Relationship between Auditory Evoked Potentials and Circadian Preference in Patients with Major Depressive Episodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Scales
2.3. Electroencephalography Methods
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Antypa, N.; Vogelzangs, N.; Meesters, Y.; Schoevers, R.; Penninx, B.W. Chronotype Associations with Depression and Anxiety Disorders in a Large Cohort Study. Depress Anxiety 2016, 33, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Takaesu, Y. Circadian rhythm in bipolar disorder: A review of the literature. Psychiatry Clin. Neurosci. 2018, 72, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Selvi, Y.; Aydin, A.; Boysan, M.; Atli, A.; Agargun, M.Y.; Besiroglu, L. Associations between chronotype, sleep quality, suicidality, and depressive symptoms in patients with major depression and healthy controls. Chronobiol. Int. 2010, 27, 1813–1828. [Google Scholar] [CrossRef] [PubMed]
- Haraden, D.A.; Mullin, B.C.; Hankin, B.L. The relationship between depression and chronotype: A longitudinal assessment during childhood and adolescence. Depress Anxiety 2017, 34, 967–976. [Google Scholar] [CrossRef]
- Romo-Nava, F.; Blom, T.J.; Cuellar-Barboza, A.B.; Winham, S.J.; Colby, C.L.; Nunez, N.A.; Biernacka, J.M.; Frye, M.A.; McElroy, S.L. Evening chronotype as a discrete clinical subphenotype in bipolar disorder. J. Affect Disord. 2020, 266, 556–562. [Google Scholar] [CrossRef]
- Park, Y.M. Chronotype is associated with emotional dysregulation influenced by childhood trauma: A retrospective study. Chronobiol. Med. 2019, 1, 21–25. [Google Scholar] [CrossRef]
- Von Schantz, M. Phenotypic effects of genetic variability in human clock genes on circadian and sleep parameters. J. Genet. 2008, 87, 513–519. [Google Scholar] [CrossRef]
- Ciarleglio, C.M.; Resuehr, H.E.; McMahon, D.G. Interactions of the serotonin and circadian systems: Nature and nurture in rhythms and blues. Neuroscience 2011, 197, 8–16. [Google Scholar] [CrossRef]
- Oikonomou, G.; Altermatt, M.; Zhang, R.W.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The Serotonergic Raphe Promote Sleep in Zebrafish and Mice. Neuron 2019, 103, 686–701 e688. [Google Scholar] [CrossRef]
- Ojeda, D.A.; Perea, C.S.; Suarez, A.; Nino, C.L.; Gutierrez, R.M.; Lopez-Leon, S.; Adan, A.; Arboleda, H.; Camargo, A.; Forero, D.A. Common functional polymorphisms in SLC6A4 and COMT genes are associated with circadian phenotypes in a South American sample. Neurol. Sci. 2014, 35, 41–47. [Google Scholar] [CrossRef]
- Hegerl, U.; Juckel, G. Identifying psychiatric patients with serotonergic dysfunctions by event-related potentials. World J. Biol. Psychiatry 2000, 1, 112–118. [Google Scholar] [CrossRef]
- Juckel, G.; Molnar, M.; Hegerl, U.; Csepe, V.; Karmos, G. Auditory-evoked potentials as indicator of brain serotonergic activity--first evidence in behaving cats. Biol. Psychiatry 1997, 41, 1181–1195. [Google Scholar] [CrossRef]
