Can Propolis Be a Useful Adjuvant in Brain and Neurological Disorders and Injuries? A Systematic Scoping Review of the Latest Experimental Evidence
Abstract
1. Introduction
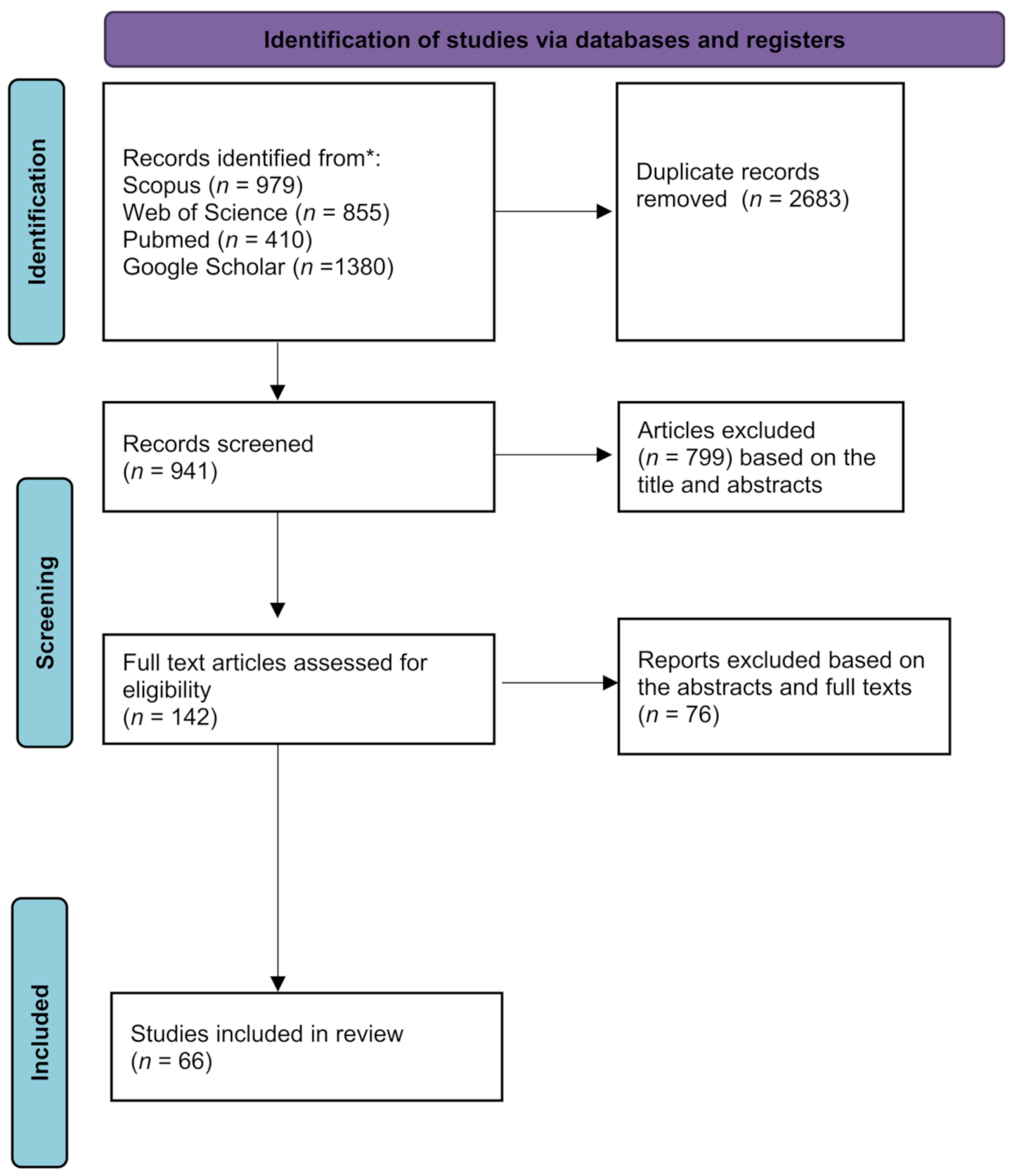
2. Methods
2.1. Search Strategy and Study Selection
2.2. Eligibility Criteria
2.3. Data Collection
3. Results
4. Discussion
5. Future Directions and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dalenberg, H.; Maes, P.; Mott, B.; Anderson, K.E.; Spivak, M. Propolis envelope promotes beneficial bacteria in the honey bee (Apis mellifera) mouthpart microbiome. Insects 2020, 11, 453. [Google Scholar] [CrossRef]
- Saelao, P.; Borba, R.S.; Ricigliano, V.; Spivak, M.; Simone-Finstrom, M. Honeybee microbiome is stabilized in the presence of propolis. Biol. Lett. 2020, 16, 20200003. [Google Scholar] [CrossRef]
- Borba, R.S.; Klyczek, K.K.; Mogen, K.L.; Spivak, M. Seasonal benefits of a natural propolis envelope to honey bee immunity and colony health. J. Exp. Biol. 2015, 218, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; De Alencar, S.M.; Rosalen, P.L. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur. J. Med. Chem. 2016, 110, 267–279. [Google Scholar] [CrossRef]
- Rojczyk, E.; Klama-Baryła, A.; Łabuś, W.; Wilemska-Kucharzewska, K.; Kucharzewski, M. Historical and modern research on propolis and its application in wound healing and other fields of medicine and contributions by Polish studies. J. Ethnopharmacol. 2020, 262, 113159. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.D.; De Jong, D.; Berretta, A.A.; dos Galvão, E.B.S.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; da Conceição, L.F.M.R.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial. Biomed. Pharmacother. 2021, 138, 111526. [Google Scholar] [CrossRef]
- Kosari, M.; Noureddini, M.; Khamechi, S.P.; Najafi, A.; Ghaderi, A.; Sehat, M.; Banafshe, H.R. The effect of propolis plus Hyoscyamus niger L. methanolic extract on clinical symptoms in patients with acute respiratory syndrome suspected to COVID-19: A clinical trial. Phyther. Res. 2021, 35, 4000–4006. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. JBI Evid. Implement. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Estarli, M.; Barrera, E.S.A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Toaleb, N.I.; El Fadaly, H.A.; Abdel-Rahman, E.H.; Barakat, A.M.A. In vivo-Cellular and Humoral Immune Response for Evaluation of Propolis Effect on Chronic Toxoplasmosis in Rats. Adv. Anim. Vet. Sci. 2021, 9, 1045–1052. [Google Scholar] [CrossRef]
- Günday, M.; Saritaş, Z.K.; Demirel, H.H.; Bülbül, A.; Saritaş, T.B.; Görücü, F.; Becit, N. Does Anzer Propolis Have a Protective Effect on Rabbit Spinal Cord Ischemia/Reperfusion Injury? Braz. J. Cardiovasc. Surg. 2021. [Google Scholar] [CrossRef]
- Bazmandegan, G.; Shamsizadeh, A.; FathiNajafi, M.; Assadollahi, Z.; Allahtavakoli, M.; Kamiab, Z.; Vakilian, A.; Moghadam-Ahmadi, A.; Amirteimoury, M.; Boroushaki, M.T. Iranian brown propolis possesses neuroprotective effect against ischemic neuronal damage in mice. J. Herbmed Pharmacol. 2020, 9, 121–129. [Google Scholar] [CrossRef]
