MIP-Modified Porous Silicon Optical Sensor for Interleukin-6 Label-Free Quantification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of IL-6 and o-PD Solution
2.3. Porous Silicon Fabrication
2.4. Reflectance Spectroscopy
2.5. Electrochemical Measurements
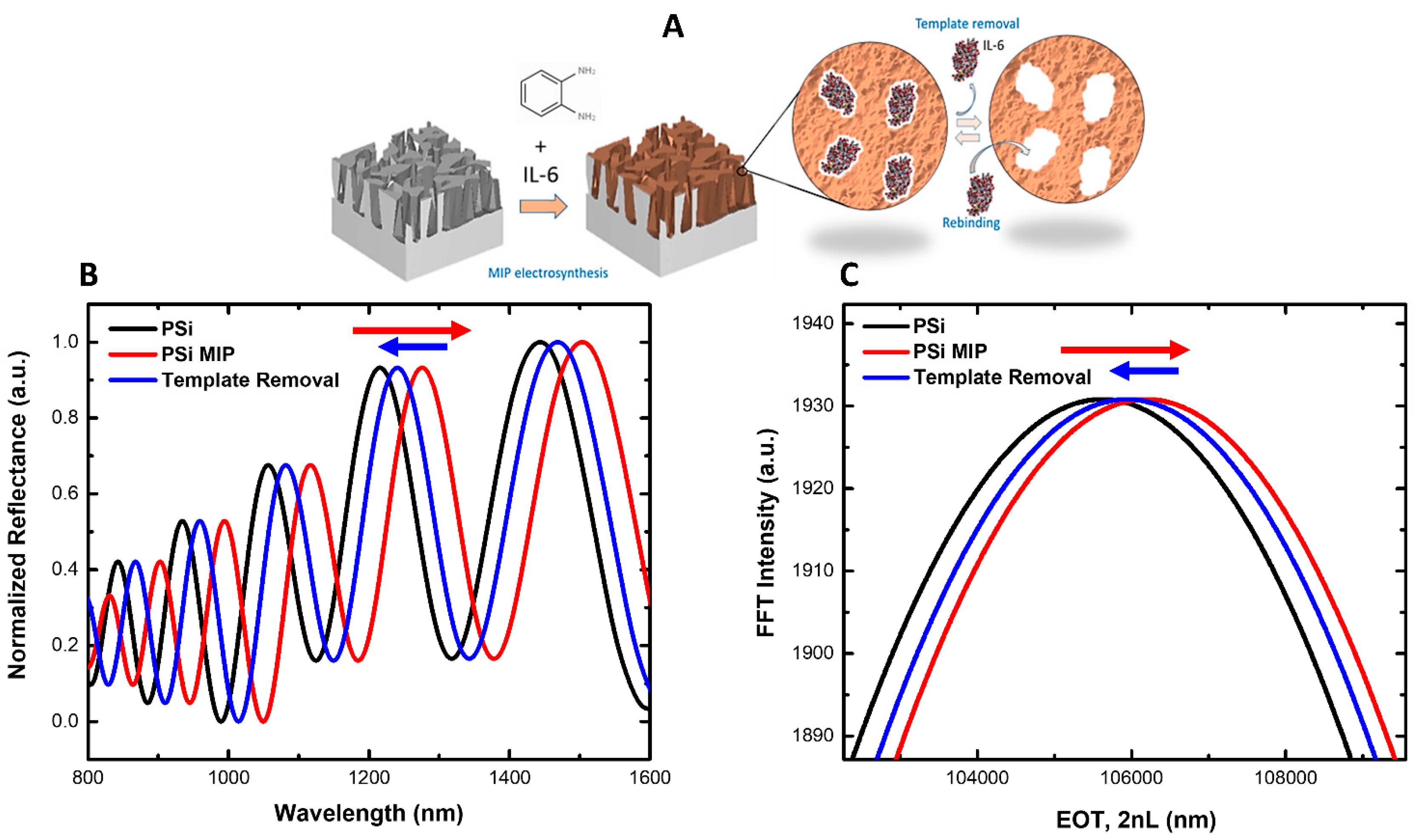
2.6. IL-6 MIP Synthesis, Template Removal, and Rebinding
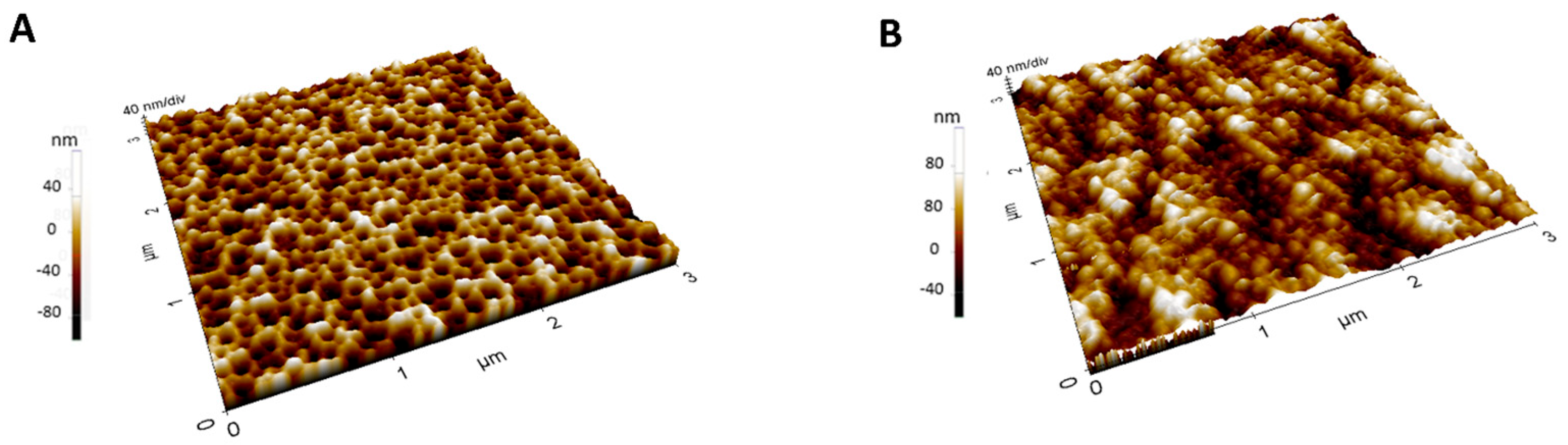
2.7. Atomic Force Microscopy
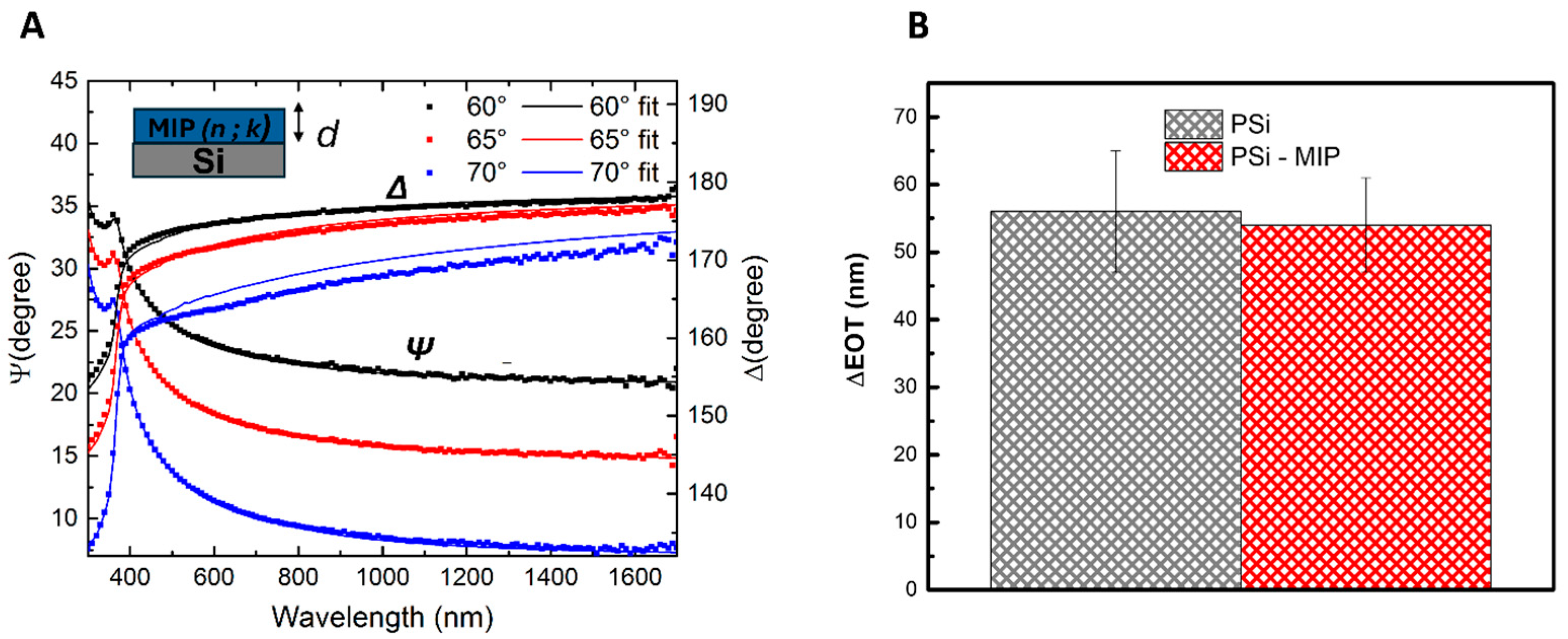
2.8. Spectroscopic Ellipsometry
2.9. QCM-D Studies
2.10. Contact Angle Measurements
3. Results and Discussion
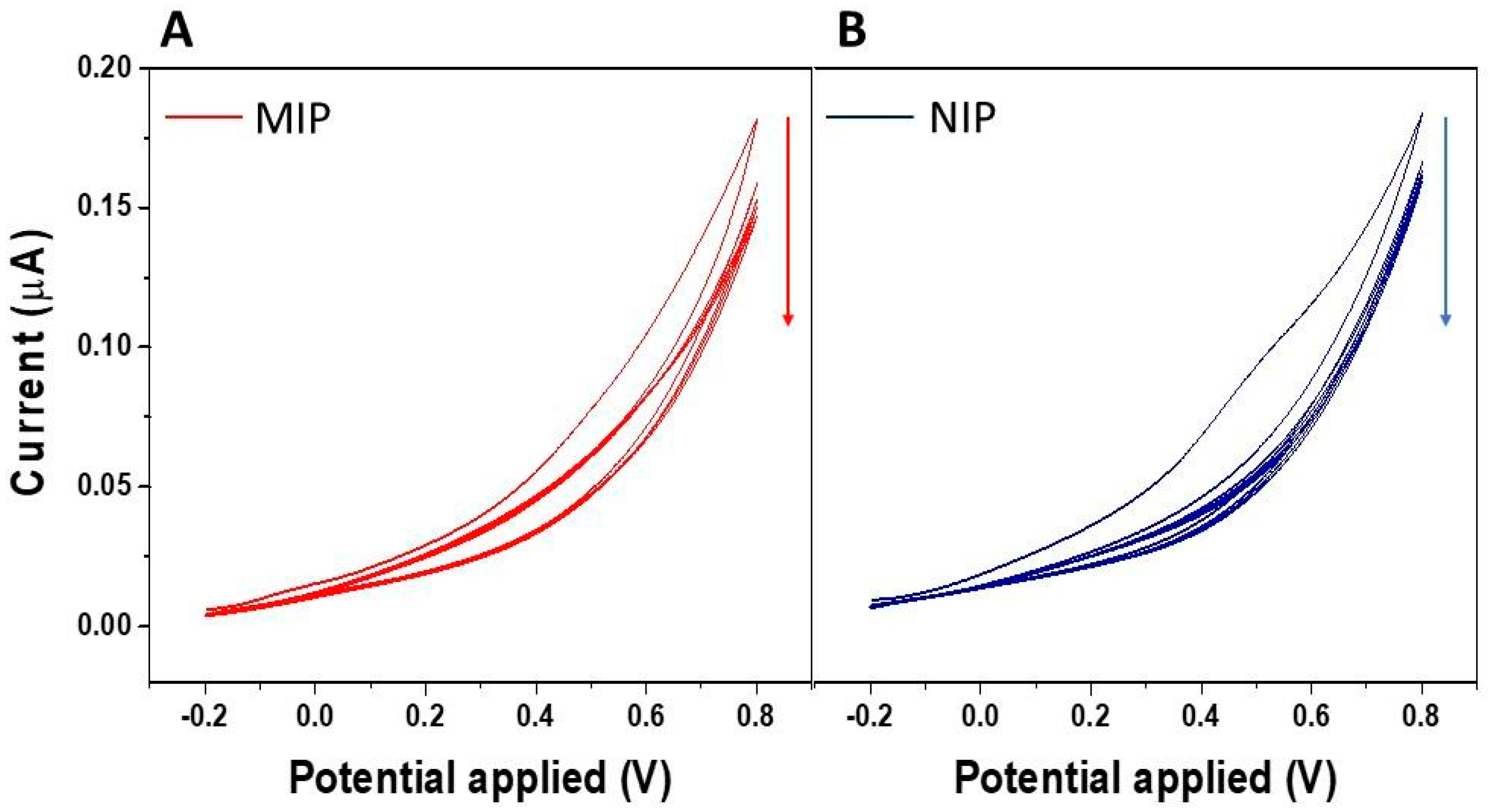
3.1. Synthesis and Characterization of the IL-6 MIP Receptor on Porous Silicon
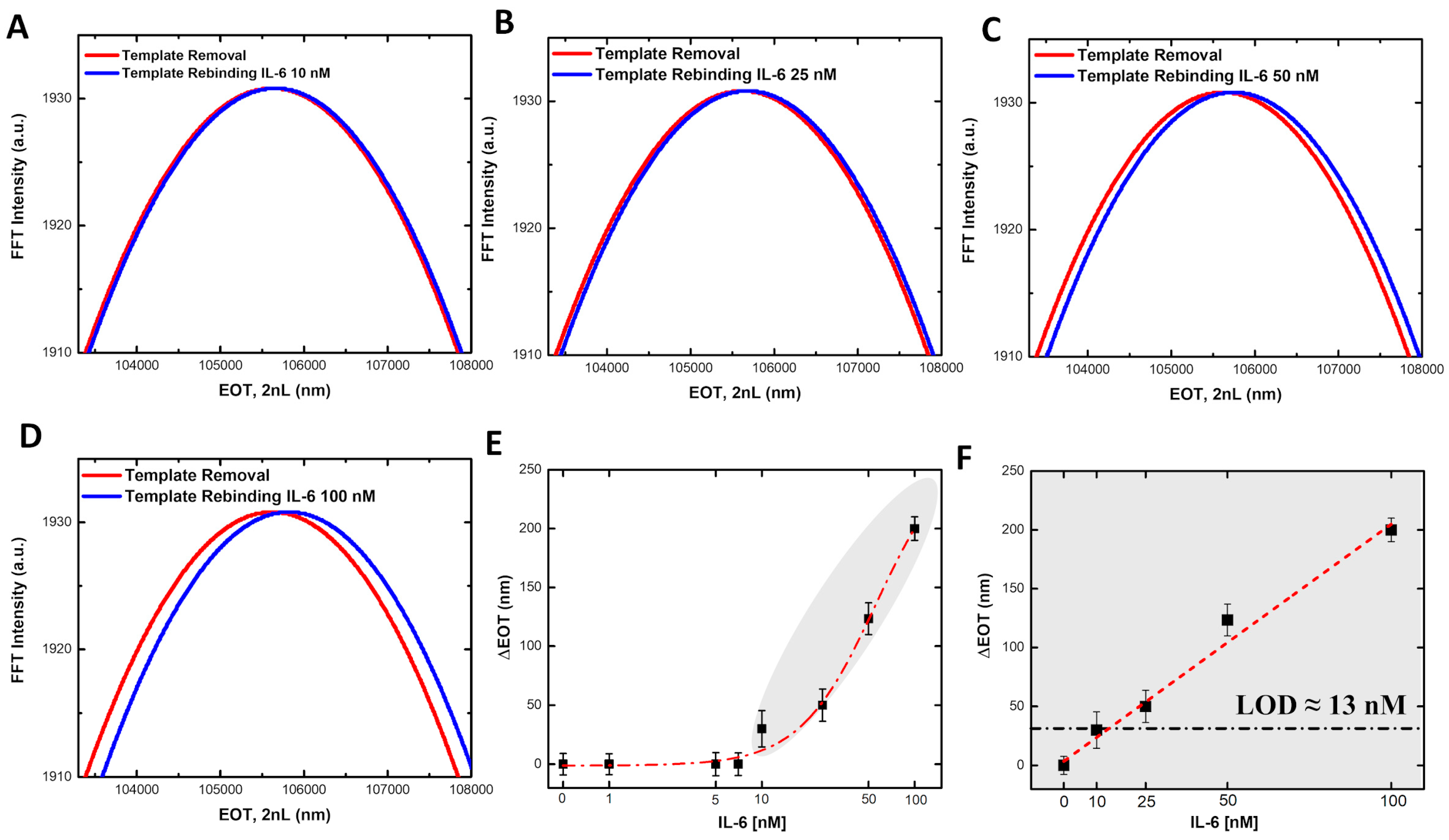
3.2. MIP-PSi Sensor Calibration and Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moretta, R.; De Stefano, L.; Terracciano, M.; Rea, I. Porous Silicon Optical Devices: Recent Advances in Biosensing Applications. Sensors 2021, 21, 1336. [Google Scholar] [CrossRef] [PubMed]
- Nocerino, V.; Rea, I.; Siciliano, G.; De Stefano, L.; Primiceri, E. Polymers Modified Porous Silicon Optical (Bio) Sensors. TrAC-Trends Anal. Chem. 2024, 177, 117811. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Massad-Ivanir, N.; Segal, E.; Weiss, S. Porous Silicon-Based Photonic Biosensors: Current Status and Emerging Applications. Anal. Chem. 2019, 91, 441–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Robbiano, V.; Paternò, G.M.; Carnicella, G.; Debrassi, A.; La Mattina, A.A.; Mariani, S.; Minotto, A.; Egri, G.; Dähne, L.; et al. Nanoscale Photoluminescence Manipulation in Monolithic Porous Silicon Oxide Microcavity Coated with Rhodamine-Labeled Polyelectrolyte via Electrostatic Nanoassembling. Adv. Opt. Mater. 2021, 9, 2100036. [Google Scholar] [CrossRef]
