stimulation and MRI-Guided Low-Intensity Focused Ultrasound Pulsation. J. Med. Imaging Health Inform. 2020, 10, 677–680. [Google Scholar] [CrossRef]
- Beisteiner, R.; Matt, E.; Fan, C.; Baldysiak, H.; Schönfeld, M.; Philippi Novak, T.; Amini, A.; Aslan, T.; Reinecke, R.; Lehrner, J.; et al. Transcranial Pulse Stimulation with Ultrasound in Alzheimer’s Disease—A New Navigated Focal Brain Therapy. Adv. Sci. 2019, 7, 1902583. [Google Scholar] [CrossRef]
- Matt, E.; Dörl, G.; Beisteiner, R. Transcranial pulse stimulation (TPS) improves depression in AD patients on state-of-the-art treatment. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12245. [Google Scholar] [CrossRef] [PubMed]
- Cont, C.; Stute, N.; Galli, A.; Schulte, C.; Logmin, K.; Trenado, C.; Wojtecki, L. Retrospective real-world pilot data on transcranial pulse stimulation in mild to severe Alzheimer’s patients. Front. Neurol. 2022, 13, 948204. [Google Scholar] [CrossRef]
- Riis, T.; Feldman, D.; Losser, A.; Mickey, B.; Kubanek, J. Device for Multifocal Delivery of Ultrasound Into Deep Brain Regions in Humans. IEEE Trans. Biomed. Eng. 2024, 71, 660–668. [Google Scholar] [CrossRef]
- Riis, T.S.; Feldman, D.A.; Vonesh, L.C.; Brown, J.R.; Solzbacher, D.; Kubanek, J.; Mickey, B.J. Durable effects of deep brain ultrasonic neuromodulation on major depression: A case report. J. Med. Case Rep. 2023, 17, 449. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Ryu, J.S.; Kim, J.; Kim, S.; Jeong, H.S.; Kim, K.R.; Kim, H.C.; Yoo, S.S.; Seok, J.H. Effect of Low-Intensity Transcranial Focused Ultrasound Stimulation in Patients With Major Depressive Disorder: A Randomized, Double-Blind, Sham-Controlled Clinical Trial. Psychiatry Investig. 2024, 21, 885–896. [Google Scholar] [CrossRef]
- Fan, J.M.; Woodworth, K.; Murphy, K.R.; Hinkley, L.; Cohen, J.L.; Yoshimura, J.; Choi, I.; Tremblay-McGaw, A.G.; Mergenthaler, J.; Good, C.H.; et al. Thalamic transcranial ultrasound stimulation in treatment resistant depression. Brain Stimul. 2024, 17, 1001–1004. [Google Scholar] [CrossRef]
- Riis, T.S.; Feldman, D.A.; Kwon, S.S.; Vonesh, L.C.; Koppelmans, V.; Brown, J.R.; Solzbacher, D.; Kubanek, J.; Mickey, B.J. Noninvasive modulation of subcallosal cingulate and depression with focused ultrasonic waves. Biol. Psychiatry 2024, 97, 825–834. [Google Scholar] [CrossRef]
- Schachtner, J.N.; Dahill-Fuchel, J.F.; Allen, K.E.; Bawiec, C.R.; Hollender, P.J.; Ornellas, S.B.; Konecky, S.D.; Achrol, A.S.; Allen, J.J.B. Transcranial focused ultrasound targeting the default mode network for the treatment of depression. Front. Psychiatry 2025, 16, 1451828. [Google Scholar] [CrossRef] [PubMed]
- Attali, D.; Tiennot, T.; Manuel, T.J.; Daniel, M.; Houdouin, A.; Annic, P.; Dizeux, A.; Haroche, A.; Dadi, G.; Henensal, A.; et al. Deep transcranial ultrasound stimulation using personalized acoustic metamaterials improves treatment-resistant depression in humans. Brain Stimul. 2025, 18, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, K.D.; Jordan, S.E.; Jordan, K.G.; Rindner, E.S.; Haroon, J.M.; Habelhah, B.; Becerra, S.A.; Surya, J.R.; Venkatraman, V.; Zielinski, M.A.; et al. A pilot study of low-intensity focused ultrasound for treatment-resistant generalized anxiety disorder. J. Psychiatr. Res. 2023, 168, 125–132. [Google Scholar] [CrossRef]
