Use of Functional Near-Infrared Spectroscopy to Predict and Measure Cochlear Implant Outcomes: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Searches
2.4. Selection of Sources of Evidence
2.5. Data Charting Process
2.6. Data Items and Synthesis of Results
3. Results
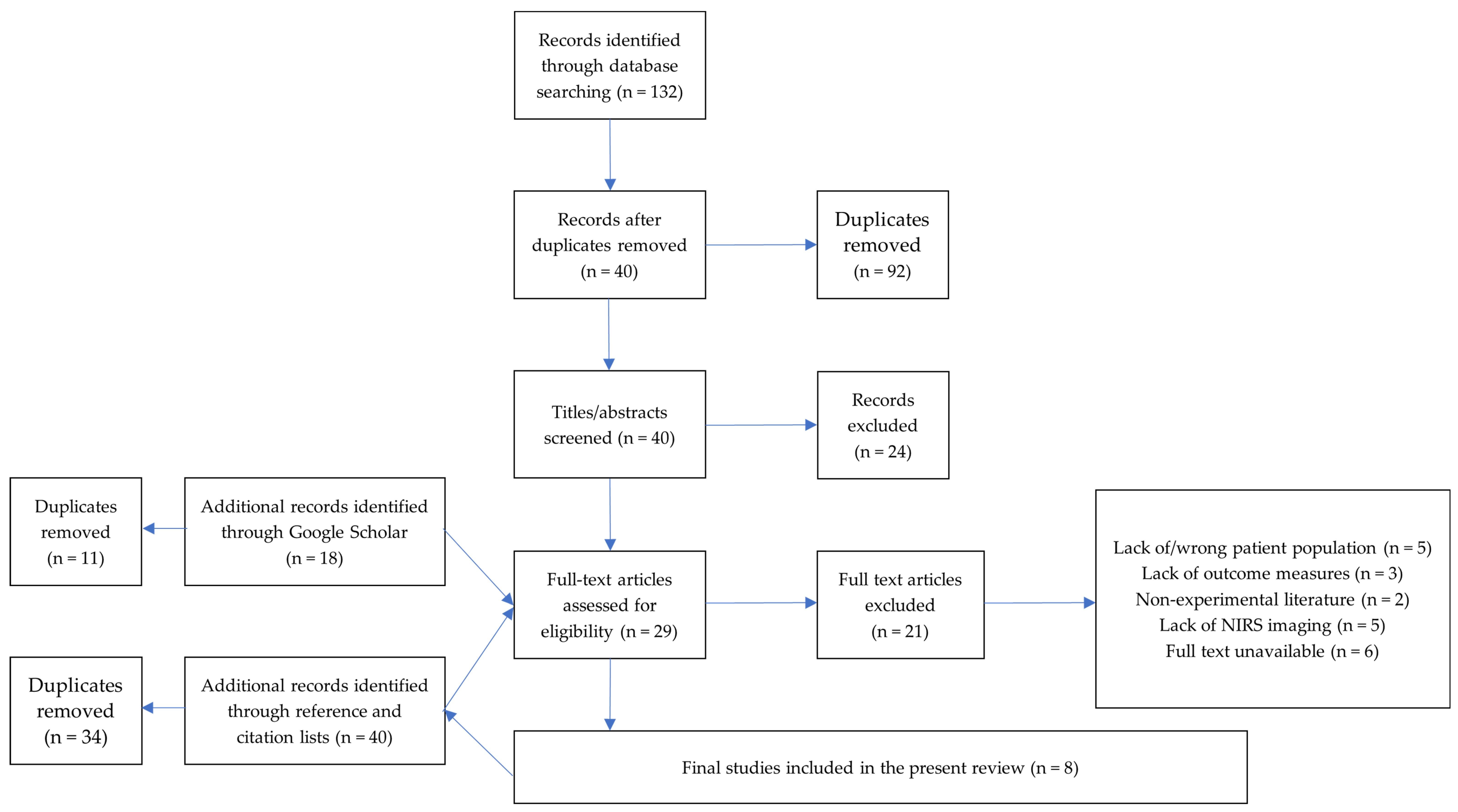
3.1. Selection of Sources of Evidence
3.2. Characteristics of Sources of Evidence
3.3. Results of Individual Sources of Evidence
3.4. Synthesis of Results
4. Discussion
4.1. Overview of Results from Research in This Field
4.2. Populations That Have Participated in Research in This Field
4.3. Clinical Outcomes the Field Has Tried to Measure or Predict with fNIRS Imaging
4.4. fNIRS Measurements
4.5. Implications for Future Research
4.5.1. Heterogenous Samples
4.5.2. Pediatric and Geriatric Research
4.5.3. Outcome Measures
4.5.4. Imaging Techniques
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Report on Disability 2011; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Addressing the Rising Prevalence of Hearing Loss; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. Newborn and Infant Hearing Screening. Available online: http://www.who.int/blindness/publications/Newborn_and_Infant_Hearing_Screening_Report.pdf (accessed on 6 March 2020).