- Tandean, S.; Japardi, I.; Loe, M.L.; Riawan, W.; July, J. Protective effects of propolis extract in a rat model of traumatic brain injury via hsp70 induction. Open Access Maced. J. Med. Sci. 2019, 7, 2763–2766. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Du, H.; Luo, X.-L.; Liu, R.-S.; Huang, L.; Cao, C.-S. Caffeic Acid Phenethyl Ester Inhibits the Progression of Elastase Induced Aortic Aneurysm in Rats. Int. J. Pharmacol. 2019, 15, 385–393. [Google Scholar] [CrossRef]
- Bazmandegan, G.; Boroushaki, M.T.; Shamsizadeh, A.; Ayoobi, F.; Hakimizadeh, E.; Allahtavakoli, M. Brown propolis attenuates cerebral ischemia-induced oxidative damage via affecting antioxidant enzyme system in mice. Biomed. Pharmacother. 2017, 85, 503–510. [Google Scholar] [CrossRef]
- Gao, L.; Xu, X.; Gao, P.; Sun, L. The Neuroprotective Effects of Propolis and Its Ingredients. J. Chin. Inst. Food Sci. Technol. 2017, 17, 60–66. [Google Scholar] [CrossRef]
- Durak, M.A.; Öztanir, M.N.; Başak Türkmen, N.; Çiftçi, O.; Taşlidere, A.; Tecellioğlu, M.; Önder, A. Chrysin prevents brain damage caused by global cerebral ischemia/reperfusion in a C57BL/J6 mouse model. Turk. J. Med. Sci. 2016, 46, 1926–1933. [Google Scholar] [CrossRef]
- Barbosa, R.A.; Nunes, T.L.G.M.; Da Paixão, A.O.; Neto, R.B.; Moura, S.; Albuquerque, R.L.C., Jr.; Cândido, E.A.F.; Padilha, F.F.; Quintans-Júnior, L.J.; Gomes, M.Z.; et al. Hydroalcoholic extract of red propolis promotes functional recovery and axon repair after sciatic nerve injury in rats. Pharm. Biol. 2016, 54, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Yüce, S.; Cemal Gökçe, E.; Işkdemir, A.; Koç, E.R.; Cemil, D.B.; Gökçe, A.; Sargon, M.F. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann. Plast. Surg. 2015, 74, 684–692. [Google Scholar] [CrossRef]
- Brasil, F.B.; de Almeida, F.J.S.; Luckachaki, M.D.; Dall’Oglio, E.L.; de Oliveira, M.R. Pinocembrin pretreatment counteracts the chlorpyrifos-induced HO-1 downregulation, mitochondrial dysfunction, and inflammation in the SH-SY5Y cells. Metab. Brain Dis. 2021, 1–15. [Google Scholar] [CrossRef]
- Ayna, A.; Özbolat, S.N.; Darendelioglu, E. Quercetin, chrysin, caffeic acid and ferulic acid ameliorate cyclophosphamide-induced toxicities in SH-SY5Y cells. Mol. Biol. Rep. 2020, 47, 8535–8543. [Google Scholar] [CrossRef]
- Darendelioglu, E. Neuroprotective Effects of Chrysin on Diclofenac-Induced Apoptosis in SH-SY5Y Cells. Neurochem. Res. 2020, 45, 1064–1071. [Google Scholar] [CrossRef]
- Omar, N.A.; Abu-Almaaty, A.H.; Abd El-Aziz, Y.M.; Abdeen, A.M.; Mohamed, F.E.Z.A.; Hashem, M.M.M.; Hammad, S. Impacts of Egyptian propolis extract on rat cerebellum intoxicated by aluminum silicate: Histopathological studies. Environ. Sci. Pollut. Res. 2019, 26, 22061–22068. [Google Scholar] [CrossRef]