- Barksdale, B.R.; Enten, L.; DeMarco, A.; Kline, R.; Doss, M.K.; Nemeroff, C.B.; Fonzo, G.A. Low-intensity transcranial focused ultrasound amygdala neuromodulation: A double-blind sham-controlled target engagement study and unblinded single-arm clinical trial. Mol. Psychiatry 2025, 30, 4497–4511. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Shapiro, M.G.; Tsao, D.Y. Ultrasonic Neuromodulation Causes Widespread Cortical Activation via an Indirect Auditory Mechanism. Neuron 2018, 98, 1031–1041.e5. [Google Scholar] [CrossRef]
- Guo, H.; Hamilton, M., 2nd; Offutt, S.J.; Gloeckner, C.D.; Li, T.; Kim, Y.; Legon, W.; Alford, J.K.; Lim, H.H. Ultrasound Produces Extensive Brain Activation via a Cochlear Pathway. Neuron 2018, 98, 1020–1030.e4. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Blackmore, J.; Cleveland, R.O.; Butler, C.R. Transcranial ultrasound stimulation in humans is associated with an auditory confound that can be effectively masked. Brain Stimul. 2020, 13, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Guo, Y.; Lin, Z.; Shi, Z.; Bian, T.; Qi, L.; Meng, L.; Grace, A.A.; Zheng, H.; Yuan, T.F. Noninvasive ultrasound deep brain stimulation of nucleus accumbens induces behavioral avoidance. Sci. China Life Sci. 2020, 63, 1328–1336. [Google Scholar] [CrossRef]
- Deveci, E.; Akbas, F.; Ergun, A.S.; Kurtulmus, A.; Kocak, A.B.; Boyraz, R.K.; Tok, O.E.; Aydin, M.S.; Kilic, O.; Bozkurt, A.; et al. The Effects of Transcranial Focused Ultrasound Stimulation of Nucleus Accumbens on Neuronal Gene Expression and Brain Tissue in High Alcohol-Preferring Rats. Mol. Neurobiol. 2023, 60, 1099–1116. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.J., 3rd; Thompson-Lake, D.G.Y.; Ranjan, M.; Marton, J.L.; Carpenter, J.S.; Zheng, W.; Berry, J.H.; Farmer, D.L.; D’Haese, P.; Finomore, V.S.; et al. Low-Intensity Focused Ultrasound Targeting the Bilateral Nucleus Accumbens as a Potential Treatment for Substance Use Disorder: A First-in-Human Report. Biol. Psychiatry 2023, 94, e41–e43. [Google Scholar] [CrossRef]
- Bian, T.; Meng, W.; Qiu, M.; Zhong, Z.; Lin, Z.; Zou, J.; Wang, Y.; Huang, X.; Xu, L.; Yuan, T.; et al. Noninvasive Ultrasound Stimulation of Ventral Tegmental Area Induces Reanimation from General Anaesthesia in Mice. Research 2021, 2021, 2674692. [Google Scholar] [CrossRef]
- Brinker, S.T.; Preiswerk, F.; White, P.J.; Mariano, T.Y.; McDannold, N.J.; Bubrick, E.J. Focused Ultrasound Platform for Investigating Therapeutic Neuromodulation Across the Human Hippocampus. Ultrasound Med. Biol. 2020, 46, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, R.; Liu, D.; Wang, X.; Yuan, W. Effect of low-intensity transcranial ultrasound stimulation on theta and gamma oscillations in the mouse hippocampal CA1. Front. Psychiatry 2023, 14, 1151351. [Google Scholar] [CrossRef]