- Aurélio, F.S.; Tochetto, T.M. Newborn hearing screening: Experiences of different countries. Int. Arch. Otorhinolaryngol. 2010, 14, 355–363. [Google Scholar]
- Dalton, D.S.; Cruickshanks, K.J.; Klein, B.E.; Klein, R.; Wiley, T.L.; Nondahl, D.M. The impact of hearing loss on quality of life in older adults. Gerontologist 2003, 43, 661–668. [Google Scholar] [CrossRef]
- Chia, E.-M.; Wang, J.J.; Rochtchina, E.; Cumming, R.R.; Newall, P.; Mitchell, P. Hearing impairment and health-related quality of life: The blue mountains hearing study. Ear Hear. 2007, 28, 187–195. [Google Scholar] [CrossRef]
- Bess, F.H.; Dodd-Murphy, J.; Parker, R.A. Children with minimal sensorineural hearing loss: Prevalence, educational performance, and functional status. Ear Hear. 1998, 19, 339–354. [Google Scholar] [CrossRef]
- Wiley, S.; Jahnke, M.; Meinzen-Derr, J.; Choo, D. Perceived qualitative benefits of cochlear implants in children with multi-handicaps. Int. J. Pediatric Otorhinolaryngol. 2005, 69, 791–798. [Google Scholar] [CrossRef]
- Kelsay, D.; Tyler, R.S. Advantages and disadvantages expected and realized by pediatric cochlear implant recipients as reported by their parents. Am. J. Otol. 1996, 17, 866–873. [Google Scholar]
- Van Hoesel, R.J. Exploring the benefits of bilateral cochlear implants. Audiol. Neurotol. 2004, 9, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.F.D.; Couto, M.I.V.; Martinho-Carvalho, A.C. Quality of life and cochlear implant: Results in adults with postlingual hearing loss☆. Braz. J. Otorhinolaryngol. 2018, 84, 494–499. [Google Scholar] [CrossRef]
- Klop, W.M.C.; Briaire, J.J.; Stiggelbout, A.M.; Frijns, J.H. Cochlear implant outcomes and quality of life in adults with prelingual deafness. Laryngoscope 2007, 117, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Damen, G.W.; Beynon, A.J.; Krabbe, P.F.; Mulder, J.J.; Mylanus, E.A. Cochlear implantation and quality of life in postlingually deaf adults: Long-term follow-up. Otolaryngol. Head Neck Surg. 2007, 136, 597–604. [Google Scholar] [CrossRef]
- Geers, A.E.; Nicholas, J.G.; Moog, J.S. Estimating the influence of cochlear implantation on language development in children. Audiol. Med. 2007, 5, 262–273. [Google Scholar] [CrossRef]
- Geers, A.E.; Nicholas, L. Persistent language delay versus late language emergence in children with early cochlear implantation. J. Speech Lang. Hear. Res. 2016, 59, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.L.; Waltzman, S.B.; Roland, J.T., Jr.; Staller, S.J.; Hoffman, R.A. Early results using the nucleus ci24m in children. Am. J. Otol. 1999, 20, 198–204. [Google Scholar]
- Lenarz, M.; Joseph, G.; Sönmez, H.; Büchner, A.; Lenarz, T. Effect of technological advances on cochlear implant performance in adults. Laryngoscope 2011, 121, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Lazard, D.S.; Bordure, P.; Lina-Granade, G.; Magnan, J.; Meller, R.; Meyer, B.; Radafy, E.; Roux, P.-E.; Gnansia, D.; Péan, V. Speech perception performance for 100 post-lingually deaf adults fitted with neurelec cochlear implants: Comparison between digisonic® convex and digisonic® sp devices after a 1-year follow-up. Acta Oto Laryngol. 2010, 130, 1267–1273. [Google Scholar] [CrossRef]
- Blamey, P.; Artieres, F.; Başkent, D.; Bergeron, F.; Beynon, A.; Burke, E.; Dillier, N.; Dowell, R.; Fraysse, B.; Gallégo, S. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiol. Neurotol. 2013, 18, 36–47. [Google Scholar] [CrossRef]
- Lazard, D.S.; Vincent, C.; Venail, F.; Van de Heyning, P.; Truy, E.; Sterkers, O.; Skarzynski, P.H.; Skarzynski, H.; Schauwers, K.; O’Leary, S. Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: A new conceptual model over time. PLoS ONE 2012, 7, e48739. [Google Scholar] [CrossRef] [PubMed]
- Blamey, P.; Arndt, P.; Bergeron, F.; Bredberg, G.; Brimacombe, J.; Facer, G.; Larky, J.; Lindström, B.; Nedzelski, J.; Peterson, A. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol. Neurotol. 1996, 1, 293–306. [Google Scholar]
- Nikolopoulos, T.P.; O’Donoghue, G.M.; Archbold, S. Age at implantation: Its importance in pediatric cochlear implantation. Laryngoscope 1999, 109, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Wiesel, T.N.; Hubel, D.H. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 1963, 26, 1003–1017. [Google Scholar] [CrossRef]
- Wiesel, T.N.; Hubel, D.H. Effects of visual deprivation on morphology and physiology of cells in the cat’s lateral geniculate body. J. Neurophysiol. 1963, 26, 978–993. [Google Scholar] [CrossRef]
- Macharadze, T.; Budinger, E.; Brosch, M.; Scheich, H.; Ohl, F.W.; Henschke, J.U. Early sensory loss alters the dendritic branching and spine density of supragranular pyramidal neurons in rodent primary sensory cortices. Front. Neural Circuits 2019, 13, 61. [Google Scholar] [CrossRef]
- Merabet, L.B.; Pascual-Leone, A. Neural reorganization following sensory loss: The opportunity of change. Nat. Rev. Neurosci. 2010, 11, 44–52. [Google Scholar] [CrossRef]
- Sadato, N.; Pascual-Leone, A.; Grafman, J.; Ibañez, V.; Deiber, M.-P.; Dold, G.; Hallett, M. Activation of the primary visual cortex by braille reading in blind subjects. Nature 1996, 380, 526–528. [Google Scholar] [CrossRef]
- Gougoux, F.; Belin, P.; Voss, P.; Lepore, F.; Lassonde, M.; Zatorre, R.J. Voice perception in blind persons: A functional magnetic resonance imaging study. Neuropsychologia 2009, 47, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Lazard, D.S.; Lee, H.J.; Truy, E.; Giraud, A.L. Bilateral reorganization of posterior temporal cortices in post-lingual deafness and its relation to cochlear implant outcome. Hum. Brain Mapp. 2013, 34, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Levänen, S. Neuromagnetic studies of human auditory cortex function and reorganization. Scand. Audiol. 1998, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Karns, C.M.; Dow, M.W.; Neville, H.J. Altered cross-modal processing in the primary auditory cortex of congenitally deaf adults: A visual-somatosensory fmri study with a double-flash illusion. J. Neurosci. 2012, 32, 9626–9638. [Google Scholar] [CrossRef]