- O’Neill, B.V.; Croft, R.J.; Nathan, P.J. The loudness dependence of the auditory evoked potential (LDAEP) as an in vivo biomarker of central serotonergic function in humans: Rationale, evaluation and review of findings. Hum. Psychopharmacol. 2008, 23, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Park, Y.M.; Lee, S.H.; Shim, M. Prediction of long-term treatment response to selective serotonin reuptake inhibitors (SSRIs) using scalp and source loudness dependence of auditory evoked potentials (LDAEP) analysis in patients with major depressive disorder. Int. J. Mol. Sci. 2015, 16, 6251–6265. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, Y.M. The association between suicidality and serotonergic dysfunction in depressed patients. J. Affect Disord. 2013, 148, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Jung, E.; Kim, H.S.; Hahn, S.W.; Lee, S.H. Differences in central serotoninergic transmission among patients with recent onset, sub-chronic, and chronic schizophrenia as assessed by the loudness dependence of auditory evoked potentials. Schizophr. Res. 2015, 168, 180–184. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, B.H. Treatment response in relation to subthreshold bipolarity in patients with major depressive disorder receiving antidepressant monotherapy: A post hoc data analysis (KOMDD study). Neuropsychiatr Dis. Treat. 2016, 12, 1221–1227. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Hirschfeld, R.M.; Williams, J.B.; Spitzer, R.L.; Calabrese, J.R.; Flynn, L.; Keck, P.E., Jr.; Lewis, L.; McElroy, S.L.; Post, R.M.; Rapport, D.J.; et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am. J. Psychiatry 2000, 157, 1873–1875. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, K.M. Long-term stability and psychometric properties of the Composite Scale of Morningness. Ergonomics 1994, 37, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.P.; Fink, L.; Handelsman, L.; Foote, J.; Lovejoy, M.; Wenzel, K.; Sapareto, E.; Ruggiero, J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 1994, 151, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Ranieri, W.F. Scale for Suicide Ideation: Psychometric properties of a self-report version. J. Clin. Psychol. 1988, 44, 499–505. [Google Scholar] [CrossRef]
- Beck, A.T.; Weissman, A.; Lester, D.; Trexler, L. The measurement of pessimism: The hopelessness scale. J. Consult. Clin. Psychol. 1974, 42, 861–865. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- Kato, Y.; Urban, R.; Saito, S.; Yoshida, K.; Kurokawa, M.; Rigo, A. Psychometric properties of a Japanese version of Composite Scale of Morningness. Heliyon 2019, 5, e01092. [Google Scholar] [CrossRef]
- Jankowski, K.S. Composite Scale of Morningness: Psychometric properties, validity with Munich ChronoType Questionnaire and age/sex differences in Poland. Eur. Psychiatry 2015, 30, 166–171. [Google Scholar] [CrossRef]