- Munoz, F.; Meaney, A.; Gross, A.; Liu, K.; Pouliopoulos, A.N.; Liu, D.; Konofagou, E.E.; Ferrera, V.P. Long term study of motivational and cognitive effects of low-intensity focused ultrasound neuromodulation in the dorsal striatum of nonhuman primates. Brain Stimul. 2022, 15, 360–372. [Google Scholar] [CrossRef]
- Chou, T.; Dougherty, D. The effects of varying pulse repetition frequencies and duty cycles for transcranial focused ultrasound (tFUS) of the ventral capsule/ventral striatum for obsessive-compulsive disorder. Brain Stimul. 2023, 16, 154. [Google Scholar] [CrossRef]
- Liu, D.; Munoz, F.; Sanatkhani, S.; Pouliopoulos, A.N.; Konofagou, E.E.; Grinband, J.; Ferrera, V.P. Alteration of functional connectivity in the cortex and major brain networks of non-human primates following focused ultrasound exposure in the dorsal striatum. Brain Stimul. 2023, 16, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Reber, J.; Tranel, D. Sex differences in the functional lateralization of emotion and decision making in the human brain. J. Neurosci. Res. 2017, 95, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Gainotti, G. A historical review of investigations on laterality of emotions in the human brain. J. Hist. Neurosci. 2019, 28, 23–41. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Nohesara, S.; Thiagalingam, S. Epigenome Defines Aberrant Brain Laterality in Major Mental Illnesses. Brain Sci. 2024, 14, 261. [Google Scholar] [CrossRef]
- Eisenstein, M. A sound solution for deep-brain imaging. Nat. Methods 2023, 20, 1623–1628. [Google Scholar] [CrossRef]
- Brown, M.D.; Generowicz, B.S.; Dijkhuizen, S.; Koekkoek, S.K.E.; Strydis, C.; Bosch, J.G.; Arvanitis, P.; Springeling, G.; Leus, G.J.T.; De Zeeuw, C.I.; et al. Four-dimensional computational ultrasound imaging of brain hemodynamics. Sci. Adv. 2024, 10, eadk7957. [Google Scholar] [CrossRef]
- El Hady, A.; Takahashi, D.; Sun, R.; Akinwale, O.; Boyd-Meredith, T.; Zhang, Y.; Charles, A.S.; Brody, C.D. Chronic brain functional ultrasound imaging in freely moving rodents performing cognitive tasks. J. Neurosci. Methods 2024, 403, 110033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gong, C.; Yang, Z.; Wei, F.; Sun, X.; Ji, J.; Zeng, Y.; Chang, C.F.; Liu, X.; Nair, D.S.R.; et al. Ultrasound Flow Imaging Study on Rat Brain with Ultrasound and Light Stimulations. Bioengineering 2024, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jimenez-Gambin, S.; Konofagou, E.E. An all-ultrasound cranial imaging method to establish the relationship between cranial FUS incidence angle and transcranial attenuation in non-human primates in 3D. Sci. Rep. 2024, 14, 1488. [Google Scholar] [CrossRef] [PubMed]
- In, A.; Strohman, A.; Payne, B.; Legon, W. Low-intensity focused ultrasound to the posterior insula reduces temporal summation of pain. Brain Stimul. 2024, 17, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Yu, K.; Yeh, C.