- Finney, E.M.; Fine, I.; Dobkins, K.R. Visual stimuli activate auditory cortex in the deaf. Nat. Neurosci. 2001, 4, 1171–1173. [Google Scholar] [CrossRef]
- Finney, E.M.; Clementz, B.A.; Hickok, G.; Dobkins, K.R. Visual stimuli activate auditory cortex in deaf subjects: Evidence from meg. Neuroreport 2003, 14, 1425–1427. [Google Scholar] [CrossRef]
- Cardin, V.; Grin, K.; Vinogradova, V.; Manini, B. Crossmodal reorganisation in deafness: Mechanisms for functional preservation and functional change. Neurosci. Biobehav. Rev. 2020, 113, 227–237. [Google Scholar] [CrossRef]
- Bottari, D.; Heimler, B.; Caclin, A.; Dalmolin, A.; Giard, M.-H.; Pavani, F. Visual change detection recruits auditory cortices in early deafness. Neuroimage 2014, 94, 172–184. [Google Scholar] [CrossRef]
- Auer, E.T., Jr.; Bernstein, L.E.; Sungkarat, W.; Singh, M. Vibrotactile activation of the auditory cortices in deaf versus hearing adults. Neuroreport 2007, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Giraud, A.L.; Truy, E. The contribution of visual areas to speech comprehension: A pet study in cochlear implants patients and normal-hearing subjects. Neuropsychologia 2002, 40, 1562–1569. [Google Scholar] [CrossRef]
- Giraud, A.-L.; Price, C.J.; Graham, J.M.; Truy, E.; Frackowiak, R.S. Cross-modal plasticity underpins language recovery after cochlear implantation. Neuron 2001, 30, 657–664. [Google Scholar] [CrossRef]
- Hauthal, N.; Thorne, J.D.; Debener, S.; Sandmann, P. Source localisation of visual evoked potentials in congenitally deaf individuals. Brain Topogr. 2014, 27, 412–424. [Google Scholar] [CrossRef]
- Goldreich, D.; Kanics, I.M. Tactile acuity is enhanced in blindness. J. Neurosci. 2003, 23, 3439–3445. [Google Scholar] [CrossRef]
- Gougoux, F.; Zatorre, R.J.; Lassonde, M.; Voss, P.; Lepore, F. A functional neuroimaging study of sound localization: Visual cortex activity predicts performance in early-blind individuals. PLoS Biol. 2005, 3, e27. [Google Scholar] [CrossRef]
- Röder, B.; Rösler, F.; Neville, H.J. Auditory memory in congenitally blind adults: A behavioral-electrophysiological investigation. Cogn. Brain Res. 2001, 11, 289–303. [Google Scholar] [CrossRef]
- Kupers, R.; Pappens, M.; de Noordhout, A.M.; Schoenen, J.; Ptito, M.; Fumal, A. Rtms of the occipital cortex abolishes braille reading and repetition priming in blind subjects. Neurology 2007, 68, 691–693. [Google Scholar] [CrossRef]
- Amedi, A.; Floel, A.; Knecht, S.; Zohary, E.; Cohen, L.G. Transcranial magnetic stimulation of the occipital pole interferes with verbal processing in blind subjects. Nat. Neurosci. 2004, 7, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Levänen, S.; Hamdorf, D. Feeling vibrations: Enhanced tactile sensitivity in congenitally deaf humans. Neurosci. Lett. 2001, 301, 75–77. [Google Scholar] [CrossRef]
- Bosworth, R.G.; Dobkins, K.R. Visual field asymmetries for motion processing in deaf and hearing signers. Brain Cogn. 2002, 49, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, R.G.; Dobkins, K.R. The effects of spatial attention on motion processing in deaf signers, hearing signers, and hearing nonsigners. Brain Cogn. 2002, 49, 152–169. [Google Scholar] [CrossRef]
- Dye, M.W.; Hauser, P.C.; Bavelier, D. Is visual selective attention in deaf individuals enhanced or deficient? The case of the useful field of view. PLoS ONE 2009, 4, e5640. [Google Scholar] [CrossRef]
- Buckley, D.; Codina, C.; Bhardwaj, P.; Pascalis, O. Action video game players and deaf observers have larger goldmann visual fields. Vis. Res. 2010, 50, 548–556. [Google Scholar] [CrossRef]
- Sandmann, P.; Dillier, N.; Eichele, T.; Meyer, M.; Kegel, A.; Pascual-Marqui, R.D.; Marcar, V.L.; Jäncke, L.; Debener, S. Visual activation of auditory cortex reflects maladaptive plasticity in cochlear implant users. Brain 2012, 135, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Strelnikov, K.; Rouger, J.; Demonet, J.-F.; Lagleyre, S.; Fraysse, B.; Deguine, O.; Barone, P. Visual activity predicts auditory recovery from deafness after adult cochlear implantation. Brain 2013, 136, 3682–3695. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, J.S.; Oh, S.H.; Kim, S.-K.; Kim, J.-W.; Chung, J.-K.; Lee, M.C.; Kim, C.S. Cross-modal plasticity and cochlear implants. Nature 2001, 409, 149–150. [Google Scholar] [CrossRef]
- Giraud, A.-L.; Lee, H.-J. Predicting cochlear implant outcome from brain organisation in the deaf. Restor. Neurol. Neurosci. 2007, 25, 381–390. [Google Scholar]
- Kim, M.-B.; Shim, H.-Y.; Jin, S.H.; Kang, S.; Woo, J.; Han, J.C.; Lee, J.Y.; Kim, M.; Cho, Y.-S.; Moon, I.J. Cross-modal and intra-modal characteristics of visual function and speech perception performance in postlingually deafened, cochlear implant users. PLoS ONE 2016, 11, e0148466. [Google Scholar] [CrossRef]
- Buckley, K.A.; Tobey, E.A. Cross-modal plasticity and speech perception in pre-and postlingually deaf cochlear implant users. Ear Hear. 2011, 32, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Heimler, B.; Weisz, N.; Collignon, O. Revisiting the adaptive and maladaptive effects of crossmodal plasticity. Neuroscience 2014, 283, 44–63. [Google Scholar] [CrossRef]
- Anderson, C.A.; Lazard, D.S.; Hartley, D.E. Plasticity in bilateral superior temporal cortex: Effects of deafness and cochlear implantation on auditory and visual speech processing. Hear. Res. 2017, 343, 138–149. [Google Scholar] [CrossRef]
- Lyness, C.R.; Woll, B.; Campbell, R.; Cardin, V. How does visual language affect crossmodal plasticity and cochlear implant success? Neurosci. Biobehav. Rev. 2013, 37, 2621–2630. [Google Scholar] [CrossRef]
- Lazard, D.S.; Lee, H.-J.; Gaebler, M.; Kell, C.A.; Truy, E.; Giraud, A.-L. Phonological processing in post-lingual deafness and cochlear implant outcome. Neuroimage 2010, 49, 3443–3451. [Google Scholar] [CrossRef]