- Aas, M.; Bellivier, F.; Bettella, F.; Henry, C.; Gard, S.; Kahn, J.P.; Lagerberg, T.V.; Aminoff, S.R.; Melle, I.; Leboyer, M.; et al. Childhood maltreatment and polygenic risk in bipolar disorders. Bipolar. Disord. 2020, 22, 174–181. [Google Scholar] [CrossRef]
- Park, H.K.; Lee, J.J.; Park, Y.M. Preserved Serotonergic Activity in Early-Onset Parkinson’s Disease. Can. J. Neurol. Sci. 2020, 47, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Karg, K.; Burmeister, M.; Shedden, K.; Sen, S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Arch. Gen. Psychiatry 2011, 68, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Perroud, N.; Zewdie, S.; Stenz, L.; Adouan, W.; Bavamian, S.; Prada, P.; Nicastro, R.; Hasler, R.; Nallet, A.; Piguet, C.; et al. Methylation of Serotonin Receptor 3a in Adhd, Borderline Personality, and Bipolar Disorders: Link with Severity of the Disorders and Childhood Maltreatment. Depress Anxiety 2016, 33, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Culverhouse, R.C.; Saccone, N.L.; Horton, A.C.; Ma, Y.; Anstey, K.J.; Banaschewski, T.; Burmeister, M.; Cohen-Woods, S.; Etain, B.; Fisher, H.L.; et al. Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol. Psychiatry 2018, 23, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, W.J.; Milaneschi, Y.; Abdellaoui, A.; Sullivan, P.F.; Hottenga, J.J.; Boomsma, D.I.; Penninx, B.W. Effect of polygenic risk scores on depression in childhood trauma. Br. J. Psychiatry 2014, 205, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mullins, N.; Power, R.A.; Fisher, H.L.; Hanscombe, K.B.; Euesden, J.; Iniesta, R.; Levinson, D.F.; Weissman, M.M.; Potash, J.B.; Shi, J.; et al. Polygenic interactions with environmental adversity in the aetiology of major depressive disorder. Psychol. Med. 2016, 46, 759–770. [Google Scholar] [CrossRef]
- Hendricks, T.; Francis, N.; Fyodorov, D.; Deneris, E.S. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J. Neurosci. 1999, 19, 10348–10356. [Google Scholar] [CrossRef]
- Malek, Z.S.; Dardente, H.; Pevet, P.; Raison, S. Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: Anatomical evidence and daily profiles. Eur. J. Neurosci. 2005, 22, 895–901. [Google Scholar] [CrossRef]
- Malek, Z.S.; Sage, D.; Pevet, P.; Raison, S. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology 2007, 148, 5165–5172. [Google Scholar] [CrossRef]
- Cagampang, F.R.; Inouye, S.T. Diurnal and circadian changes of serotonin in the suprachiasmatic nuclei: Regulation by light and an endogenous pacemaker. Brain Res. 1994, 639, 175–179. [Google Scholar] [CrossRef]
- Donner, N.C.; Johnson, P.L.; Fitz, S.D.; Kellen, K.E.; Shekhar, A.; Lowry, C.A. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress Anxiety 2012, 29, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hortnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Nakamaru-Ogiso, E.; Hamada, K.; Hensch, T.K. Serotonergic integration of circadian clock and ultradian sleep-wake cycles. J. Neurosci. 2012, 32, 14794–14803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGlashan, E.M.; Drummond, S.P.A.; Cain, S.W. Evening types demonstrate reduced SSRI treatment efficacy. Chronobiol. Int. 2018, 35, 1175–1178. [Google Scholar] [CrossRef]