- Hussein, U.K.; Hassan, N.E.-H.Y.; Elhalwagy, M.E.A.; Zaki, A.R.; Abubakr, H.O.; Venkata, K.C.N.; Jang, K.Y.; Bishayee, A. Ginger and propolis exert neuroprotective effects against monosodium glutamate-induced neurotoxicity in rats. Molecules 2017, 22, 1928. [Google Scholar] [CrossRef] [PubMed]
- Kassab, A.A.; Elkaliny, H.H. The possible role of propolis in ameliorating paclitaxel-induced peripheral neuropathy in sciatic nerve of adult male albino rats. Egypt. J. Histol. 2017, 40, 141–155. [Google Scholar] [CrossRef][Green Version]
- Mostafa, R.E.; Salama, A.A.A.; Abdel-Rahman, R.F.; Ogaly, H.A. Hepato-and neuro-protective influences of biopropolis on thioacetamide-induced acute hepatic encephalopathy in rats. Can. J. Physiol. Pharmacol. 2017, 95, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Khalil, F.A.; EL-Kirsh, A.A.A.; Kamel, E.A.; EL-Rahmany, N.G. Beneficial Effect of Propolis Extract (Bee Glue) against Methotrexate-Induced Stress in Liver and Brain of Albino Rats. Indian J. Med. Res. Pharm. Sci. 2016, 3, 24–35. [Google Scholar] [CrossRef]
- Taysi, S.; Demir, E.; Cinar, K.; Tarakcioglu, M. The Radioprotective Effects of Propolis and Caffeic Acid Phenethyl Ester on Radiation-Induced Oxidative/nitrosative Stress in Brain Tissue. Free Radic. Biol. Med. 2016, 100, S111. [Google Scholar] [CrossRef]
- Thangarajan, S.; Ramachandran, S.; Krishnamurthy, P. Chrysin exerts neuroprotective effects against 3-Nitropropionic acid induced behavioral despair—Mitochondrial dysfunction and striatal apoptosis via upregulating Bcl-2 gene and downregulating Bax—Bad genes in male wistar rats. Biomed. Pharmacother. 2016, 84, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Gocmez, S.; Demirtas, T.; Aksoz, E.; Utkan, T.; Gacar, N. P.1.h.009 Protective effects of an extract of propolis in scopolamine-induced cognitive impairment in rats. Eur. Neuropsychopharmacol. 2015, 25, S286. [Google Scholar] [CrossRef]
- Alkis, H.E.E.; Kuzhan, A.; Dirier, A.; Tarakcioglu, M.; Demir, E.; Saricicek, E.; Demir, T.; Ahlatci, A.; Demirci, A.; Cinar, K.; et al. Neuroprotective effects of propolis and caffeic acid phenethyl ester (CAPE) on the radiation-injured brain tissue (neuroprotective effects of propolis and CAPE). Int. J. Radiat. Res. 2015, 13, 297–303. [Google Scholar] [CrossRef]
- Dos Santos, N.A.G.; Martins, N.M.; de Silva, R.B.; Ferreira, R.S.; Sisti, F.M.; dos Santos, A.C. Caffeic acid phenethyl ester (CAPE) protects PC12 cells from MPP+ toxicity by inducing the expression of neuron-typical proteins. Neurotoxicology 2014, 45, 131–138. [Google Scholar] [CrossRef]
- Swamy, M.; Suhaili, D.; Sirajudeen, K.N.S.; Mustapha, Z.; Govindasamy, C. Propolis ameliorates tumor nerosis factor-α, nitric oxide levels, caspase-3 and nitric oxide synthase activities in kainic acid mediated excitotoxicity in rat brain. African J. Tradit. Complement. Altern. Med. 2014, 11, 48–53. [Google Scholar] [CrossRef]
- Newairy, A.A.; Abdou, H.M. Effect of propolis consumption on hepatotoxicity and brain damage in male rats exposed to chlorpyrifos. African J. Biotechnol. 2013, 12, 5232–5243. [Google Scholar] [CrossRef]