- Feng, G.; Ingvalson, E.M.; Grieco-Calub, T.M.; Roberts, M.Y.; Ryan, M.E.; Birmingham, P.; Burrowes, D.; Young, N.M.; Wong, P.C. Neural preservation underlies speech improvement from auditory deprivation in young cochlear implant recipients. Proc. Natl. Acad. Sci. USA 2018, 115, E1022–E1031. [Google Scholar] [CrossRef] [PubMed]
- Gaab, N.; Gabrieli, J.D.; Glover, G.H. Assessing the influence of scanner background noise on auditory processing. I. An fmri study comparing three experimental designs with varying degrees of scanner noise. Hum. Brain Mapp. 2007, 28, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Scarff, C.J.; Dort, J.C.; Eggermont, J.J.; Goodyear, B.G. The effect of mr scanner noise on auditory cortex activity using fmri. Hum. Brain Mapp. 2004, 22, 341–349. [Google Scholar] [CrossRef] [PubMed]
- BinKhamis, G.; Perugia, E.; O’Driscoll, M.; Kluk, K. Speech-abrs in cochlear implant recipients: Feasibility study. Int. J. Audiol. 2019, 58, 678–684. [Google Scholar] [CrossRef]
- Deprez, H.; Gransier, R.; Hofmann, M.; van Wieringen, A.; Wouters, J.; Moonen, M. Independent component analysis for cochlear implant artifacts attenuation from electrically evoked auditory steady-state response measurements. J. Neural Eng. 2017, 15, 016006. [Google Scholar] [CrossRef]
- Fujiki, N.; Naito, Y.; Hirano, S.; Kojima, H.; Shiomi, Y.; Nishizawa, S.; Konishi, J.; Honjo, I. Correlation between rcbf and speech perception in cochlear implant users. Auris Nasus Larynx 1999, 26, 229–236. [Google Scholar] [CrossRef]
- Green, K.M.; Julyan, P.J.; Hastings, D.L.; Ramsden, R.T. Auditory cortical activation and speech perception in cochlear implant users: Effects of implant experience and duration of deafness. Hear. Res. 2005, 205, 184–192. [Google Scholar] [CrossRef]
- Mortensen, M.V.; Mirz, F.; Gjedde, A. Restored speech comprehension linked to activity in left inferior prefrontal and right temporal cortices in postlingual deafness. Neuroimage 2006, 31, 842–852. [Google Scholar] [CrossRef]
- Boas, D.A.; Dale, A.M.; Franceschini, M.A. Diffuse optical imaging of brain activation: Approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage 2004, 23, S275–S288. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fnirs) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Harrison, S.; Hartley, D. Shedding light on the human auditory cortex: A review of the advances in near infrared spectroscopy (nirs). Rep. Med. Imaging 2019, 12, 31–42. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. Homer: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Fox, S.; Blasi, A.; Elwell, C. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010, 34, 269–284. [Google Scholar] [CrossRef]
- Saliba, J.; Bortfeld, H.; Levitin, D.J.; Oghalai, J.S. Functional near-infrared spectroscopy for neuroimaging in cochlear implant recipients. Hear. Res. 2016, 338, 64–75. [Google Scholar] [CrossRef]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Pavia, J.M.; Wolf, U.; Wolf, M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 2014, 85, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Tak, S.; Ye, J.C. Statistical analysis of fnirs data: A comprehensive review. Neuroimage 2014, 85, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Dewey, R.S.; Hartley, D.E. Cortical cross-modal plasticity following deafness measured using functional near-infrared spectroscopy. Hear. Res. 2015, 325, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Bisconti, S.; Shulkin, M.; Hu, X.; Basura, G.J.; Kileny, P.R.; Kovelman, I. Functional near-infrared spectroscopy brain imaging investigation of phonological awareness and passage comprehension abilities in adult recipients of cochlear implants. J. Speech Lang. Hear. Res. 2016, 59, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Wiggins, I.M.; Kitterick, P.T.; Anderson, C.A.; Hartley, D.E. The benefit of cross-modal reorganization on speech perception in pediatric cochlear implant recipients revealed using functional near-infrared spectroscopy. Front. Hum. Neurosci. 2020, 14, 308. [Google Scholar] [CrossRef]
- Sevy, A.B.; Bortfeld, H.; Huppert, T.J.; Beauchamp, M.S.; Tonini, R.E.; Oghalai, J.S. Neuroimaging with near-infrared spectroscopy demonstrates speech-evoked activity in the auditory cortex of deaf children following cochlear implantation. Hear. Res. 2010, 270, 39–47. [Google Scholar] [CrossRef]
- Van de Rijt, L.P.; van Opstal, A.J.; Mylanus, E.A.; Straatman, L.V.; Hu, H.Y.; Snik, A.F.; van Wanrooij, M.M. Temporal cortex activation to audiovisual speech in normal-hearing and cochlear implant users measured with functional near-infrared spectroscopy. Front. Hum. Neurosci. 2016, 10, 48. [Google Scholar] [CrossRef]
- Quaresima, V.; Bisconti, S.; Ferrari, M. A brief review on the use of functional near-infrared spectroscopy (fnirs) for language imaging studies in human newborns and adults. Brain Lang. 2012, 121, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bortfeld, H. Functional near-infrared spectroscopy as a tool for assessing speech and spoken language processing in pediatric and adult cochlear implant users. Dev. Psychobiol. 2019, 61, 430–443. [Google Scholar] [CrossRef]
- Daudt, H.M.; van Mossel, C.; Scott, S.J. Enhancing the scoping study methodology: A large, inter-professional team’s experience with arksey and o’malley’s framework. BMC Med. Res. Methodol. 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Reprint—preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. Prisma extension for scoping reviews (prisma-scr): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Anderson, C.A.; Wiggins, I.M.; Kitterick, P.T.; Hartley, D.E. Adaptive benefit of cross-modal plasticity following cochlear implantation in deaf adults. Proc. Natl. Acad. Sci. USA 2017, 114, 10256–10261. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Wiggins, I.M.; Kitterick, P.T.; Hartley, D.E. Pre-operative brain imaging using functional near-infrared spectroscopy helps predict cochlear implant outcome in deaf adults. J. Assoc. Res. Otolaryngol. 2019, 20, 511–528. [Google Scholar] [CrossRef]
- Chen, L.-C.; Puschmann, S.; Debener, S. Increased cross-modal functional connectivity in cochlear implant users. Sci. Rep. 2017, 7, 1–10. [Google Scholar]
