Not Only Mania or Depression: Mixed States/Mixed Features in Paediatric Bipolar Disorders
Abstract
:1. Introduction
2. Materials and Methods
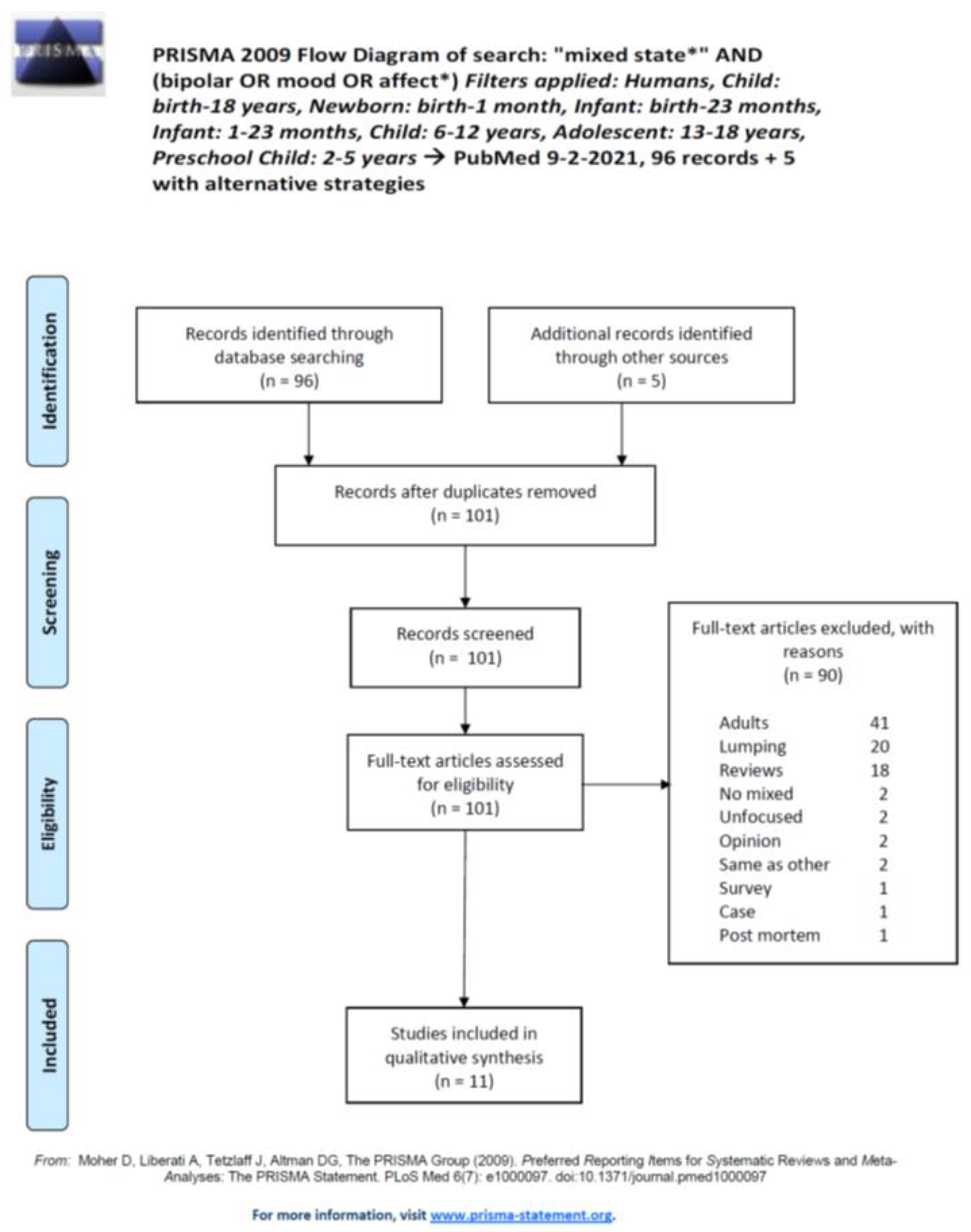
2.1. Search Strategy
2.2. Eligibility and Study Selection
2.3. Data Extraction and Synthesis
3. Results
| Study | Design/Clinical Assessment | Population | Results | Conclusions |
|---|---|---|---|---|
| Strober et al., 1995 [25], Los Angeles, CA | LS; 5-yr follow-up; clinical observation, RDC (a predecessor of the DSM-III with stricter criteria) snd SADS | N = 54 BD-I; 29 ♀, 25 ♂; Age, x = 16.0 years; range 13–17 years | On admission, 20 were manic, 14 were depressed, 10 were mixed, and 10 were cycling; manic or mixed presentation recovered in 9 or 11 weeks, respectively, whereas depressive recovered in a median time of 26 weeks and rapid cycling recovered in 15 weeks | About 19% of adolescents hospitalised for BD-I present with mixed symptoms; the presence of a depressive core is a factor delaying recovery during a 5-year observation period |
| Wozniak et al., 1995 [26], Boston, MA | CS; referrals assessed for symptoms; DSM-III-R | N = 262 referrals for outpatient psychopharmacological treatment, of whom 207 had either mania (N = 43, age x = 7.9 ± 2.6 years; 36 ♂, 7 ♀) or ADHD (N = 164, age x = 8.8± 2.2 years; 141 ♂, 23 ♀); compared with 84 non-ADHD controls (age x = 9.0± 2.1 years; 71 ♂, 13 ♀); age, <12 | Of children with mania, 14% (N = 6) had mania only, 84% (N = 36) had a mixed presentation, and 2% (N = 1) had nonoverlapping episodes of mania and major depression | Most children below age 12 present with mixed episodes (84%) |
| Biederman et al., 2000 [21], Boston, MA | LS, RChR; effect of treatments; DSM-III-R, K-SADS-E | N = 59 referrals for outpatient psychopharmacological treatment with BD (49 ♂, 10 ♀; Age, x = 10.8 ± 3.7 years; range 3.5–17 years) | The occurrence of mixed episodes was frequent in the sample. The patients received TCAs (45%), stimulants (20%), SSRIs (20%), mood stabilisers (40%), or FGAs (10%) during their follow-up visits. Depression was more responsive to SSRIs than to other treatments | This study supports the use of antidepressant monotherapy in youths with mixed states. The study was conducted during 1991–1995; at those times, SSRIs were not completely introduced in the US market. This justifies the lower rate of SSRI prescription compared to TCAs. |
| Frazier et al., 2001 [22], Boston, MA | LS; 20 mg/d open-label olanzapine × 8 weeks at 1 site; DSM-IV; assessment through the CGI-S and the YMRS | 23 BD-I and -II (manic, mixed, or hypomanic; age, x = 10.3 ± 2.9 years, range 5–14 years; 13 ♂, 10 ♀) | Improvement criteria: at least 30% drop of YMRS score from baseline levels plus maximum 3 on the CGI-S mania, 61% responder rate; 17 patients (74%) with mixed presentation | No reported differential response for patients with mixed symptoms |