- Corsi, M.; Paghi, A.; Mariani, S.; Golinelli, G.; Debrassi, A.; Egri, G.; Leo, G.; Vandini, E.; Vilella, A.; Dähne, L.; et al. Bioresorbable Nanostructured Chemical Sensor for Monitoring of PH Level In Vivo. Adv. Sci. 2022, 9, 2202062. [Google Scholar] [CrossRef]
- Kilian, K.A.; Lai, L.M.H.; Magenau, A.; Cartland, S.; Bocking, T.; Girolamo, N.D.; Gal, M.; Gaus, K.; Gooding, J.J. Smart Tissue Culture: In Situ Monitoring of the Activity of Protease Enzymes Secreted from Live Cells Using Nanostructured Photonic Crystals. Nano Lett. 2009, 9, 2021–2025. [Google Scholar] [CrossRef]
- Rea, I.; Iodice, M.; Coppola, G.; Rendina, I.; Marino, A.; De Stefano, L. A Porous Silicon-Based Bragg Grating Waveguide Sensor for Chemical Monitoring. Sens. Actuators B Chem. 2009, 139, 39–43. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, R.; Sharma, P.; Voelcker, N.H. Advancements in Porous Silicon Biosensors for Point of Care, Wearable, and Implantable Applications. ACS Appl. Mater. Interfaces 2025, 17, 2814–2843. [Google Scholar] [CrossRef]
- Di Giulio, T.; Asif, I.M.; Corsi, M.; Rajpal, S.; Mizaikoff, B.; Ditaranto, N.; De Benedetto, G.E.; Malitesta, C.; Barillaro, G.; Mazzotta, E. A Molecularly Imprinted Polymer-Based Porous Silicon Optical Sensor for Quercetin Detection in Wines. ACS Appl. Mater. Interfaces 2025, 17, 12663–12675. [Google Scholar] [CrossRef]
- Mazzotta, E.; Di Giulio, T.; Mariani, S.; Corsi, M.; Malitesta, C.; Barillaro, G. Vapor-Phase Synthesis of Molecularly Imprinted Polymers on Nanostructured Materials at Room-Temperature. Small 2023, 19, 2302274. [Google Scholar] [CrossRef]
- Harraz, F.A. Porous Silicon Chemical Sensors and Biosensors: A Review. Sens. Actuators B Chem. 2014, 202, 897–912. [Google Scholar] [CrossRef]