-Y.; Fouda, R.; Argueta, D.; Kiven, S.; Ni, Y.; Niu, X.; Chen, Q.; Kim, K.; et al. Low-intensity transcranial focused ultrasound suppresses pain by modulating pain-processing brain circuits. Blood 2024, 144, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Chen, K.T.; Hsu, C.C.; Liu, H.L.; Jiang, Y.T.; Ho, C.W.; Chen, J.C.; Li, H.Y.; Weng, C.C.; Hsu, P.H. Stimulation of dorsal root ganglion with low-intensity focused ultrasound ameliorates pain responses through the GABA inhibitory pathway. Life Sci. 2025, 361, 123323. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.C.; Huang, C.S.; Ing, S.Z.; Yu, H.Y.; Fisher, R.S.; Liu, H.L. Pulsed Focused Ultrasound Reduces Hippocampal Volume Loss and Improves Behavioral Performance in the Kainic Acid Rat Model of Epilepsy. Neurotherapeutics 2023, 20, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, Y.; Xiao, X.; Wang, L.; Wei, G.; Guo, M.; Song, X.; Tian, Y.; Ming, D.; Yang, J.; et al. Low-intensity focused ultrasound stimulation reverses social avoidance behavior in mice experiencing social defeat stress. Cereb. Cortex 2022, 32, 5580–5596. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xu, X.; Yan, C.S.; Mao, M.C.; Luo, K.X.; Zhang, X.F.; Liang, Q.H.; Long, X.J.; Ao, L.J.; Chen, M.X. Case Report: Low-intensity transcranial focused ultrasound stimulation improves social interaction and stereotyped behavior in a boy with autism spectrum disorder. Front. Psychiatry 2025, 16, 1606300. [Google Scholar] [CrossRef] [PubMed]

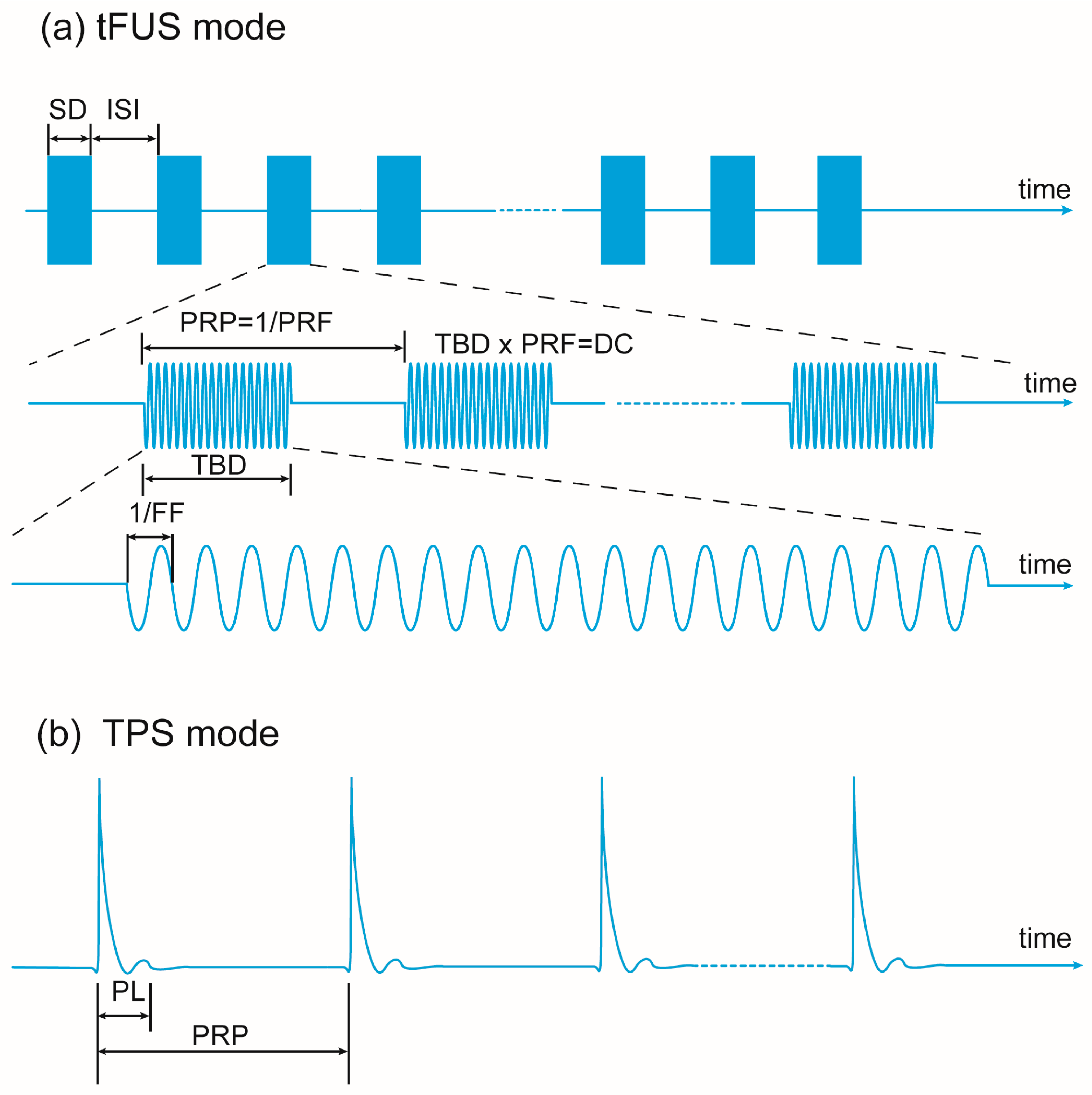

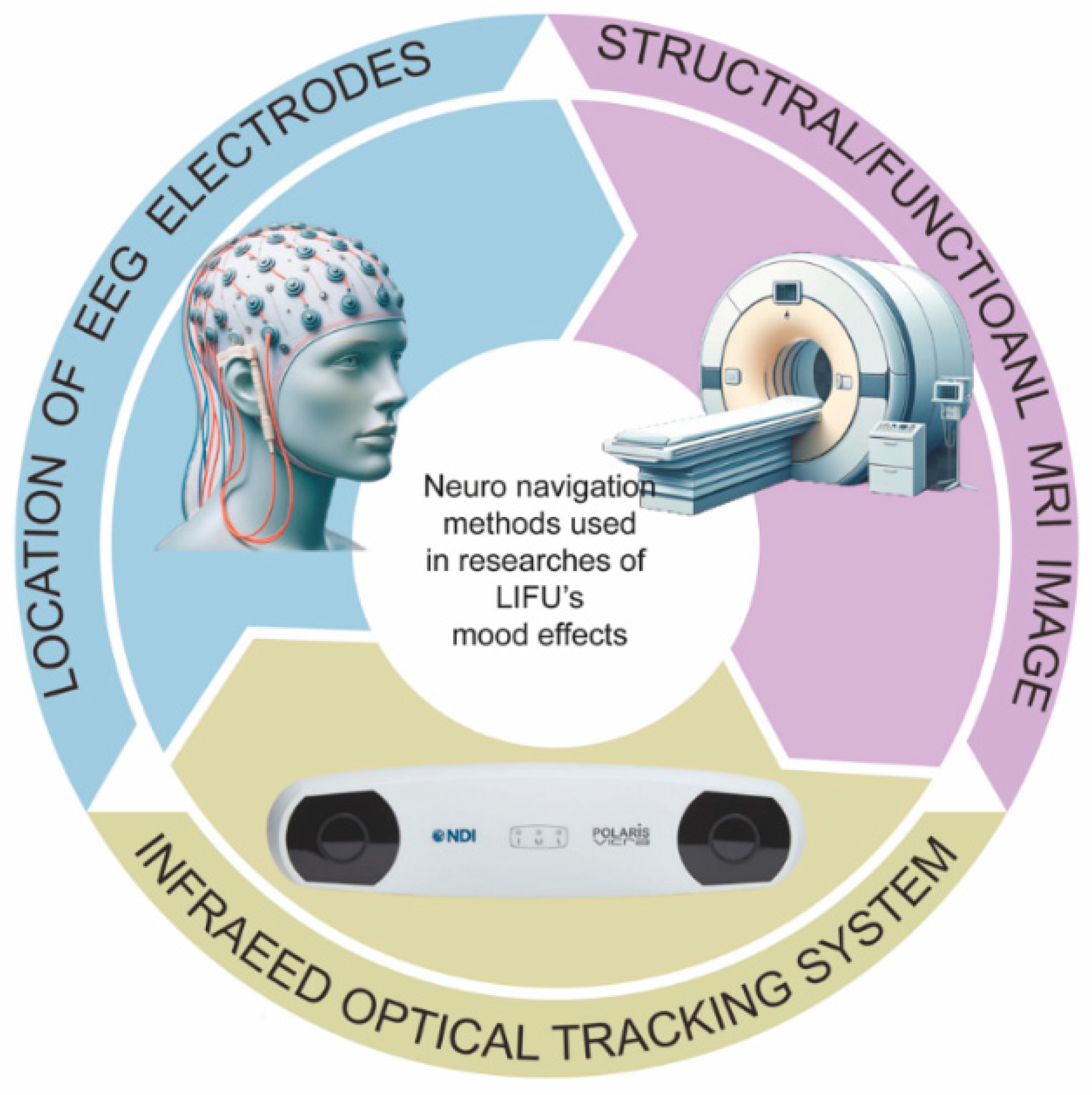
| Study | Animal Species | Animal Model | Target | Sonication Parameters | Protocol | Main Results | Behavior Tests | Damage Report |
|---|---|---|---|---|---|---|---|---|
| Zhang et al. (2019) [44] | S-D rats (n = 76) | depressive model: RS | l-PLC | FF = 0.5 MHz, SD = 0.4 s, ISI = 3 s, TBD = 0.4 ms, PRF = 1.5 KHz, DC = 60%, Pmax = 0.38 MPa, ISPTA = 4.55 W/cm2, ISPPA = 7.59 W/cm2 (without skull) | 15 min per day for 2 weeks | DLB↓, anhedonia↓, exploratory behavior↑; BDNF in left hippocampus↑ | SPT, OFT, FST | none |
| Zhang et al. (2020) [45] | S-D rats (n = 30) | depressive model: CUS | vmPFC | FF = 0.8 MHz, SD = 1 s, ISI = 3 s, TBD = 0.2 ms, PRF = 0.2 KHz, DC = 4%, Pmax = 0.28 MPa, ISPTA = 248 mW/cm2, ISPPA = 6.2 W/cm2 (without skull); ISPTA = 154 mW/cm2, ISPPA = 3.84 W/cm2, MI = 0.28 (with skull) | 20 min per day for 4 weeks | DLB↓, anhedonia↓, despair↓; ERK, mTORC1, S6K, BDNF, TrkB↑ | SPT, OFT, FST | none |
| Yi et al. (2022) [46] | C57 mice (n = 47) | depressive model: lipopolysaccharide-induced | PFC | FF = 0.5 MHz, SD = 60 s, ISI = 120 s, TBD = 5 ms, PRF = 0.1 KHz, DC = 50%, Pmax = 0.62 MPa, ISPPA = 10.09 W/cm2 (without skull) | 30 min single session | ALB↓, DLB↓, despair↓; IL-6, IL-1β, TNF-α in PFC↓ | OFT, FST, TST, EPM | none |
| Zhu et al. (2023) [47] | C57 mice (n = 50) | depressive model: CRS | DRN | FF = 1.1 MHz, SD = 1 s, ISI = 1 s, TBD = 0.5 ms, PRF = 1 KHz, DC = 50%, Pmax = 0.44 MPa, ISPPA = 5.68 W/cm2 (with skull) | 30 min per day for 2 weeks | DLB↓, anhedonia↓, despair↓; 5-HT/c-Fos in DRN↑ | SPT, TST | none |
| Zhu et al. (2022) [48] | C57 mice (n = 10) | depressive model: CRS | PLC and DRN | FF = 1.1 MHz, SD = 1 s, ISI = 1 s, TBD = 0.5 ms, PRF = 1 KHz, DC = 50% | 30 min per day for 1 week | BDNF in PLC↑; 5-HT in DRN↑ | \ | \ |