- Lovenberg, T.W.; Baron, B.M.; de Lecea, L.; Miller, J.D.; Prosser, R.A.; Rea, M.A.; Foye, P.E.; Racke, M.; Slone, A.L.; Siegel, B.W.; et al. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 1993, 11, 449–458. [Google Scholar] [CrossRef]
- Sprouse, J.; Reynolds, L.; Li, X.; Braselton, J.; Schmidt, A. 8-OH-DPAT as a 5-HT7 agonist: Phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology 2004, 46, 52–62. [Google Scholar] [CrossRef]
- Vadnie, C.A.; McClung, C.A. Circadian Rhythm Disturbances in Mood Disorders: Insights into the Role of the Suprachiasmatic Nucleus. Neural. Plast 2017, 2017, 1504507. [Google Scholar] [CrossRef]
- Park, Y.M. The Hypothesis on the Prediction of Treatment Response with Buspirone Augmentation along with Serotonergic Antidepressant in Patients with Major Depressive Disorder Using Loudness Dependence of Auditory Evoked Potentials: Two Cases and Review of the Literature for Evidence. Psychiatry Investig. 2020, 17, 222–224. [Google Scholar] [CrossRef]
- Hegerl, U.; Gallinat, J.; Juckel, G. Event-related potentials. Do they reflect central serotonergic neurotransmission and do they predict clinical response to serotonin agonists? J. Affect Disord. 2001, 62, 93–100. [Google Scholar] [CrossRef]
- Pillai, R.L.I.; Bartlett, E.A.; Ananth, M.R.; Zhu, C.; Yang, J.; Hajcak, G.; Parsey, R.V.; DeLorenzo, C. Examining the underpinnings of loudness dependence of auditory evoked potentials with positron emission tomography. Neuroimage 2020, 213, 116733. [Google Scholar] [CrossRef]
- Daut, R.A.; Fonken, L.K. Circadian regulation of depression: A role for serotonin. Front. Neuroendocr. 2019, 54, 100746. [Google Scholar] [CrossRef] [PubMed]
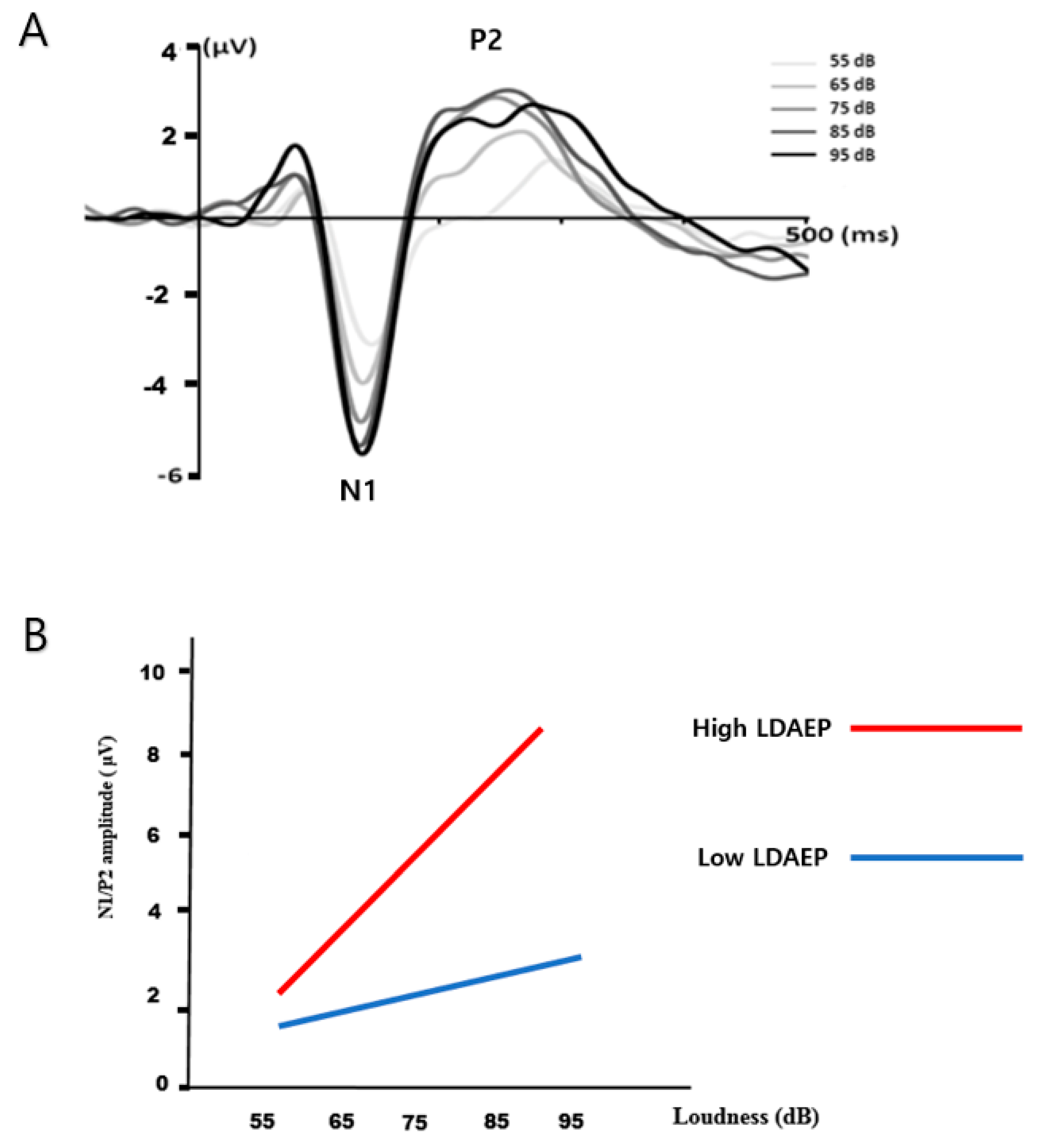
| Variable | Morningness (n = 10) | Intermediate (n = 19) | Eveningness (n = 19) | p |
|---|---|---|---|---|
| Age at assessment, years | 40.0 ± 13.9 | 41.1 ± 13.0 | 34.2 ± 12.9 | 0.24 |
| a Sex ratio, males/females | 4/6 | 1/18 | 4/15 | 0.065 |
| Age at onset, years | 42.83 ± 7.99 | 32.90 ± 13.10 | 25.21 ± 11.45 | < 0.01 |
| b Number of episodes | 2.83 ± 4.02 | 5.05 ± 4.29 | 4.90 ± 4.47 | 0.11 |
| a Presence of bipolarity | 8/2 | 16/3 | 5/14 | < 0.001 |
| LDAEP (µV/10 dB) | 0.84 ± 0.53 | 1.38 ± 0.77 | 1.07 ± 0.73 | 0.14 |
| BIS | 84.67 ± 20.37 | 71.68 ± 21.37 | 84.42 ± 12.90 | 0.077 |
| BDI | 25.50 ± 10.19 | 26.58 ± 9.97 | 32.58 ± 6.56 | 0.057 |
| b BSS | 8.67 ± 11.09 | 9.53 ± 7.84 | 17.90 ± 9.15 | < 0.05 |
| b BHS | 7.67 ± 6.35 | 8.68 ± 6.11 | 16.50 ± 3.13 | < 0.001 |
| HAMD | 17.17 ± 7.08 | 18.79 ± 4.89 | 19.05 ± 5.79 | 0.77 |
| HAMA | 17.67 ± 7.34 | 20.11 ± 6.65 | 21.74 ± 6.79 | 0.43 |
| b K-MDQ | 4.80 ± 1.75 | 4.47 ± 2.55 | 8.32 ± 3.13 | < 0.001 |