- Chen, L.-C.; Sandmann, P.; Thorne, J.D.; Bleichner, M.G.; Debener, S. Cross-modal functional reorganization of visual and auditory cortex in adult cochlear implant users identified with fnirs. Neural Plast. 2016, 2016, 4382656. [Google Scholar] [CrossRef]
- Chen, L.-C.; Stropahl, M.; Schönwiesner, M.; Debener, S. Enhanced visual adaptation in cochlear implant users revealed by concurrent eeg-fnirs. Neuroimage 2017, 146, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Olds, C.; Pollonini, L.; Abaya, H.; Larky, J.; Loy, M.; Bortfeld, H.; Beauchamp, M.S.; Oghalai, J.S. Cortical activation patterns correlate with speech understanding after cochlear implantation. Ear Hear. 2016, 37, e160. [Google Scholar] [CrossRef]
- Zhou, X.; Seghouane, A.-K.; Shah, A.; Innes-Brown, H.; Cross, W.; Litovsky, R.; McKay, C.M. Cortical speech processing in postlingually deaf adult cochlear implant users, as revealed by functional near-infrared spectroscopy. Trends Hear. 2018, 22, 2331216518786850. [Google Scholar] [CrossRef]
- Boothroyd, A.; Hanin, L.; Hnath, T. A Sentence Test of Speech Perception: Reliability, Set Equivalence, and Short Term Learning; City University of New York: New York, NY, USA, 1985. [Google Scholar]
- Wagener, K.; Brand, T.; Kollmeier, B. Development and evaluation of a german sentence test part iii: Evaluation of the oldenburg sentence test. Z. Fur Audiol. 1999, 38, 86–95. [Google Scholar]
- Wallace, I.F.; Gravel, J.S.; McCarton, C.M.; Ruben, R.J. Otitis media and language development at 1 year of age. J. Speech Hear. Disord. 1988, 53, 245–251. [Google Scholar] [CrossRef]
- Cejas, I.; Hoffman, M.F.; Quittner, A.L. Outcomes and benefits of pediatric cochlear implantation in children with additional disabilities: A review and report of family influences on outcomes. Pediatric Health Med. Ther. 2015, 6, 45. [Google Scholar] [CrossRef]
- Chilosi, A.M.; Comparini, A.; Scusa, M.F.; Berrettini, S.; Forli, F.; Battini, R.; Cipriani, P.; Cioni, G. Neurodevelopmental disorders in children with severe to profound sensorineural hearing loss: A clinical study. Dev. Med. Child Neurol. 2010, 52, 856–862. [Google Scholar] [CrossRef]
- Besser, J.; Stropahl, M.; Urry, E.; Launer, S. Comorbidities of hearing loss and the implications of multimorbidity for audiological care. Hear. Res. 2018, 369, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-M.; Zhang, X.; Hoffman, H.J.; Cotch, M.F.; Themann, C.L.; Wilson, M.R. Hearing impairment associated with depression in us adults, national health and nutrition examination survey 2005–2010. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Gantz, B.J.; Turner, C.; Gfeller, K.E.; Lowder, M.W. Preservation of hearing in cochlear implant surgery: Advantages of combined electrical and acoustical speech processing. Laryngoscope 2005, 115, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.; Hill, T.; Mahon, M. Quality of life in children and adolescents with cochlear implants and additional needs. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 851–857. [Google Scholar] [CrossRef]
- Schorr, E.A.; Roth, F.P.; Fox, N.A. Quality of life for children with cochlear implants: Perceived benefits and problems and the perception of single words and emotional sounds. J. Speech Lang. Hear. Res. 2009, 52, 141–152. [Google Scholar] [CrossRef]
- Behrendt, H.F.; Firk, C.; Nelson III, C.A.; Perdue, K.L. Motion correction for infant functional near-infrared spectroscopy with an application to live interaction data. Neurophotonics 2018, 5, 015004. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Pirazzoli, L.; Blasi, A.; Bulgarelli, C.; Hakuno, Y.; Minagawa, Y.; Brigadoi, S. Recommendations for motion correction of infant fnirs data applicable to multiple data sets and acquisition systems. NeuroImage 2019, 200, 511–527. [Google Scholar] [CrossRef]
- Abboub, N.; Nazzi, T.; Gervain, J. Prosodic grouping at birth. Brain Lang. 2016, 162, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Telkemeyer, S.; Rossi, S.; Koch, S.P.; Nierhaus, T.; Steinbrink, J.; Poeppel, D.; Obrig, H.; Wartenburger, I. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J. Neurosci. 2009, 29, 14726–14733. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.B.; Fox, S.E.; Tager-Flusberg, H.; Nelson, C.A. Neural processing of repetition and non-repetition grammars in 7- and 9-month-old infants. Front. Psychol. 2011, 2, 168. [Google Scholar] [CrossRef]
- Kozberg, M.; Hillman, E. Neurovascular coupling and energy metabolism in the developing brain. Prog. Brain Res. 2016, 225, 213–242. [Google Scholar] [PubMed]
- De Roever, I.; Bale, G.; Mitra, S.; Meek, J.; Robertson, N.J.; Tachtsidis, I. Investigation of the pattern of the hemodynamic response as measured by functional near-infrared spectroscopy (fnirs) studies in newborns, less than a month old: A systematic review. Front. Hum. Neurosci. 2018, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Issard, C.; Gervain, J. Variability of the hemodynamic response in infants: Influence of experimental design and stimulus complexity. Dev. Cogn. Neurosci. 2018, 33, 182–193. [Google Scholar] [CrossRef]
- Budenz, C.L.; Cosetti, M.K.; Coelho, D.H.; Birenbaum, B.; Babb, J.; Waltzman, S.B.; Roehm, P.C. The effects of cochlear implantation on speech perception in older adults. J. Am. Geriatr. Soc. 2011, 59, 446–453. [Google Scholar] [CrossRef]
- Friedland, D.R.; Runge-Samuelson, C.; Baig, H.; Jensen, J. Case-control analysis of cochlear implant performance in elderly patients. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.S.; Lin, H.W.; Herrmann, B.S.; Lee, D.J. Differential cochlear implant outcomes in older adults. Laryngoscope 2013, 123, 1952–1956. [Google Scholar] [CrossRef]
- Vermeire, K.; Brokx, J.P.; Wuyts, F.L.; Cochet, E.; Hofkens, A.; Van de Heyning, P.H. Quality-of-life benefit from cochlear implantation in the elderly. Otol. Neurotol. 2005, 26, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Salant, S. Hearing loss and aging: New research findings and clinical implications. J. Rehabil. Res. Dev. 2005, 42, 9–24. [Google Scholar] [CrossRef]