- Mahmoud, S.M.; El-Yamany, N.A. The protective effect of propolis on norepinephrine, dopamine and 5-hydroxytryptamine content in thalamus-hypothalamus and cerebellum of endotoxin-intoxicated adult male albino rats. Life Sci. J. 2012, 9, 3372–3379. [Google Scholar]
- Ramezani, M.; Hesami, M.D.; Rafiei, Y.; Ghareghozloo, E.R.; Meratan, A.A.; Nikfarjam, N. Efficient Amyloid Fibrillation Inhibition and Remodeling of Preformed Fibrils of Bovine Insulin by Propolis Polyphenols-Based Nanosheets. ACS Appl. Bio Mater. 2021, 4, 3547–3560. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Fotsing, M.T.; Talla, E.; Ozturk, M.; Mbafor, J.T.; Duru, M.E.; Shaheen, F. Chemical composition and evaluation of anticholinesterase activity of essential oil from Cameroonian propolis. Issues Biol. Sci. Pharm. Res. 2019, 7, 58–63. [Google Scholar] [CrossRef]
- Boulechfar, S.; Zellagui, A.; Chemsa, A.E.; Bensouici, C.; Segueni, N.; Lahouel, M.; Öztürk, M.; Duru, M.E. Investigation of Antioxidant and Anticholinesterase Potential of Essential Oil and Methanolic Extract of Propolis from Mila Region. J. Biol. Act. Prod. Nat. 2019, 9, 434–444. [Google Scholar] [CrossRef]
- Nanaware, S.; Shelar, M.; Sinnathambi, A.; Mahadik, K.R.R.; Lohidasan, S. Neuroprotective effect of Indian propolis in β-amyloid induced memory deficit: Impact on behavioral and biochemical parameters in rats. Biomed. Pharmacother. 2017, 93, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Scozzafava, A.; Supuran, C.T.; Akıncıoğlu, H.; Koksal, Z.; Turkan, F.; Alwasel, S. The effect of caffeic acid phenethyl ester (CAPE) on metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, glutathione S-transferase, lactoperoxidase, and carbonic anhydrase isoenzymes I, II, IX, and XII. J. Enzym. Inhib. Med. Chem. 2016, 31, 1095–1101. [Google Scholar] [CrossRef]
- Liu, R.; Wu, C.X.; Zhou, D.; Yang, F.; Tian, S.; Zhang, L.L.; Zhang, T.-T.; Du, G.-H. Pinocembrin protects against β-amyloid-induced toxicity in neurons through inhibiting receptor for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis. BMC Med. 2012, 10, 1–21. [Google Scholar] [CrossRef]
- Ayikobua, E.T.; Kasolo, J.; Kasozi, K.I.; Eze, E.D.; Safiriyu, A.; Ninsiima, H.I.; Kiyimba, K.; Namulema, J.; Jjesero, E.; Ssempijja, F.; et al. Synergistic action of propolis with levodopa in the management of Parkinsonism in Drosophila melanogaster. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef]
- Gonçalves, V.C.; Pinheiro, D.J.L.L.; de la Rosa, T.; de Almeida, A.C.G.; Scorza, F.A.; Scorza, C.A. Propolis as a potential disease-modifying strategy in parkinson’s disease: Cardioprotective and neuroprotective effects in the 6-ohda rat model. Nutrients 2020, 12, 1551. [Google Scholar] [CrossRef]
- Gomes, M.; Barroso, S.; de Oliveira, J.; Marques, D.; Santos, A.; Cardoso, J. Neuroprotective actions of red propolis extract and formononetin in a rat model of Parkinson’s disease. Mov. Disord. 2019, 34, S502. [Google Scholar]