| K-CTQ | 40.50 ± 14.52 | 50.63 ± 17.29 | 55.95 ± 22.80 | 0.13 |
| b Emotional abuse | 6.60 ± 2.37 | 10.58 ± 5.00 | 12.26 ± 5.58 | < 0.05 |
| b Physical abuse | 7.70 ± 3.16 | 9.53 ± 4.82 | 11.26 ± 6.40 | 0.34 |
| b Sexual abuse | 5.80 ± 1.14 | 5.79 ± 1.48 | 8.00 ± 5.13 | 0.71 |
| Variable | Higher K-CSM (n = 25) (Toward Morningness) | Lower K-CSM (n = 23) (Toward Eveningness) | p |
|---|---|---|---|
| Age at assessment, years (mean ± SD) | 40.5 ± 13.9 | 35.5 ± 12.3 | 0.20 |
| a Sex ratio, males/females | 5/20 | 4/19 | 0.82 |
| Age at onset, years | 35.05 ± 13.42 | 27.17 ± 11.73 | < 0.05 |
| b Number of episodes | 4.24 ± 4.02 | 5.09 ± 4.59 | 0.52 |
| a Presence of bipolarity | 22/3 | 7/16 | < 0.001 |
| LDAEP (µV/10 dB) | 1.16 ± 0.74 | 1.12 ± 0.73 | 0.86 |
| BIS | 72.33 ± 21.33 | 85.00 ± 13.82 | < 0.05 |
| BDI | 25.12 ± 9.67 | 32.65 ± 6.91 | < 0.01 |
| b BSS | 9.00 ± 8.49 | 17.90 ± 9.15 | < 0.01 |
| b BHS | 8.14 ± 6.05 | 15.32 ± 4.57 | < 0.001 |
| HAMD | 19.41 ± 6.71 | 19.09 ± 5.69 | 0.62 |
| HAMA | 17.67 ± 7.34 | 21.70 ± 6.75 | 0.22 |
| b K-MDQ | 4.40 ± 2.10 | 7.87 ± 3.24 | < 0.001 |
| K-CTQ | 46.24 ± 16.17 | 55.39 ± 22.28 | 0.11 |
| b Emotional abuse | 8.84 ± 4.43 | 12.13 ± 5.52 | < 0.05 |
| b Physical abuse | 9.04 ± 4.49 | 10.70 ± 6.09 | 0.43 |
| b Sexual abuse | 5.92 ± 1.41 | 7.48 ± 4.79 | 0.93 |
| Variables | Coefficient | SE | t | p |
|---|---|---|---|---|
| K-CSM Score | ||||
| Age | 0.023 | 0.13 | 0.17 | 0.87 |
| Gender | −1.25 | 3.19 | −0.39 | 0.70 |
| BDI | −0.25 | 0.13 | −1.91 | 0.064 |
| K-MDQ | −1.71 | 0.38 | −4.55 | <0.001 |
| K-CTQ | −0.01 | 0.063 | −0.15 | 0.88 |
| Age at onset | 0.015 | 0.15 | 0.098 | 0.92 |
| Groups based on low and high LDAEP | −7.56 | 2.51 | −3.01 | <0.01 |
| Variables | Coefficient | SE | t | p |
|---|---|---|---|---|
| Age at Onset | ||||
| Gender | 0.097 | 5.38 | 0.018 | 0.99 |
| K-CTQ | −0.26 | 0.20 | −1.31 | 0.20 |
| Types of circadian preference | −5.51 | 6.09 | −0.90 | 0.37 |
| LDAEP | −10.25 | 7.67 | −1.34 | 0.19 |
| Interaction of K-CTQ and LDAEP | −14.08 | 5.87 | −2.40 | <0.05 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-M. Relationship between Auditory Evoked Potentials and Circadian Preference in Patients with Major Depressive Episodes. Brain Sci. 2020, 10, 370. https://doi.org/10.3390/brainsci10060370
Park Y-M. Relationship between Auditory Evoked Potentials and Circadian Preference in Patients with Major Depressive Episodes. Brain Sciences. 2020; 10(6):370. https://doi.org/10.3390/brainsci10060370
Chicago/Turabian StylePark, Young-Min. 2020. "Relationship between Auditory Evoked Potentials and Circadian Preference in Patients with Major Depressive Episodes" Brain Sciences 10, no. 6: 370. https://doi.org/10.3390/brainsci10060370
APA StylePark, Y.-M. (2020). Relationship between Auditory Evoked Potentials and Circadian Preference in Patients with Major Depressive Episodes. Brain Sciences, 10(6), 370. https://doi.org/10.3390/brainsci10060370