| Wu et al. (2023) [49] | C57 mice (n = 24) | depressive and anxious model: corticosterone-induced | DRN | FF = 1.1 MHz, SD = 1 s, ISI = 1 s, TBD = 0.5 ms, PRF = 1 KHz, DC = 50%, Pmax = 283 KPa, ISPPA = 2.42 W/cm2 (with skull) | 30 min per day for 3 weeks | ALB↓, DLB↓, anhedonia↓, despair↓; 5-HT, NE↑, c-Fos in DRN↑ | SPT, TST, EPM | none |
| Wang et al. (2023) [58] | S-D rats (n = 57) | depressive model: CUS | mPFC | FF = 0.5 MHz, SD = 0.5 s, ISI = 2 s, TBD = 0.3 ms, PRF = 2 KHz, DC = 60%, ISPTA1 = 0.5 W/cm2, ISPTA2 = 0.23 W/cm2 (without skull) | 15 min per day for 2 weeks | DLB↓; ALB unchanged; anhedonia↓; despair↓ PLV of delta and theta rhythm↑ | SPT, FST, EPM | none |
| Wang et al. (2024) [59] | C57 mice (n = 44) | depressive model: CRS | VTA | FF = 0.5 MHz, SD = 0.2 s, ISI = 1.6 s, TBD = 0.3 ms, PRF = 1.5 KHz, DC = 45%, ISPTA = 150 mW/cm2 (without skull); ISPTA = 90 mW/cm2 (with skull) | 15 min per day for 10 days | DLB↓, anhedonia↓, despair↓; DA level in mPFC↑; increased level of DA in mPFC positively correlated with improved behavior in SPI | SPT, TST, NFT, OFT, EPM, SIT | none |
| Study | Participants | Target | Sonication Parameters | Protocol | Evaluation Methods | Main Results | Navigation Method | Sham or Control Condition Included |
|---|---|---|---|---|---|---|---|---|
| Sanguinetti et al. (2020) [60] | experiment 1: healthy volunteers (n = 51) experiment 2: healthy volunteers (n = 9) | r-IFG | FF = 0.5 MHz, PRF = 40 Hz Experiment 1: TBD = 65 us, DC = 0.26%, Pmax = 1.27 MPa, ISPTA = 130 mW/cm2, ISPPA = 54 W/cm2, MI = 1.79 (without skull); Experiment 2: TBD = 125 us, DC = 0.5%, Pmax = 1.26 MPa, ISPTA = 272 mW/cm2, ISPPA = 54 W/cm2, MI = 1.79 (without skull) | experiment 1: single session 30 s experiment 2: single session 2 min | VAMS, GA, GV, fMRI | experiment 1: 30 s LIFU can induce positive mood effects for at least 30 min; experiment 2: FC in r-IFG↓,FC in DMN↓, FC of r-IFG and r-MFG↑ | location of EEG F8 electrode | sham group |
| Fini et al. (2020) [62] | healthy volunteers (n = 28) | dACC | FF = 0.5 MHz, PRF = 1 KHz, DC = 24%, Pmax = 1 MPa, ISPPA = 20.4 W/cm2 (without skull) | single session 500 ms | PANAS, HRV, EEG | parasympathetic markers of HRV↑, emotional processing altered, sustained attention enhanced, significant effects on ERPs, alteration of alpha/delta/theta band of EEG | MRI + infrared optical tracking system | sham group |
| Fini et al. (2020) [63] | healthy volunteers (n = 28) | AINS/FO | FF = 1 MHz, SD = 0.5 s, ISI < 2.8 s, TBD = 240 us, PRF = 1 KHz, DC = 24%, Pmax = 1 MPa, ISPPA = 20.4 W/cm2 (without skull) | single session | PANAS, HRV, EEG | fear response/HRV/performance of emotional distraction interference altered; ERP/delta/alpha/beta/theta band of EEG altered | MRI + infrared optical tracking system | sham group |
| Forster et al. (2023) [64] | healthy volunteers (n = 55) | r-DLPFC | FF = 0.5 MHz, TBD = 125 us, PRF = 40 Hz, DC = 0.5%, Pmax = 1.09 MPa, ISPTA = 199 mW/cm2, ISPPA = 40 W/cm2, MI = 1.54 (without skull) | single session 120 s | EEG, ECG, VAMS, BDI-V | offline effects lasted 30–90 min, suppression of midline delta, better than average performance in learned helpless task | location of EEG F8 electrode | sham and control group |