- Safari, M.; Sameni, H.R.; Badban, L.; Bandegi, A.R.; Vafaei, A.A.; Pour, A.R.; Ghahari, L. Protective effects of water extract of propolis on dopaminergic neurons, brain derived neurotrophic factor and stress oxidative factors in the rat model of parkinson’s disease. Int. J. Pharmacol. 2015, 11, 300–308. [Google Scholar] [CrossRef]
- Barros Silva, R.; Santos, N.A.G.; Martins, N.M.; Ferreira, D.A.S.; Barbosa, F., Jr.; Oliveira Souza, V.C.; Kinoshita, Â.; Baffa, O.; Del-Bel, E.; Santos, A.C. Caffeic acid phenethyl ester protects against the dopaminergic neuronal loss induced by 6-hydroxydopamine in rats. Neuroscience 2013, 233, 86–94. [Google Scholar] [CrossRef]
- Yilmaz, U.C.; Bagca, B.G.; Karaca, E.; Durmaz, A.; Durmaz, B.; Aykut, A.; Kayalar, H.; Avci, C.B.; Susluer, S.Y.; Pariltay, E.; et al. Propolis Extract Regulate microRNA Expression in Glioblastoma and Brain Cancer Stem Cells. Anticancer. Agents Med. Chem. 2021, 21. [Google Scholar] [CrossRef]
- Keskin, S.; Çetin, E.A. Lavender Volatile Oil: A New Solvent for Propolis Extraction, Chemical Composition, Antioxidant Activity and Cytotoxicity on T98G Glioblastoma Cell Line. J. Essent. Oil-Bear. Plants 2020, 23, 514–521. [Google Scholar] [CrossRef]
- Agca, C.A.; Tykhomyrov, A.A.; Baydas, G.; Nedzvetsky, V.S. Effects of a Propolis Extract on the Viability of and Levels of Cytoskeletal and Regulatory Proteins in Rat Brain Astrocytes: An In Vitro Study. Neurophysiology 2017, 49, 261–271. [Google Scholar] [CrossRef]
- Kalia, P.; Kumar, N.R.; Harjai, K. Studies on the effect of ethanolic extract of propolis in BALB/c mice. J. Appl. Nat. Sci. 2014, 6, 638–643. [Google Scholar] [CrossRef]
- Markiewicz-Zukowska, R.; Car, H.; Naliwajko, S.K.; Sawicka, D.; Szynaka, B.; Chyczewski, L.; Isidorov, V.; Borawska, M.H. Ethanolic extract of propolis, chrysin, CAPE inhibit human astroglia cells. Adv. Med. Sci. 2012, 57, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Asama, T.; Hiraoka, T.; Ohkuma, A.; Okumura, N.; Yamaki, A.; Urakami, K. Cognitive Improvement and Safety Assessment of a Dietary Supplement Containing Propolis Extract in Elderly Japanese: A Placebo-Controlled, Randomized, Parallel-Group, Double-Blind Human Clinical Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 6664217. [Google Scholar] [CrossRef] [PubMed]
- Gocmez, S.S.; Celebi, G.; Demirtaş-Şahin, T.; Aksoz, E.; Utkan, T.P. 480 The effects of propolis extract on age-associated cognitive deficits in rats. Eur. Neuropsychopharmacol. 2019, 29, S337–S338. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.; Zhu, A.; Nakanishi, H.; Ni, J.; Zhong, X.; Meng, J.; Wu, S. Brazilian green propolis improves cognitive functions and modulates systemic cytokines in elderly people living at high altitude. J. Neurol. Sci. 2017, 381, 678–679. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhong, X.; Du, C.; Li, H.J.; Wu, Z.; Nakanishi, H.; Zhu, A.Q. Clinical significance of brazilian propolis interference in patients with mild cognitive impairment (MCI) in plateau area of serum c-reactive protein (HS-CRP) and lipid metabolism disorders. J. Am. Geriatr. Soc. 2014, 62, S361. [Google Scholar]