| DelBello et al., 2002 [15], Cincinnati, OH | LS; add-on quetiapine vs. placebo to valproate; random allocation × 6 weeks, double-blind, parallel; DSM-IV; YMRS primary outcome | 30 BD-I patients with manic or mixed episodes randomised to valproate + quetiapine (N = 15; age, x = 14.1 ± 2 years; 8 ♂, 7 ♀) or valproate + placebo (N = 15; age, x = 14.5 years ± 2; 8 ♂, 7 ♀) | 13 (87%) of patients randomised to valproate + placebo and 10 (67%) to valproate + quetiapine. Valproate + quetiapine reduced YMRS significantly better than valproate + placebo (but 7 patients in the former group quit the study before endpoint) | High attrition rate in the valproate + quetiapine group was not addressed through LOCF. No differential data provided for mixed episode BD-I patients. Small sample size |
| Wilens et al., 2003 [27], Boston, MA | CS, RChR; included if meeting DSM-III-R criteria. Assessed with K-SADS-E preschoolers 4-6-yr-old referred to a psychopharmacology clinic for BD and BD probands 7–9-yr-old | 44 preschoolers with BD (age, x = 5.1 ± 0.8 years; 35 ♂, 9 ♀) 29 BD probands (age, x = 7.8 ± 0.9 years; 24 ♂, 5 ♀) | The two samples did not differ for psychopathology, other than conduct disorders, largely explained by oppositive-defiant disorder; 80% of preschoolers and 76% of school-age probands had mixed episodes | Children 4–6-year-old and those of 7–9 years of age with BD share psychopathological features and report similar rates of mixed states |
| Jerrell & Shugart, 2004 [19], USA | CS, RChR; utilised a cross-national database of clinical records of children with various psychopathological disorders diagnosed with the DSM-IV | Identified 83 patients with BD (age, x = 15 years; 43 ♂, 40 ♀); 16 were aged 7–12 years, 67 were 13–20-years-old | Most patients shared ADHD and conduct disorder symptoms; the two age groups did not differ in symptoms, save for the fact that very early onset children were more distractible, excitable, irritable, and fidgety and that early onset BD children displayed more depression; 23% had mixed episodes | About one fourth of patients with preadolescent and adolescent paediatric BD have mixed symptoms; in both, high rates of ADHD and conduct disorder comorbidity |
| Dilsaver & Akiskal, 2005 [28], Merced, CA | CS; assessment with SCID-DSM-IV of adolescents referred for MDD (MDE present) | 49 consecutive Hispanic adolescents (age range 23–27 years; 33 ♀ with age x = 14.8 ± 1.6 years, 16 ♂ with age x = 14.8 ± 1.8 years) | Most patients were rediagnosed; 55% BD, 8.2% BD-I, 6.1% BD-II, and 40.9% mixed state. Half of the ♀ sample had family history positive for mood disorder and about one fourth had family history positive for MDD; 17 (51.5%) ♀ and 10 (62.5%) ♂ met DSM-IV criteria for BD. Psychotic features were more present in MDD than BD girls; the opposite was true for boys; irritability was shared by about 80% of the sample and hostility by about 50% | Most adolescents have more mixed states than pure depressive or manic/hypomanic presentations during a MDE. More than 40% of Hispanic adolescents present with mixed episodes, in line with results of other adolescent populations |
| Dilsaver et al., 2005 [24], Merced, CA | CS; acreening adolescent Hispanic (99%) destitues for mixed states with the SCID-DSM-IV | 247 adolescents with MDE screened for mixed states (age, x = 14.7 ± 1.5 years; range, 12–17 years; 108 ♂, 139 ♀) | 100 patients were with BD (40.5%); of them, 82 had a mixed state (46 ♂, 36 ♀, 33.1% of the whole sample); 147 met MDD criteria; 99 displayed psychotic features; 164 had suicidal ideation, 118 had past suicide attempts; 101 had positive family history for mood disorders (57 MDD, 44 BD). Only 11 were purely depressive BD-I and 7 purely depressive with BD-II | Confirmed was the high occurrence of mixed states in the presentation of adolescent patients with BD; the nonmixed samples did not differ from the mixed one for suicidal ideation or suicidal attempts |