- Humes, L.E.; Christopherson, L. Speech identification difficulties of hearing-impaired elderly persons: The contributions of auditory processing deficits. J. Speech Lang. Hear. Res. 1991, 34, 686–693. [Google Scholar] [CrossRef]
- Wong, P.C.; Jin, J.X.; Gunasekera, G.M.; Abel, R.; Lee, E.R.; Dhar, S. Aging and cortical mechanisms of speech perception in noise. Neuropsychologia 2009, 47, 693–703. [Google Scholar] [CrossRef]
- Wong, P.C.; Ettlinger, M.; Sheppard, J.P.; Gunasekera, G.M.; Dhar, S. Neuroanatomical characteristics and speech perception in noise in older adults. Ear Hear. 2010, 31, 471. [Google Scholar] [CrossRef]
- Gallagher, A.; Béland, R.; Lassonde, M. The contribution of functional near-infrared spectroscopy (fnirs) to the presurgical assessment of language function in children. Brain Lang. 2012, 121, 124–129. [Google Scholar] [CrossRef]
- Watson, N.F.; Dodrill, C.; Farrell, D.; Holmes, M.D.; Miller, J.W. Determination of language dominance with near-infrared spectroscopy: Comparison with the intracarotid amobarbital procedure. Seizure 2004, 13, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Minagawa-Kawai, Y.; Mori, K.; Furuya, I.; Hayashi, R.; Sato, Y. Assessing cerebral representations of short and long vowel categories by nirs. Neuroreport 2002, 13, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Petitto, L.-A.; Berens, M.S.; Kovelman, I.; Dubins, M.H.; Jasinska, K.; Shalinsky, M. The “perceptual wedge hypothesis” as the basis for bilingual babies’ phonetic processing advantage: New insights from fnirs brain imaging. Brain Lang. 2012, 121, 130–143. [Google Scholar] [CrossRef]
- Weder, S.; Shoushtarian, M.; Olivares, V.; Zhou, X.; Innes-Brown, H.; McKay, C. Cortical fnirs responses can be better explained by loudness percept than sound intensity. Ear Hear. 2020, 41, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Homae, F.; Watanabe, H.; Nakano, T.; Taga, G. Prosodic processing in the developing brain. Neurosci. Res. 2007, 59, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sulpizio, S.; Doi, H.; Bornstein, M.H.; Cui, J.; Esposito, G.; Shinohara, K. Fnirs reveals enhanced brain activation to female (versus male) infant directed speech (relative to adult directed speech) in young human infants. Infant Behav. Dev. 2018, 52, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wartenburger, I.; Steinbrink, J.; Telkemeyer, S.; Friedrich, M.; Friederici, A.D.; Obrig, H. The processing of prosody: Evidence of interhemispheric specialization at the age of four. Neuroimage 2007, 34, 416–425. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Y.; Hou, X.; Cui, Y.; Zhou, C. Discrimination of emotional prosodies in human neonates: A pilot fnirs study. Neurosci. Lett. 2017, 658, 62–66. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, M.; Chen, F. Characterizing the Area of Significant Brain Activation Region to Spectrally-Degraded Music: A Functional Near-Infrared Spectroscopy Study. In Proceedings of the 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER), Virtual, 4–6 May 2021; pp. 205–208. [Google Scholar]
- Santosa, H.; Hong, M.J.; Hong, K.-S. Lateralization of music processing with noises in the auditory cortex: An fnirs study. Front. Behav. Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Beer, J.; Kronenberger, W.G.; Castellanos, I.; Colson, B.G.; Henning, S.C.; Pisoni, D.B. Executive functioning skills in preschool-age children with cochlear implants. J. Speech Lang. Hear. Res. 2014, 57, 1521–1534. [Google Scholar] [CrossRef]
- Kronenberger, W.G.; Beer, J.; Castellanos, I.; Pisoni, D.B.; Miyamoto, R.T. Neurocognitive risk in children with cochlear implants. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 608–615. [Google Scholar] [CrossRef]
- Blasi, A.; Lloyd-Fox, S.; Johnson, M.H.; Elwell, C. Test–retest reliability of functional near infrared spectroscopy in infants. Neurophotonics 2014, 1, 025005. [Google Scholar] [CrossRef]
- Wiggins, I.M.; Anderson, C.A.; Kitterick, P.T.; Hartley, D.E. Speech-evoked activation in adult temporal cortex measured using functional near-infrared spectroscopy (fnirs): Are the measurements reliable? Hear. Res. 2016, 339, 142–154. [Google Scholar] [CrossRef]
- Kakimoto, Y.; Nishimura, Y.; Hara, N.; Okada, M.; Tanii, H.; Okazaki, Y. Intrasubject reproducibility of prefrontal cortex activities during a verbal fluency task over two repeated sessions using multi-channel near-infrared spectroscopy. Psychiatry Clin. Neurosci. 2009, 63, 491–499. [Google Scholar] [CrossRef]
- Schecklmann, M.; Ehlis, A.-C.; Plichta, M.M.; Fallgatter, A.J. Functional near-infrared spectroscopy: A long-term reliable tool for measuring brain activity during verbal fluency. Neuroimage 2008, 43, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Plichta, M.; Herrmann, M.; Baehne, C.; Ehlis, A.C.; Richter, M.; Pauli, P.; Fallgatter, A. Event-related functional near-infrared spectroscopy (fnirs) based on craniocerebral correlations: Reproducibility of activation? Hum. Brain Mapp. 2007, 28, 733–741. [Google Scholar] [CrossRef]
- Plichta, M.M.; Herrmann, M.J.; Baehne, C.; Ehlis, A.-C.; Richter, M.; Pauli, P.; Fallgatter, A.J. Event-related functional near-infrared spectroscopy (fnirs): Are the measurements reliable? Neuroimage 2006, 31, 116–124. [Google Scholar] [CrossRef]
- Shin, J.; Kwon, J.; Choi, J.; Im, C.-H. Performance enhancement of a brain-computer interface using high-density multi-distance nirs. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Udagawa, H.; Masuda, Y.; Kohno, S.; Amita, T.; Inoue, Y. Development of Double Density Whole Brain Fnirs with Eeg System for Brain Machine Interface. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 6118–6122. [Google Scholar]
- Chen, L.-C.; Sandmann, P.; Thorne, J.D.; Herrmann, C.S.; Debener, S. Association of concurrent fnirs and eeg signatures in response to auditory and visual stimuli. Brain Topogr. 2015, 28, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Putze, F.; Hesslinger, S.; Tse, C.-Y.; Huang, Y.; Herff, C.; Guan, C.; Schultz, T. Hybrid fnirs-eeg based classification of auditory and visual perception processes. Front. Neurosci. 2014, 8, 373. [Google Scholar] [CrossRef] [PubMed]