- Nosratiyan, M.; Farjah, G.H.; Karimipour, M.; Pourheidar, B. The Effect of Propolis-Gum Arabic as a Novel Nerve Guidance Channel on Regeneration of Sciatic Nerve in Male Rats. Turk. Neurosurg. 2021, 31, 361–367. [Google Scholar] [CrossRef]
- Shao, Q.; Zhao, M.; Pei, W.; Pu, Y.; Liu, M.; Liu, W.; Yu, Z.; Chen, K.; Liu, H.; Deng, B.; et al. Pinocembrin Promotes OPC Differentiation and Remyelination via the mTOR Signaling Pathway. Neurosci. Bull. 2021, 37, 1314–1324. [Google Scholar] [CrossRef]
- Li, Z.; Chu, S.; He, W.; Zhang, Z.; Liu, J.; Cui, L.; Yan, X.X.; Li, D.; Chen, N. A20 as a novel target for the anti-neuroinflammatory effect of chrysin via inhibition of NF-κB signaling pathway. Brain. Behav. Immun. 2019, 79, 228–235. [Google Scholar] [CrossRef]
- Kuswati, K.; Handayani, E.S.; Nugraha, Z.S.; Rahmanti, F.A.; Wicaksana, Z.L.; Zhafirrahman, M. Propolis inhibited Bax expression and increased neuronal count of hippocampal area CA1 in rats receiving sodium nitrite. Universa Med. 2019, 38, 73–78. [Google Scholar] [CrossRef]
- Lee, B.K.; Lee, W.J.; Jung, Y.-S. Chrysin attenuates VCAM-1 expression and monocyte adhesion in lipopolysaccharide-stimulated brain endothelial cells by preventing NF-κB signaling. Int. J. Mol. Sci. 2017, 18, 1424. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Y.; Zhu, A.; Wu, S.; Nakanishi, H. Brazilian green propolis suppresses microglia-mediated neuroinflammation by inhibiting NF-kB activation. J. Neurol. Sci. 2017, 381, 678. [Google Scholar] [CrossRef]
- Cavendish, R.L.; Santos, J.d.S.; Neto, R.B.; Paixão, A.O.; Oliveira, J.V.; Araujo, E.D.D.; Silva, A.A.B.; Thomazzi, S.M.; Cardoso, J.C.; Gomes, M.Z. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. J. Ethnopharmacol. 2015, 173, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Mountassir, M.; Chaib, S.; Selami, Y.; Khalki, H.; Ouachrif, A.; Moubtakir, S.; Aboufatima, R.; Zyad, A.; Benharref, A.; Chait, A. Antinociceptive Activity and Acute Toxicity of Moroccan Black Propolis. Int. J. Eng. Res. Technol. 2014, 3, 2393–2397. [Google Scholar]
- Wu, Z.; Zhu, A.; Takayama, F.; Okada, R.; Liu, Y.; Harada, Y.; Wu, S.; Nakanishi, H. Brazilian green propolis suppresses the hypoxia-induced neuroinflammatory responses by inhibiting NF-B activation in microglia. Oxid. Med. Cell. Longev. 2013, 2013, 906726. [Google Scholar] [CrossRef] [PubMed]
- Franchin, M.; Da Cunha, M.G.; Denny, C.; Napimoga, M.H.; Cunha, T.M.; Koo, H.; de Alencar, S.M.; Ikegaki, M.; Rosalen, P.L. Geopropolis from Melipona scutellaris decreases the mechanical inflammatory hypernociception by inhibiting the production of IL-1β and TNF-α. J. Ethnopharmacol. 2012, 143, 709–715. [Google Scholar] [CrossRef]