- De Stefano, L.; De Tommasi, E.; Rea, I.; Rotiroti, L.; Canciello, M.; Maglio, G.; Palumbo, R. A Nanostructured Hybrid Material Based on Polymer Infiltrated Porous Silicon Layer. Appl. Phys. A Mater. Sci. Process. 2010, 98, 525–530. [Google Scholar] [CrossRef]
- Mariani, S.; Paghi, A.; La Mattina, A.A.; Debrassi, A.; Dähne, L.; Barillaro, G. Decoration of Porous Silicon with Gold Nanoparticles via Layer-by-Layer Nanoassembly for Interferometric and Hybrid Photonic/Plasmonic (Bio) Sensing. ACS Appl. Mater. Interfaces 2019, 11, 43731–43740. [Google Scholar] [CrossRef] [PubMed]
- Alhusaini, Q.; Scheld, W.S.; Jia, Z.; Das, D.; Afzal, F.; Müller, M.; Schönherr, H. Bare Eye Detection of Bacterial Enzymes of Pseudomonas Aeruginosa with Polymer Modified Nanoporous Silicon Rugate Filters. Biosensors 2022, 12, 1064. [Google Scholar] [CrossRef]
- Chin, G.P.; Guo, K.; Vasani, R.; Voelcker, N.H.; Prieto-Simón, B. Carbon-Stabilized Porous Silicon Biosensor for the Ultrasensitive Label-Free Electrochemical Detection of Bacterial RNA Gene Fragments. Biosens. Bioelectron. X 2024, 16, 100438. [Google Scholar] [CrossRef]
- De Stefano, L.; Rea, I.; Giardina, P.; Armenante, A.; Rendina, I. Protein-Modified Porous Silicon Nanostructures. Adv. Mater. 2008, 20, 1529–1533. [Google Scholar] [CrossRef]
- Kilian, K.A.; Böcking, T.; Gooding, J.J. The Importance of Surface Chemistry in Mesoporous Materials: Lessons from Porous Silicon Biosensors. Chem. Commun. 2009, 45, 630–640. [Google Scholar] [CrossRef]
- Gostkowska-Lekner, N.; Kojda, D.; Hoffmann, J.E.; May, M.; Huber, P.; Habicht, K.; Hofmann, T. Synthesis of Organic–Inorganic Hybrids Based on the Conjugated Polymer P3HT and Mesoporous Silicon. Microporous Mesoporous Mater. 2022, 343, 112155. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, D. Recent Advances in Hybrid System of Porous Silicon Nanoparticles and Biocompatible Polymers for Biomedical Applications. Biomed. Eng. Lett. 2021, 11, 171–181. [Google Scholar] [CrossRef]