| Record | Sample | Stimuli/Imaging Paradigm | Cortical ROIs | Outcome & Measurements | Study Design |
|---|---|---|---|---|---|
| Anderson et al., 2017 [86] | Patient group: 17. Bilaterally profoundly deaf, pre-surgical. Two pre-lingually, three peri-lingually, and twelve post-lingually deaf. Age 36–78 (mean = 58). Controls: 17. Mean age = 57 years. | IHR number sentences (normal speech, male and female speakers). Split into visual-only, auditory-only. All at 65 dB for 24 s blocks | Bilateral fNIRS with lowermost optode close to preauricular point and uppermost optode aligned towards Cz. Targets temporal lobe, specifically superior temporal cortex (STC) | Speech understanding: CUNY (City University of New York) Sentence lists in quiet. Measured via speech reading pre-implantation and via auditory performance post-implantation. | Longitudinal repeated measures |
| Anderson et al., 2019 [87] | Patient group: 17. Bilaterally profoundly deaf, pre-surgical. Mix of pre- and post-lingually deaf. Age 36–78 (mean = 58). Controls: 17. Mean age = 57 years. | IHR number sentences (normal speech, male and female speakers). Split into visual-only, auditory-only, audio-visual. All at 65 dB for 24 s blocks | Bilateral fNIRS with lowermost optode close to preauricular point and uppermost optode aligned towards Cz. Targets temporal lobe, specifically superior temporal cortex (STC) | Speech understanding: CUNY (City University of New York) Sentence lists in quiet. Measured via speech reading pre-implantation, and via auditory performance post-implantation. | Longitudinal repeated measures |
| Chen et al., 2017 [88] | Patient group: 20. Unilaterally implanted post-lingually deaf CI users with ≥6 months experience. Age 24–77 (mean = 54.58). Controls: 20. Age 24–78 (mean = 54.89). | Visual stimuli consisting of circular checkerboard patterns in 10 s blocks. Auditory stimuli consisting of normal speech and reversed speech in 5 s blocks and tonal bursts in 3 s blocks. Loudness levels for auditory stimuli were adjusted to subjective comfortable levels. | Bilateral fNIRS. Temporal lobe headset centered at T7/T8. Occipital lobe headset centered at O1/O2. | Speech recognition: Freiburg monosyllabic words test, Oldenburg sentences test (OLSA) in quiet, OLSA test in noise. | Cross-sectional |
| Chen et al., 2016 [89] | Patient group: 20. Unilaterally implanted post-lingually deaf CI users with ≥6 months experience. Age 24–77 (mean = 54.58). Controls: 20. Age 24–78 (mean = 54.89). | Visual stimuli consisting of circular checkerboard patterns in 10 s blocks. Auditory stimuli consisting of normal speech and reversed speech in 5 s blocks and tonal bursts in 3 s blocks. Loudness levels for auditory stimuli were adjusted to subjective comfortable levels. | Bilateral fNIRS. Temporal lobe ROI centered at T7/T8. Occipital lobe ROI centered at O1/O2. | Speech recognition: Oldenburg sentences test (OLSA) in quiet and noise | Cross-sectional |
| Chen et al., 2017 [90] | Patient group: 20. Unilaterally implanted post-lingually deaf CI users with ≥6 months experience. Age 24–77 (mean = 54.58). Controls: 20. Age 24–78 (mean = 54.89). | Visual stimuli consisting of circular checkerboard patterns in 10 s blocks. Auditory stimuli consisting of tonal bursts in 3 s blocks. Loudness levels for auditory stimuli were adjusted to subjective comfortable levels. | Bilateral fNIRS. Left and right temporal lobe and occipital lobe. Simultaneous EEG. | Speech recognition: Oldenburg sentences test (OLSA) in quiet and noise | Cross-sectional |
| Mushtaq et al., 2020 [78] | Patient group: 19. Bilaterally implanted CI users with 29–123 months experience. Age 6–11 (mean = 8.4). Controls: 20. Age 6–12 (mean = 9.5). | Visual speech, auditory speech, signal correlated noise, and steady speech shaped noise. On average 2.97 s long. | Bilateral fNIRS with lowermost optode close to preauricular point and uppermost optode aligned towards Cz. | Speech understanding: Bamford–Kowal–Bench (BKB) sentences in silence and in noise | Cross-sectional |
| Old et al., 2016 [91] | CI users: 32. Post-lingually deaf adults. Experience range 1 day–12 years. Age 23–86. Controls: 35. Adults aged 24–65 | Normal speech, channelized speech, scrambled speech, environmental sounds. All at 60 dB for 20 s blocks | Bilateral fNIRS with headset centered at T7/T8. Targets lateral temporal lobe and superior temporal gyrus (LTL/STG) | Hearing level: Speech recognition threshold (SRT). Speech perception: Consonant-Nucleus-Consonant (CNC) words, AzBio Sentence Test. Both presented in quiet at 60 dB | Cross-sectional |
| Zhou et al., 2018 [92] | Patient group: 20. Post-lingually deaf CI users with >12 months experience with right-sided implant. Mix of unilaterally and bilaterally implanted individuals. Age 46–79 (mean = 64.2) Controls: 19. Age 33–70 (mean = 53.5). | Auditory and visual speech stimuli. 11 s long blocks. Auditory at 65 dBA. | Bilateral fNIRS. Left middle superior temporal lobe, right anterior temporal lobe, superior temporal sulcus/gyrus. | Speech understanding: Open-set consonant-nucleus-consonant (CNC) words and CUNY sentences. CNC presented in quiet at 60 dBA. CUNY presented in quiet at 60 dBA and in noise of 5–15 dB SNR. | Cross-sectional |
| Record | Key Purpose/Questions | Summary of Main Results |
|---|---|---|
| Anderson et al., 2017 [86] | How does cross-modal activation of auditory brain regions by visual speech change from pre- to post-implantation? How does this relate to the ability to understand speech with a cochlear implant (CI)? What is the relationship between post-implant cortical plasticity within auditory brain regions and the ability of these regions to respond to auditory speech stimulation? | Increased cross-modal activation of auditory brain regions by lip-reading pre-implantation is not associated with post-implantation cortical responsiveness to auditory speech. Differences in pre- to post-implantation activation by visual speech is associated with speech understanding outcomes (r = 0.77) and with increased cross-modal activation post-implantation associated with increased auditory responsiveness and better speech understanding outcomes. |