- Kaya, G.B.; Emre, M.H. Effects of Propolis on Spatial Memory Induced by Lithium Pilocarpine in the Experimental Status Epilepticus Model in the Rats. J. Nat. Sci. Res. 2019, 215, 19. [Google Scholar] [CrossRef]
- Yiş, U.; Topçu, Y.; Özbal, S.; Tuǧyan, K.; Bayram, E.; Karakaya, P.; Yilmaz, O.; Kurul, S.H. Caffeic acid phenethyl ester prevents apoptotic cell death in the developing rat brain after pentylenetetrazole-induced status epilepticus. Epilepsy Behav. 2013, 29, 275–280. [Google Scholar] [CrossRef]
- Gocmez, S.S.; Ozer, C.; Celebi, G.; Yazir, Y.; Duruksu, G.; Demirtaş-Şahin, T.; Uckan, F.; Utkan, T.P. 217 The effects of propolis on cognitive impairment in chronic unpredictable mild stress-induced depression model of rats. Eur. Neuropsychopharmacol. 2019, 29, S167–S168. [Google Scholar] [CrossRef]
- Nugroho, K.; Handayani, E.S.; Nugraha, Z.S. Propolis increases neuronal count in hippocampal area CA1 and prefrontal cortex in stressed rats. Universa Med. 2017, 36, 214–220. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Giacomeli, R.; Antunes, M.; Luchese, C.; et al. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur. J. Pharmacol. 2016, 791, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; Gomes, M.G.D.; Goes, A.T.R.; Souza, L.C.; Boeira, S.P. Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem. Biol. Interact. 2016, 260, 154–162. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, Y.H.; Lee, B.R.; Kwon, S.H.; Moon, W.J.; Hong, K.S.; Song, Y.S.; Morita, K.; Hahm, D.H.; Shim, I.; et al. Novel antidepressant-like activity of caffeic acid phenethyl ester is mediated by enhanced glucocorticoid receptor function in the hippocampus. Evid.-Based Complement. Altern. Med. 2014, 2014, 646039. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, Y.H.; Park, W.S.; Ahn, W.G.; Park, O.K.; Kwon, S.H.; Morita, K.; Shim, I.; Her, S. Novel antidepressant-like activity of propolis extract mediated by enhanced glucocorticoid receptor function in the hippocampus. Evid.-Based Complement. Altern. Med. 2013, 2013, 217853. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, H.; Ulkevan, T. Propolis induced mania with psychotic features: A case report. Klin. Psikofarmakol. Bul. 2015, 25, 207–208. [Google Scholar] [CrossRef]
- Yang, Z.H.; Sun, X.; Qi, Y.; Mei, C.; Sun, X.B.; Du, G.H. Uptake characteristics of pinocembrin and its effect on p-glycoprotein at the blood-brain barrier in in vitro cell experiments. J. Asian Nat. Prod. Res. 2012, 14, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant status, antidiabetic properties and effects on Caco-2 cells of colored and non-colored enriched extracts of sweet cherry fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef]
- Silveira, M.; Teles, F.; Melo, E.; Borges, V.; Miranda, F.; Dutra, F.; Berretta, A.; Cezar, R.; Silva, J.; Santos, H.; et al. P1574Effects of Brazilian Green Propolis Extract (Epp-Af) on Inflammation in Hemodialysis Patients. Nephrol. Dial. Transpl. 2020, 35 (Suppl. 3), gfaa142.P1574. [Google Scholar] [CrossRef]