- De Stefano, L.; Rea, I.; Armenante, A.; Giardina, P.; Giocondo, M.; Rendina, I. Self-Assembled Biofilm of Hydrophobins Protects the Silicon Surface in the KOH Wet Etch Process. Langmuir 2007, 23, 7920–7922. [Google Scholar] [CrossRef]
- Mariani, S.; Robbiano, V.; Strambini, L.M.; Debrassi, A.; Egri, G.; Dähne, L.; Barillaro, G. Layer-by-Layer Biofunctionalization of Nanostructured Porous Silicon for High-Sensitivity and High-Selectivity Label-Free Affinity Biosensing. Nat. Commun. 2018, 9, 5256. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zheng, Y.; Xue, Q.; Wen, Z.; Chen, L.; Huang, Y.; Hang, T.; Wu, Y.; Li, M. Covalent Grafting of PMMA Organic Film on Porous Silicon for Achieving Ultralow-k Organic Films. ACS Appl. Electron. Mater. 2022, 4, 6047–6057. [Google Scholar] [CrossRef]
- Sayed, F.A.; Elsayed, H.A.; Mehaney, A.; Eissa, M.F.; Aly, A.H. A Doped-Polymer Based Porous Silicon Photonic Crystal Sensor for the Detection of Gamma-Ray Radiation. RSC Adv. 2023, 13, 3123–3138. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Sarkar, D. Electrical Properties of Polyaniline (PANI)-Porous Silicon (PS) Heterostructure. Polym. Bull. 2023, 81, 2329–2343. [Google Scholar] [CrossRef]
- Agnishwaran, B.; Manivasagam, G.; Udduttula, A. Molecularly Imprinted Polymers: Shaping the Future of Early-Stage Bone Loss Detection—A Review. ACS Omega 2024, 9, 8730–8742. [Google Scholar] [CrossRef]
- Kor, K.; Zarei, K. Development and Characterization of an Electrochemical Sensor for Furosemide Detection Based on Electropolymerized Molecularly Imprinted Polymer. Talanta 2016, 146, 181–187. [Google Scholar] [CrossRef]