| Ziebell et al. (2023) [65] | healthy volunteers (n = 152) | r-PFC | FF = 0.5 MHz, TBD = 125 us, PRF = 40 Hz, DC = 0.5%, Pmax = 1.09 MPa, ISPTA = 199 mW/cm2, ISPPA = 40 W/cm2, MI = 1.54 (without skull) | single session 120 s | EEG, objective behavioral measurement, SAM | anxiety↓, long-lasting r-PFC TUS effects (50–100 min) on physiology and behavior among large sample | location of EEG F8 electrode | sham stimulation |
| Chou et al. (2024) [66] | healthy volunteers (n = 30) | l-amygdala | FF = 0.65 MHz, SD = 30 s, ISI = 30 s, TBD = 5 ms, PRF = 10 Hz, DC = 5%, ISPTA = 0.72 W/cm2, ISPPA = 14.4 W/cm2 (without skull) | single session 20 min | fMRI, subjective anxiety rating scale | anxiety rating↓, amygdala BOLD signal during fear task↓, amygdala activation correlated with decreased subjective anxiety↓ | offline MRI with fiducial markers on the transducer | sham group |
| Study | Participants | Target | Sonication Parameters | Protocol | Evaluation Method | Main Results | Side Effects | Navigation Method | Sham or Control Condition Included |
|---|---|---|---|---|---|---|---|---|---|
| Reznik et al. (2020) [67] | college students with mild to moderate depression (n = 24) | r-IFG | FF = 0.5 MHz, Pmax = 0.65 MPa, ISPTA = 71 mW/cm2, ISPPA = 14 W/cm2, MI = 0.9 (without skull) | 30 s per session, 5 LIFU sessions within 7 days | BDI, OASIS, RRS, and PSWQ | severity of depression and anxiety not reduced, trait worry↓, happiness↑, global affect↑ | not reported | EEG F8 location | sham group |
| LI et al. (2020) [68] | MDD patients (n = 60) | lateral orbitofrontal cortex; cuneiform lobe; dorsal prefrontal cortex | FF = 0.65 MHz, SD = 30 s, ISI = 30 s, TBD = 0.5 ms, PRF = 100 Hz, ISPTA = 720 mW/cm2 (without skull) | 15 min per session, 5 days in a week for 2 months | HRSD, GDS, PSQI | HRSD↓, GDS↓, PSQI↑ | 10% adverse reaction (dizziness, vomiting) | fMRI-guided | control group |
| Beisteiner et al. (2019) [69] | AD patients (n = 35) | center1: dorsolateral prefrontal cortex, bilateral frontal cortex (dorsolateral prefrontal cortex and inferior frontal cortex), bilateral lateral parietal cortex, extended precuneus cortex; center2: global brain stimulation (no specific target) | center1: PL = 3 us, PRF = 4 Hz, EFD = 0.25 mJ/mm2, Pmax = 25 MPa, ISPTA = 0.1 W/cm2 (without skull), NOPs = 6000 center2: PL = 3 us, PRF = 5 Hz, EFD = 0.2 mJ/mm2, Pmax = 25 MPa, ISPTA = 0.1 W/cm2 (without skull), NOPs = 6000 | every ROI stimulated twice per session, 3 sessions per week for 2–4 weeks | CERAD scores, GDS, BDI, fMRI | memory and verbal ability↑; FC for hippocampus, parahippocampal cortex, parietal cortex, and precuneus↑; increased of FC is correlated with improved CERAD scores, LIFU effects lasted for 3 months | 4% headache, 3% mood deterioration, no hemorrhages and edema | infrared camera tracking system, MRI-based neuronavigation system | sham stimulation |