| Potter et al., 2009 [20], Boston, MA | CS, RChR; youths diagnosed with DSM-IV BD at a paediatric psychopharmacology clinic; assessed severity according to treatment received; responder was a patient with CGI-I 1 or 2 | 53 (age, x = 13 ± 3.6 years, range 4–19 years; 18 (34%) <12-year-old, 39 (74%) ♂, 14 (26%) ♀) | Treated for comorbidity, 32 (68%); for ADHD, 32 (60%); for depression, 18 (34%); and for anxiety, 14 (26%). Monotherapy in 23% of patients, mostly SGAs. Responder rates, 80% of mania, 57% of mixed states, 56% of ADHD, 61% of anxiety, and 90% of depression | Provided response for mixed state but pooled numbers of patients with mania with those with mixed state. Results show mania to be a more unstable state than the mixed and a more responsive to SGAs |
| Findling et al., 2015 [23], multicentre (76 US; 10 Russia) | LS; double-blind randomised 1:1:1:1 assignment to 5, 10, and 20 mg/day asenapine or placebo to patients with manic or mixed episodes (DSM-IV-R) × 3 weeks; assessment with K-SADS, YMRS, CGI-BP; responder: who dropped ≥50% on the YMRS from baseline | 403 patients (age, x = 13.8 ± 2.0 212; range, 10–17 years; ♀ (52.6%)) with mania (N = 171) or mixed state (N = 232) randomised to placebo (N = 101), 5 mg (N = 104), 10 mg (N = 99), or 20 mg asenapine (N = 99) | ADHD comorbidity N = 220 (54.6%), total comorbidities, N = 256 (63.5%), with stimulant use N = 96 (23.8%); asenapine performed better than placebo (lower and higher dosages, but all doses were equally efficacious on the CGI-BP). Responder rates, 42–54% with asenapine, 28% with placebo | Mixed states still constitute the majority of BD presentations in children and adolescents; more than one fourth of patients are likely to respond to placebo at the 3-week endpoint. Site effects analysed but not reported; separate analyses carried out for ADHD comorbidity (with or without) but not for pure mania vs. mixed state responsiveness |
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kraepelin, E. Psychiatrie: Ein Lehrbuch für Studirende und Aerzte; Verlag von Johann Ambrosius Barth: Leipzig, Germany, 1899. [Google Scholar]
- Weygandt, W. Über die Mischzustände des Manisch-Depressiven Irreseins: Ein Beitrag zur Klinischen Psychiatrie; J. F. Lehmann: Munich, Germany, 1899. [Google Scholar]
- Cassano, G.B.; Micheli, C. Psychotic mixed states. Clin. Neuropharmacol. 1992, 15. [Google Scholar] [CrossRef]
- Koukopoulos, A.; Faedda, G.; Proietti, R.; D’Amico, S.; de Pisa, E.; Simonetto, C. Mixed depressive syndrome. L’Encéphale 1992, 18, 19–21. [Google Scholar] [CrossRef]
- Perugi, G.; Akiskal, H.S.; Micheli, C.; Musetti, L.; Paiano, A.; Quilici, C.; Rossi, L.; Cassano, G.B. Clinical subtypes of bipolar mixed states: Validating a broader European definition in 143 cases. J. Affect. Disord. 1997, 43, 169–180. [Google Scholar] [CrossRef]
- Akiskal, H.S. The distinctive mixed states of bipolar I, II, and III. Clin. Neuropharmacol. 1992, 15. [Google Scholar] [CrossRef] [PubMed]