- Siciliano, G.; Chiriacò, M.S.; Ferrara, F.; Turco, A.; Velardi, L.; Signore, M.A.; Esposito, M.; Gigli, G.; Primiceri, E. Development of an MIP Based Electrochemical Sensor for TGF-Β1 Detection and Its Application in Liquid Biopsy. Analyst 2023, 148, 4447–4455. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Turner, A.P.F.; Tiwari, A. Electrochemical Evaluation of Troponin T Imprinted Polymer Receptor. Biosens. Bioelectron. 2014, 59, 160–165. [Google Scholar] [CrossRef]
- Shi, J.; Huo, R.; Li, N.; Li, H.; Zhai, T.; Li, H.; Shen, B.; Ye, J.; Fu, R.; Di, W. CYR61, a Potential Biomarker of Tumor Inflammatory Response in Epithelial Ovarian Cancer Microenvironment of Tumor Progress. BMC Cancer 2019, 19, 1140. [Google Scholar] [CrossRef]
- Seki, E.; Schwabe, R.F. Hepatic Inflammation and Fibrosis: Functional Links and Key Pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. Recent Advances in Electrochemical and Optical Biosensors Designed for Detection of Interleukin 6. Sensors 2020, 20, 646. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 Cytokine: An Overview of the Immune Regulation, Immune Dysregulation, and Therapeutic Approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Dalal, V.; Kumar, R.; Kumar, S.; Sharma, A.; Kumar, L.; Sharma, J.B.; Roy, K.K.; Singh, N.; Vanamail, P. Biomarker Potential of IL-6 and VEGF-A in Ascitic Fluid of Epithelial Ovarian Cancer Patients. Clin. Chim. Acta 2018, 482, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Papiris, S.A.; Tomos, I.P.; Karakatsani, A.; Spathis, A.; Korbila, I.; Analitis, A.; Kolilekas, L.; Kagouridis, K.; Loukides, S.; Karakitsos, P.; et al. High Levels of IL-6 and IL-8 Characterize Early-on Idiopathic Pulmonary Fibrosis Acute Exacerbations. Cytokine 2018, 102, 168–172. [Google Scholar] [CrossRef]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic Literature Review of IL-6 as a Biomarker or Treatment Target in Patients with Gastric, Bile