| Matt et al. (2022) [70] | AD patients (n = 18) | bilateral frontal cortex (dorsolateral prefrontal cortex and inferior frontal cortex), bilateral lateral parietal cortex, extended precuneus cortex | PL = 3 us, PRF = 5 Hz, EFD = 0.2 mJ/mm2 (without skull), NOPs = 6000 | 3 sessions per week for 2–4 weeks | BDI-II, fMRI | FC between L-FOrb and R-AIsula decreased, and negative correlation with BDI improvements; FC between VMN and SN trended to be normalized | not reported | infrared camera tracking system, MRI-based neuronavigation system | none |
| Cont et al. (2022) [71] | AD patients (n = 11) | bilateral frontal cortex, bilateral lateral parietal cortex, extended precuneus cortex, bilateral temporal cortex | PRF = 4 Hz, EFD = 0.20 mJ/mm2 | 6000 pulses per session, six sessions over 2 weeks; or 3000 pulses per session, 12 sessions every day | ADAS, MMSE, MoCA, NRSs | ADAS and ADAS improved, self-reported symptom severity↓, depressive symptoms↓, cognition↑ | shown in 4% sessions (pain in jaw, nausea, drowsiness) | MRI-guided system | none |
| Riis et al. (2024) [72] | MDD patients (n = 2) | SGC, ventral striatum | FF = 0.65 MHz, TBD = 30 ms, PRP = 4.03 s, Pmax = 1.0 MPa, ISPTA = 0.233 W/cm2, ISPPA = 31 W/cm2 (without skull), MI = 1.2 | single session for 60–180 s | 7-point scale, GASE | depression symptom↓, anxiety symptom↓ | none | single anatomical MRI scan | sham stimulation |
| Riis et al. (2023) [73] | severe TRD patient with family history of mood disorders (n = 1) | SCC (posterior SCC, anterior SCC), pregenual cingulate | FF = 0.65 MHz, SD = 1 min, ISI = 1 min, TBD = 30 ms, PRP = 4.03 s, DC = 0.75%, Pmax = 1.0 MPa | single session treatment: each target was sonicated for 2 min, 10 times in random order | fMRI, HRSD-6, GASE | SCC BOLD signal suppressed; rapid and sustained remission of depressive symptoms | none | single anatomical MRI scan | sham stimulation |
| Oh et al. (2024) [74] | MDD patients (n = 40) | l-DLPFC | FF = 0.25 MHz, SD = 0.3 s, ISI = 6 s, TBD = 1 ms, PRF = 500 Hz, DC = 50%, Pmax = 0.3 MPa, ISPTA = 3 W/cm2 (without skull) | 20 min per session, 6 sessions in 2 weeks (thrice a week) | fMRI, MADRS, QIDS-SR, STAI, SSI, K-POMS | depression↓, anxiety↓, suicidal ideation↓; FC between sgACC and associated brain regions ↑ | none | CT/MRI | sham group |
| Fan et al. (2024) [75] | TRD patient (n = 1) | VC, BNST, ANT | FF = 0.5 MHz, SD = 5.2 ms, ISI = 34.8 ms, PRF = 25 Hz, DC = 13%, Pmax = 1.169 MPa, ISPTA = 10.6 W/cm2, ISPPA = 42.2–50.2 W/cm2, MI= 1.654 (without skull) | single session treatment: 300 s per stimulation, 8 stimulations per session with 10 min interval, | VAS, HRSD-6 | DMN connectivity ↓; depression↓ | none | MRI image | control stimulation (unfocused ultrasound) |
| Riis et al. (2024) [76] | TRD patients (n = 22) | SCC (anterior, middle, posterior) | FF = 0.65 MHz, SD = 30 ms, ISI = 0.7 s or 1.4 s, TBD = 5 ms, PRF = 100 Hz, DC = 50%, Pmax = 1 MPa, ISPPA = 31.1 W/cm2 | 2 sessions in a week; one session contained 3 blocks. block A: tolerability test block B: 6 three-minute stimulation block C: 6 three-minute stimulation | PANAS-X, HRSD-6, IDS-SR, GAD-7, fMRI | activity of SCC↓; activity of l-vlPFC, right-superior temporal gyrus↑; depression↓ | no severe adverse effects within 24 h; 2 significant mood swings within 24–72 h | MRI | sham group |