- Koukopoulos, A.; Sani, G.; Koukopoulos, A.E.; Manfredi, G.; Pacchiarotti, I.; Girardi, P. Melancholia agitata and mixed depression. Acta Psychiatr. Scand. 2007, 115, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Koukopoulos, A.; Sani, G. DSM-5 criteria for depression with mixed features: A farewell to mixed depression. Acta Psychiatr. Scand. 2014, 129, 4–16. [Google Scholar] [CrossRef]
- Pacchiarotti, I.; Nivoli, A.M.A.; Mazzarini, L.; Kotzalidis, G.D.; Sani, G.; Koukopoulos, A.; Scott, J.; Strejilevich, S.; Sánchez-Moreno, J.; Murru, A.; et al. The symptom structure of bipolar acute episodes: In search for the mixing link. J. Affect. Disord. 2013, 149, 56–66. [Google Scholar] [CrossRef]
- Vieta, E.; Valentì, M. Mixed states in DSM-5: Implications for clinical care, education, and research. J. Affect. Disord. 2013, 148, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, A.; Vieta, E.; Tohen, M.; Baldessarini, R.J. Factors modifying drug and placebo responses in randomized trials for bipolar mania. Int. J. Neuropsychopharmacol. 2011, 14, 863–875. [Google Scholar] [CrossRef] [Green Version]
- Dagani, J.; Baldessarini, R.J.; Signorini, G.; Nielssen, O.; de Girolamo, G.; Large, M. The age of onset of bipolar disorders. In Age of Onset of Mental Disorders; Springer: Cham, Switzerland, 2019; pp. 75–110. ISBN 9783319726199. [Google Scholar]
- Baldessarini, R.J.; Tondo, L.; Vázquez, G.H.; Undurraga, J.; Bolzani, L.; Yildiz, A.; Khalsa, H.M.K.; Lai, M.; Lepri, B.; Lolich, M.; et al. Age at onset versus family history and clinical outcomes in 1665 international bipolar-I disorder patients. World Psychiatry 2012, 11, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, B.I.; Birmaher, B. Prevalence, clinical presentation and differential diagnosis of pediatric bipolar disorder. Isr. J. Psychiatry Relat. Sci. 2012, 49, 3–14. [Google Scholar]
- DelBello, M.P.; Schwiers, M.L.; Rosenberg, H.L.; Strakowski, S.M. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J. Am. Acad. Child. Adolesc. Psychiatry 2002, 41, 1216–1223. [Google Scholar] [CrossRef]
- Frazier, E.A.; Swenson, L.P.; Mullare, T.; Dickstein, D.P.; Hunt, J.I. Depression with mixed features in adolescent psychiatric patients. Child. Psychiatry Hum. Dev. 2017, 48, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Dilsaver, S.C.; Benazzi, F.; Akiskal, H.S. Mixed states: The most common outpatient presentation of bipolar depressed adolescents? Psychopathology 2005, 38, 268–272. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerrell, J.M.; Shugart, M.A. Community-based care for youths with early and very-early onset bipolar I disorder. Bipolar Disord. 2004, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.P.; Liu, H.Y.; Monuteaux, M.C.; Henderson, C.S.; Wozniak, J.; Wilens, T.E.; Biederman, J. Prescribing patterns for treatment of pediatric bipolar disorder in a specialty clinic. J. Child. Adolesc. Psychopharmacol. 2009, 19, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Biederman, J.; Mick, E.; Spencer, T.J.; Wilens, T.E.; Faraone, S.V. Therapeutic dilemmas in the pharmacotherapy of bipolar depression in the young. J. Child. Adolesc. Psychopharmacol. 2000, 10, 185–192. [Google Scholar] [CrossRef]