Duct, Pancreatic and Colorectal Cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, X.; Fan, M.; Ruan, S.; Peng, S.; You, R.; Chen, J.; Lu, Y. SERS-Based Self-Calibrating Aptamer Sensor for Selective Detection of IL-6. Sens. Actuators B Chem. 2023, 374, 132828. [Google Scholar] [CrossRef]
- Dolin, H.H.; Papadimos, T.J.; Stepkowski, S.; Chen, X.; Pan, Z.K. A Novel Combination of Biomarkers to Herald the Onset of Sepsis Prior to the Manifestation of Symptoms. Shock 2018, 49, 364–370. [Google Scholar] [CrossRef]
- Nocerino, V.; Siciliano, G.; Rea, I.; Dardano, P.; Chiriacò, M.S.; Ferrara, F.; Primiceri, E.; De Stefano, L. Porous Silicon Modified by Molecularly Imprinted Polymers for Interleukin-6 (IL-6) Detection. EPJ Web Conf. 2024, 309, 04019. [Google Scholar] [CrossRef]
- Bianco, M.; Guarino, V.; Maruccio, G.; Galli, G.; Martinelli, E.; Montani, G.; Rinaldi, R.; Arima, V. Non-Biofouling Fluorinated Block Copolymer Coatings for Contact Lenses. Sci. Adv. Mater. 2015, 7, 1387–1394. [Google Scholar] [CrossRef]
- Gupta, P.; Banerjee, A.; Gupta, N.J. Spatio-Temporal Study on Changing Trend of Land Use and Land Cover Pattern in Munnar Area, Idukki District, Western Ghats, India. Indian J. Geo-Marine Sci. 2020, 49, 1055–1067. [Google Scholar]
- Bianco, M.; Aloisi, A.; Arima, V.; Capello, M.; Ferri-Borgogno, S.; Novelli, F.; Leporatti, S.; Rinaldi, R. Quartz Crystal Microbalance with Dissipation (QCM-D) as Tool to Exploit Antigen-Antibody Interactions in Pancreatic Ductal Adenocarcinoma Detection. Biosens. Bioelectron. 2013, 42, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Moretta, R.; Terracciano, M.; Borbone, N.; Oliviero, G.; Schiattarella, C.; Piccialli, G.; Falanga, A.P.; Marzano, M.; Dardano, P.; De Stefano, L.; et al. Pna-Based Graphene Oxide/Porous Silicon Hybrid Biosensor: Towards a Label-Free Optical Assay for Brugada Syndrome. Nanomaterials 2020, 10, 2233. [Google Scholar] [CrossRef]