| Schachtner et al. (2025) [77] | MDD patients (n = 20) | amPFC | FF = 400 kHz, TBD = 5 ms, PRF = 10 Hz, Pmax = 820 kPa, ISPTA = 670 mW/cm2 (without skull) | 11 sessions in up to 3 weeks | BDI-II, HRSD, PTQ | depression↑; RNT↓; rapid antidepressant effects | no serious adverse events | MRI | none |
| Attali et al. (2025) [78] | MDD (n = 5) | intersection of 3 white matter tracts in l-SCC: FM, UF, CB | FF = 500 kHz, SD = 5 s, ISI = 10 s, TBD = 4.5 ms, PRF = 14 Hz, DC = 6%, Pmax = MPa, ISPTA = 184.4 ± 74.0 mW/cm2 (with metalens), MI =1.61 | 5 min per session, 25 sessions in 5 days | MADRS, HRSD, QIDS-SR | depression↓; FC between L-SCC and l-DLPFC↑; FC between SCC and right hippocampal, r-parahippocampal region↓ | no serious adverse events | MRI, CT, optical neuronavigator system | none |
| Study | Participants | Target | Sonication Parameters | Protocol | Evaluation Method | Main Results | Side Effects | Navigation Method | Sham or Control Condition Included |
|---|---|---|---|---|---|---|---|---|---|
| Mahdavi et al. (2023) [79] | severe trGAD patients (n = 25) | centromedial nucleus of r-amygdala | FF = 0.65 MHz, SD = 30 s, ISI = 30 s, TBD = 5 ms, PRF = 10 Hz, DC = 5%, Pmax = 0.61 MPa, ISPTA = 719.73 mW/cm2, ISPPA = 14.39 W/cm2, MI = 0.75 (without skull) | 10 min per session, weekly for 8 weeks | HAM-A, BAI, PGI-I | anxiety symptoms↓, 64% gained significant benefit, 32% achieved remission of GAD | none | functional and structural MRI, optical neuronavigation system | none |
| Barksdale et al. (2025) [80] | MATRDs patients (n = 29); HC (n = 23) | l-amygdala | SD = 30 s, ISI = 30 s, TBD = 5 ms, PRF = 10 Hz, DC = 5%, Pmax = 0.64 MPa, ISPTA = 719.91 mW/cm2, ISPPA = 14.4 W/cm2 | 10 min per session, 15 sessions daily per week for 3 weeks | MASQ-GD | activity in l-amygdala and r-amygdala↓; MASQ-GD↓ | non-severe side effects | MRI, optical neuronavigation system | sham stimulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, A.; Huang, M.; Wang, Z.; Zhou, H.; Duan, H.; Hu, S.; Zheng, Y. Using Low-Intensity Focused Ultrasound to Treat Depression and Anxiety Disorders: A Review of Current Evidence. Brain Sci. 2025, 15, 1129. https://doi.org/10.3390/brainsci15101129
Du A, Huang M, Wang Z, Zhou H, Duan H, Hu S, Zheng Y. Using Low-Intensity Focused Ultrasound to Treat Depression and Anxiety Disorders: A Review of Current Evidence. Brain Sciences. 2025; 15(10):1129. https://doi.org/10.3390/brainsci15101129
Chicago/Turabian StyleDu, Ao, Manli Huang, Zheng Wang, Hetong Zhou, Huilong Duan, Shaohua Hu, and Yinfei Zheng. 2025. "Using Low-Intensity Focused Ultrasound to Treat Depression and Anxiety Disorders: A Review of Current Evidence" Brain Sciences 15, no. 10: 1129. https://doi.org/10.3390/brainsci15101129
APA StyleDu, A., Huang, M., Wang, Z., Zhou, H., Duan, H., Hu, S., & Zheng, Y. (2025). Using Low-Intensity Focused Ultrasound to Treat Depression and Anxiety Disorders: A Review of Current Evidence. Brain Sciences, 15(10), 1129. https://doi.org/10.3390/brainsci15101129