- Frazier, J.A.; Biederman, J.; Tohen, M.; Feldman, P.D.; Jacobs, T.G.; Toma, V.; Rater, M.A.; Tarazi, R.A.; Kim, G.S.; Garfield, S.B.; et al. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J. Child. Adolesc. Psychopharmacol. 2001, 11, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Findling, R.L.; Landbloom, R.L.; Szegedi, A.; Koppenhaver, J.; Braat, S.; Zhu, Q.; Mackle, M.; Chang, K.; Mathews, M. Asenapine for the acute treatment of pediatric manic or mixed episode of bipolar I disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2015, 54, 1032–1041. [Google Scholar] [CrossRef] [Green Version]
- Dilsaver, S.C.; Benazzi, F.; Rihmer, Z.; Akiskal, K.K.; Akiskal, H.S. Gender, suicidality and bipolar mixed states in adolescents. J. Affect. Disord. 2005, 87, 11–16. [Google Scholar] [CrossRef]
- Strober, M.; Schmidt-Lackner, S.; Freeman, R.; Bower, S.; Lampert, C.; DeAntonio, M. Recovery and relapse in adolescents with bipolar affective illness: A five-year naturalistic, prospective follow-up. J. Am. Acad. Child. Adolesc. Psychiatry 1995, 34, 724–731. [Google Scholar] [CrossRef]
- Wozniak, J.; Biederman, J.; Kiely, K.; Ablon, J.S.; Faraone, S.V.; Mundy, E.; Mennin, D. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J. Am. Acad. Child. Adolesc. Psychiatry 1995, 34, 867–876. [Google Scholar] [CrossRef]
- Wilens, T.E.; Biederman, J.; Forkner, P.; Ditterline, J.; Morris, M.; Moore, H.; Galdo, M.; Spencer, T.J.; Wozniak, J. Patterns of comorbidity and dysfunction in clinically referred preschool and school-age children with bipolar disorder. J. Child. Adolesc. Psychopharmacol. 2003, 13, 495–505. [Google Scholar] [CrossRef]
- Dilsaver, S.C.; Akiskal, H.S. High rate of unrecognized bipolar mixed states among destitute Hispanic adolescents referred for “major depressive disorder”. J. Affect. Disord. 2005, 84, 179–186. [Google Scholar] [CrossRef]
- Janiri, D.; Doucet, G.E.; Pompili, M.; Sani, G.; Luna, B.; Brent, D.A.; Frangou, S. Risk and protective factors for childhood suicidality: A US population-based study. Lancet Psychiatry 2020, 7, 317–326. [Google Scholar] [CrossRef]
- Gratz, K.L.; Roemer, L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. J. Psychopathol. Behav. Assess. 2004, 26, 41–54. [Google Scholar] [CrossRef]
- Silvers, J.A.; McRae, K.; Gabrieli, J.D.E.; Gross, J.J.; Remy, K.A.; Ochsner, K.N. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion 2012, 12, 1235–1247. [Google Scholar] [CrossRef] [Green Version]
- Hare, T.A.; Tottenham, N.; Galvan, A.; Voss, H.U.; Glover, G.H.; Casey, B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry 2008, 63, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Veroude, K.; Jolles, J.; Croiset, G.; Krabbendam, L. Changes in neural mechanisms of cognitive control during the transition from late adolescence to young adulthood. Dev. Cogn. Neurosci. 2013, 5, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Hulvershorn, L.A.; Mennes, M.; Castellanos, F.X.; di Martino, A.; Milham, M.P.; Hummer, T.A.; Roy, A.K. Abnormal amygdala functional connectivity associated with emotional lability in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2014, 53, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Hibar, D.P.; Westlye, L.T.; van Erp, T.G.M.; Rasmussen, J.; Leonardo, C.D.; Faskowitz, J.; Haukvik, U.K.; Hartberg, C.B.; Doan, N.T.; Agartz, I.; et al. Subcortical volumetric abnormalities in bipolar disorder. Mol. Psychiatry 2016, 21, 1710–1716. [Google Scholar] [CrossRef] [Green Version]