| Anderson et al., 2019 [87] | To understand whether fNIRS measures of cross-modal activation obtained pre-operatively could predict future clinical outcomes for CI candidates. To explore whether pre-operative brain imaging using fNIRS could offer incremental prognostic information and value above that already provided by known clinical factors. To explore underlying mechanisms of the relationship between pre-operative brain activation and post-operative outcomes. | Stronger activation to visual speech pre-operatively was predictive of poorer speech understanding outcomes post-implantation (r = −0.75). fNIRS measures can provide additional prognostic information about future CI outcome. Relationship between fNIRS measurements and outcomes driven by clinical factors (i.e., whether participants were pre- or post-lingually deaf). |
| Chen et al., 2017 [88] | To investigate whether cross-modal functional connectivity between visual and auditory cortices is elevated in CI users. To assess the relationship between cross-modal functional connectivity and speech recognition abilities in CI users. | CI users exhibited reduced intra-modal connectivity within visual and auditory areas and greater cross-modal connectivity between visual and auditory areas in the left hemisphere. Cross-modal functional connectivity was correlated with Freiburg speech recognition scores but not OLSA scores (r = −0.525). |
| Chen et al., 2016 [89] | How does the combination of visual and auditory cortex reorganization within the same CI user jointly affect their speech recognition performance? | CI users with more reorganization of the visual cortex compared to reorganization of the auditory cortex performed better in the speech recognition tasks than CI users with the opposite pattern of reorganization (R = 0.518). |
| Chen et al., 2017 [90] | To investigate whether stimulus-specific adaptation in the visual system is enhanced in CI users compared to NH controls and whether such enhanced adaptation corresponds to decreased activity in visual cortex during visual processing. | Reduced visually evoked activation in the visual cortex and reduced auditory-evoked activation in the auditory cortex were observed in CI users compared to NH controls when fNIRS-measured latency was analyzed. CI users showed enhanced stimulus-specific adaptation for visual stimuli but decreased adaptation for auditory stimuli compared to NH controls. EEG adaptation for auditory stimuli and speech recognition scores did not correlate. |
| Mushtaq et al., 2020 [78] | To investigate the influence of cross-modal plasticity on speech understanding in children with CIs. To explore the relationship between speech understanding ability and intelligibility and amplitude modulation processing. | Significant activation to signal correlated noise was noted only in the CI group. Responses to visual speech were larger in the CI group than in the NH group. Responses to auditory speech were larger than responses to signal correlated noise, which were larger than responses to steady speech shaped noise. No significant correlations were noted between speech understanding scores and visual speech activation (ԏb = 0.236); auditory speech activation (ԏb = 0.189); intelligibility processing (ԏb = −0.047); nor amplitude modulation processing (ԏb = −0.142). |
| Old et al., 2016 [91] | To better understand speech–understanding variability in outcomes. To explore the use of fNIRS as an objective measure of speech perception. | Greater activation to speech stimuli compared to unintelligible speech in good users. Poor users showed no distinguishable differences. Ratio of activation to speech:scrambled speech was directly correlated with CNC (R2 = 0.53 to 0.68) and AzBio scores (R2 = 0.55 to 0.66). Cortical activation measures did not correlate with their general auditory sensitivity (SRT scores). |
| Zhou et al., 2018 [92] | To determine whether fNIRS responses to auditory or visualspeech in different brain regions correlated with speech understanding abilities in CI users. | fNIRS responses to auditory stimuli in the left middle superior temporal lobe and the right anterior temporal lobe were negatively correlated with auditory speech understanding tests scores (r = −0.650 and −0.620). Responses to visual stimuli in the left STS/STG were negatively correlated with auditory speech understanding scores (r = −0.668). Combination of the above responses produced a better prediction of auditory speech understanding ability than the activity in any one area alone (R2 = 0.709). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrison, S.C.; Lawrence, R.; Hoare, D.J.; Wiggins, I.M.; Hartley, D.E.H. Use of Functional Near-Infrared Spectroscopy to Predict and Measure Cochlear Implant Outcomes: A Scoping Review. Brain Sci. 2021, 11, 1439. https://doi.org/10.3390/brainsci11111439
Harrison SC, Lawrence R, Hoare DJ, Wiggins IM, Hartley DEH. Use of Functional Near-Infrared Spectroscopy to Predict and Measure Cochlear Implant Outcomes: A Scoping Review. Brain Sciences. 2021; 11(11):1439. https://doi.org/10.3390/brainsci11111439
Chicago/Turabian StyleHarrison, Samantha C., Rachael Lawrence, Derek J. Hoare, Ian M. Wiggins, and Douglas E. H. Hartley. 2021. "Use of Functional Near-Infrared Spectroscopy to Predict and Measure Cochlear Implant Outcomes: A Scoping Review" Brain Sciences 11, no. 11: 1439. https://doi.org/10.3390/brainsci11111439
APA StyleHarrison, S. C., Lawrence, R., Hoare, D. J., Wiggins, I. M., & Hartley, D. E. H. (2021). Use of Functional Near-Infrared Spectroscopy to Predict and Measure Cochlear Implant Outcomes: A Scoping Review. Brain Sciences, 11(11), 1439. https://doi.org/10.3390/brainsci11111439