- Rea, I.; Giardina, P.; Longobardi, S.; Porro, F.; Casuscelli, V.; Rendina, I.; De Stefano, L. Hydrophobin Vmh2-Glucose Complexes Self-Assemble in Nanometric Biofilms. J. R. Soc. Interface 2012, 9, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Bahri, M.; Fredj, Z.; Qin, P.; Sawan, M. DNA-Coupled AuNPs@CuMOF for Sensitive Electrochemical Detection of Carcinoembryonic Antigen. ACS Appl. Nano Mater. 2024, 7, 11921–11930. [Google Scholar] [CrossRef]
- Bahri, M.; Shi, B.; Elaguech, M.A.; Djebbi, K.; Zhou, D.; Liang, L.; Tlili, C.; Wang, D. Tungsten Disulfide Nanosheet-Based Field-Effect Transistor Biosensor for DNA Hybridization Detection. ACS Appl. Nano Mater. 2022, 5, 5035–5044. [Google Scholar] [CrossRef]
- Zito, G.; Siciliano, G.; Seifalinezhad, A.; Miranda, B.; Lanzio, V.; Schwartzberg, A.; Gigli, G.; Turco, A.; Rendina, I.; Mocella, V.; et al. Molecularly Imprinted Polymer Sensor Empowered by Bound States in the Continuum for Selective Trace-Detection of TGF-Beta. Adv. Sci. 2024, 11, 2401843. [Google Scholar] [CrossRef] [PubMed]
- Giorgi-Coll, S.; Marín, M.J.; Sule, O.; Hutchinson, P.J.; Carpenter, K.L.H. Aptamer-Modified Gold Nanoparticles for Rapid Aggregation-Based Detection of Inflammation: An Optical Assay for Interleukin-6. Microchim. Acta 2020, 187, 13. [Google Scholar] [CrossRef]
- Xie, T.; Xu, D.; Shang, Y.; Li, Y.; Gu, Y.; Yang, G.; Qu, L. Highly Sensitive SERS Detection of IL-6 in Serum by Au@Fe3O4 Nanoring-Based Sandwich Immunoassay. Sens. Actuators B Chem. 2023, 375, 132897. [Google Scholar] [CrossRef]
- Ting, W.T.; Wang, M.J.; Howlader, M.M.R. Interleukin-6 Electrochemical Sensor Using Poly(o-Phenylenediamine)-Based Molecularly Imprinted Polymer. Sens. Actuators B Chem. 2024, 404, 135282. [Google Scholar] [CrossRef]