- Brancati, G.E.; Vieta, E.; Azorin, J.-M.; Angst, J.; Bowden, C.L.; Mosolov, S.; Young, A.H.; Perugi, G. The role of overlapping excitatory symptoms in major depression: Are they relevant for the diagnosis of mixed state? J. Psychiatr. Res. 2019, 115, 151–157. [Google Scholar] [CrossRef]
- Perugi, G.; Pacchiarotti, I.; Mainardi, C.; Verdolini, N.; Menculini, G.; Barbuti, M.; Angst, J.; Azorin, J.-M.; Bowden, C.L.; Mosolov, S.; et al. Patterns of response to antidepressants in major depressive disorder: Drug resistance or worsening of depression are associated with a bipolar diathesis. Eur. Neuropsychopharmacol. 2019, 29, 825–834. [Google Scholar] [CrossRef]
- Janiri, D.; Sani, G.; de Rossi, P.; Piras, F.; Iorio, M.; Banaj, N.; Giuseppin, G.; Spinazzola, E.; Maggiora, M.; Ambrosi, E.; et al. Amygdala and hippocampus volumes are differently affected by childhood trauma in patients with bipolar disorders and healthy controls. Bipolar Disord. 2017, 19, 353–362. [Google Scholar] [CrossRef]
- Janiri, D.; Sani, G.; de Rossi, P.; Piras, F.; Banaj, N.; Ciullo, V.; Simonetti, A.; Arciniegas, D.B.; Spalletta, G. Hippocampal subfield volumes and childhood trauma in bipolar disorders. J. Affect. Disord. 2019, 253, 35–43. [Google Scholar] [CrossRef]
- Janiri, D.; Kotzalidis, G.D.; de Chiara, L.; Koukopoulos, A.E.; Aas, M.; Sani, G. The ring of fire: Childhood trauma, emotional reactivity, and mixed states in mood disorders. Psychiatr. Clin. N. Am. 2020, 43, 69–82. [Google Scholar] [CrossRef]
- Bipolar Genome Study Consortium (BiGS). Polygenic risk for anxiety influences anxiety comorbidity and suicidal behavior in bipolar disorder. Transl. Psychiatry 2020, 10, 298. [Google Scholar] [CrossRef]
- Spoorthy, M.S.; Chakrabarti, S.; Grover, S. Comorbidity of bipolar and anxiety disorders: An overview of trends in research. World J. Psychiatry 2019, 9, 7–29. [Google Scholar] [CrossRef]
- Yapıcı Eser, H.; Taşkıran, A.S.; Ertınmaz, B.; Mutluer, T.; Kılıç, Ö.; Özcan Morey, A.; Necef, I.; Yalçınay İnan, M.; Öngür, D. Anxiety disorders comorbidity in pediatric bipolar disorder: A meta-analysis and meta-regression study. Acta Psychiatr. Scand. 2020, 141, 327–339. [Google Scholar] [CrossRef]
- Masi, G.; Perugi, G.; Toni, C.; Millepiedi, S.; Mucci, M.; Bertini, N.; Pfanner, C. Attention-deficit hyperactivity disorder—Bipolar comorbidity in children and adolescents. Bipolar Disord. 2006, 8, 373–381. [Google Scholar] [CrossRef]
- Hassan, A.; Agha, S.S.; Langley, K.; Thapar, A. Prevalence of bipolar disorder in children and adolescents with attention-deficit hyperactivity disorder. Br. J. Psychiatry 2011, 198, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Biederman, J.; Faraone, S.; Mick, E.; Wozniak, J.; Chen, L.; Ouellette, C.; Marks, A.; Moore, P.; Garcia, J.; Mennin, D.; et al. Attention-deficit hyperactivity disorder and juvenile mania: An overlooked comorbidity? J. Am. Acad. Child. Adolesc. Psychiatry 1996, 35, 997–1008. [Google Scholar] [CrossRef]
- Maire, J.; Galera, C.; Bioulac, S.; Bouvard, M.; Michel, G. Emotional lability and irritability have specific associations with symptomatology in children with attention deficit hyperactivity disorder. Psychiatry Res. 2020, 285, 112789. [Google Scholar] [CrossRef]