- Oliveira, D.; Correia, B.P.; Sharma, S.; Moreira, F.T.C. Molecular Imprinted Polymers on Microneedle Arrays for Point of Care Transdermal Sampling and Sensing of Inflammatory Biomarkers. ACS Omega 2022, 7, 39039–39044. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, F.; Wang, C.; Li, H.; Xu, Y.; Sun, S. Ratiometric Electrochemical Detection of Interleukin-6 Using Electropolymerized Methylene Blue and a Multi-Walled Carbon-Nanotube-Modified Screen-Printed Carbon Electrode. Biosensors 2024, 14, 457. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocerino, V.; Siciliano, G.; Bianco, M.; Rea, I.; Dardano, P.; Chiriacò, M.S.; Ferrara, F.; Gigli, G.; Primiceri, E.; De Stefano, L. MIP-Modified Porous Silicon Optical Sensor for Interleukin-6 Label-Free Quantification. Biosensors 2025, 15, 320. https://doi.org/10.3390/bios15050320
Nocerino V, Siciliano G, Bianco M, Rea I, Dardano P, Chiriacò MS, Ferrara F, Gigli G, Primiceri E, De Stefano L. MIP-Modified Porous Silicon Optical Sensor for Interleukin-6 Label-Free Quantification. Biosensors. 2025; 15(5):320. https://doi.org/10.3390/bios15050320
Chicago/Turabian StyleNocerino, Valeria, Giulia Siciliano, Monica Bianco, Ilaria Rea, Principia Dardano, Maria Serena Chiriacò, Francesco Ferrara, Giuseppe Gigli, Elisabetta Primiceri, and Luca De Stefano. 2025. "MIP-Modified Porous Silicon Optical Sensor for Interleukin-6 Label-Free Quantification" Biosensors 15, no. 5: 320. https://doi.org/10.3390/bios15050320
APA StyleNocerino, V., Siciliano, G., Bianco, M., Rea, I., Dardano, P., Chiriacò, M. S., Ferrara, F., Gigli, G., Primiceri, E., & De Stefano, L. (2025). MIP-Modified Porous Silicon Optical Sensor for Interleukin-6 Label-Free Quantification. Biosensors, 15(5), 320. https://doi.org/10.3390/bios15050320