- Gisbert, L.; Richarte, V.; Corrales, M.; Ibáñez, P.; Bosch, R.; Casas, M.; Ramos-Quiroga, J.A. The impact of emotional lability symptoms during childhood in adults with ADHD. J. Atten. Disord. 2018, 22, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Janiri, D.; Moser, D.A.; Doucet, G.E.; Luber, M.J.; Rasgon, A.; Lee, W.H.; Murrough, J.W.; Sani, G.; Eickhoff, S.B.; Frangou, S. Shared neural phenotypes for mood and anxiety disorders: A meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry 2020, 77, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Doucet, G.E.; Janiri, D.; Howard, R.; O’Brien, M.; Andrews-Hanna, J.R.; Frangou, S. Transdiagnostic and disease-specific abnormalities in the default-mode network hubs in psychiatric disorders: A meta-analysis of resting-state functional imaging studies. Eur. Psychiatry 2020, 63, e57. [Google Scholar] [CrossRef]
- Janiri, D.; di Nicola, M.; Martinotti, G.; Janiri, L. Who’s the leader, mania or depression? Predominant polarity and alcohol/polysubstance use in bipolar disorders. Curr. Neuropharmacol. 2017, 15, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Sani, G.; Simonetti, A.; Janiri, D.; Banaj, N.; Ambrosi, E.; de Rossi, P.; Ciullo, V.; Arciniegas, D.B.; Piras, F.; Spalletta, G. Association between duration of lithium exposure and hippocampus/amygdala volumes in type I bipolar disorder. J. Affect. Disord. 2018, 232, 341–348. [Google Scholar] [CrossRef]
- Luciano, M.; Janiri, D.; Fiorillo, A.; Sani, G. Clinical picture, temperament, and personality of patients with mixed states. Psychiatr. Clin. N. Am. 2020, 43, 15–26. [Google Scholar] [CrossRef]
- Sani, G.; Tondo, L.; Koukopoulos, A.; Reginaldi, D.; Kotzalidis, G.D.; Koukopoulos, A.E.; Manfredi, G.; Mazzarini, L.; Pacchiarotti, I.; Simonetti, A.; et al. Suicide in a large population of former psychiatric inpatients. Psychiatry Clin. Neurosci. 2011, 65, 286–295. [Google Scholar] [CrossRef]
- Verdolini, N.; Dean, J.; Elisei, S.; Quartesan, R.; Zaman, R.; Agius, M. Bipolar disorder: The importance of clinical assessment in identifying prognostic factors—An Audit. Part 2: Mixed state features and rapid cycling. Psychiatr. Danub. 2014, 26, 301–308. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiri, D.; Conte, E.; De Luca, I.; Simone, M.V.; Moccia, L.; Simonetti, A.; Mazza, M.; Marconi, E.; Monti, L.; Chieffo, D.P.R.; et al. Not Only Mania or Depression: Mixed States/Mixed Features in Paediatric Bipolar Disorders. Brain Sci. 2021, 11, 434. https://doi.org/10.3390/brainsci11040434
Janiri D, Conte E, De Luca I, Simone MV, Moccia L, Simonetti A, Mazza M, Marconi E, Monti L, Chieffo DPR, et al. Not Only Mania or Depression: Mixed States/Mixed Features in Paediatric Bipolar Disorders. Brain Sciences. 2021; 11(4):434. https://doi.org/10.3390/brainsci11040434
Chicago/Turabian StyleJaniri, Delfina, Eliana Conte, Ilaria De Luca, Maria Velia Simone, Lorenzo Moccia, Alessio Simonetti, Marianna Mazza, Elisa Marconi, Laura Monti, Daniela Pia Rosaria Chieffo, and et al. 2021. "Not Only Mania or Depression: Mixed States/Mixed Features in Paediatric Bipolar Disorders" Brain Sciences 11, no. 4: 434. https://doi.org/10.3390/brainsci11040434