- Hesami, S.; Hashemipour, S.; Shiri-Shahsavar, M.R.; Koushan, Y.; Haghighian, H.K. Administration of Iranian Propolis attenuates oxidative stress and blood glucose in type II diabetic patients: A randomized, double-blind, placebo-controlled, clinical trial. Casp. J. Intern. Med. 2019, 10, 48–54. [Google Scholar] [CrossRef]
- El-Sharkawy, H.M.; Anees, M.M.; Van Dyke, T.E. Propolis Improves Periodontal Status and Glycemic Control in Patients with Type 2 Diabetes Mellitus and Chronic Periodontitis: A Randomized Clinical Trial. J. Periodontol. 2016, 87, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Bhagavathula, A.S.; Aldhaleei, W.A.; Rahmani, J.; Karam, G.; Rinaldi, G.; Clark, C.; Salehisahlabadi, A.; Yuan, Q. Effect of propolis supplementation on C-reactive protein levels and other inflammatory factors: A systematic review and meta-analysis of randomized controlled trials. J. King Saud Univ.-Sci. 2020, 32, 1694–1701. [Google Scholar] [CrossRef]
- Zulhendri, F.; Felitti, R.; Fearnley, J.; Ravalia, M. The Use of Propolis in Dentistry, Oral Health, and Medicine: A Review. J. Oral Biosci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Šuran, J.; Cepanec, I.; Mašek, T.; Starčević, K.; Gajger, I.T.; Vranješ, M.; Radić, B.; Radić, S.; Kosalec, I.; Vlainić, J. Nonaqueous polyethylene glycol as a safer alternative to ethanolic propolis extracts with comparable antioxidant and antimicrobial activity. Antioxidants 2021, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Tlak Gajger, I.; Vlainić, J. Propolis Extract and Its Bioactive Compounds—From Traditional to Modern Extraction Technologies. Molecules 2021, 26, 2930. [Google Scholar] [CrossRef]
- Grozdanova, T.; Trusheva, B.; Alipieva, K.; Popova, M.; Dimitrova, L.; Najdenski, H.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Miloshev, G.; et al. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020, 14, 73. [Google Scholar] [CrossRef]


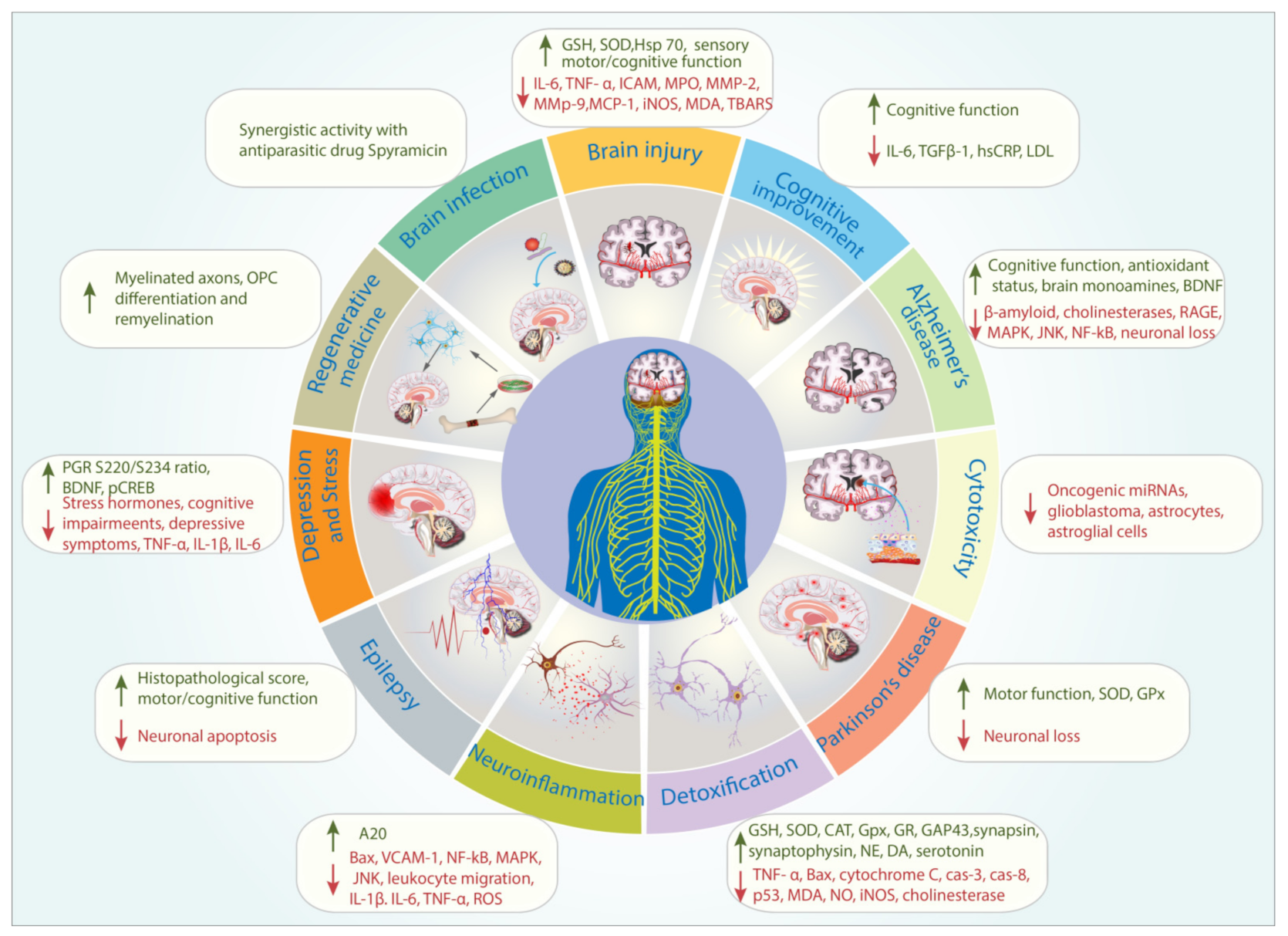
| Themes | Percentage (%) |
|---|---|
| Adverse effects | 2 |
| Alzheimer’s disease model | 9 |
| Brain infection | 2 |
| Cognitive Improvement | 6 |
| Cytotoxicity | 8 |
| Depression and Stress models | 9 |
| Detoxification | 24 |
| Epilepsy model | 3 |
| Ischemia/Ischemia-Reperfusion injury/Traumatic Brain Injury/Aneurysm | 14 |
| Neuroinflammation, Pain, and Oxidative stress | 12 |
| Parkinson’s disease model | 8 |
| Regenerative medicine | 3 |
| Others | 2 |
| Themes | References | Types of Study | Types of Propolis Extracts and/or Propolis Bioactive Compounds | Geographic Locations of the Propolis Source | Measured Outcome |
|---|---|---|---|---|---|
| Brain Infection | [13] | Animal model (n = 140, rats) | Hydroethanolic extract 0.1 mL of 1:10 w/v (25 g in 250 mL) | Egypt | Propolis enhanced the anti-Toxoplasma gondii activity of Spiramycin |
| Ischemia/Ischaemia-Reperfusion injury/ Traumatic Brain Injury/Aneurysm | [14] | Animal model (n = 12, rabbit) | Ethanolic extract 100 mg/kg BW | Turkey | Neuroprotective properties of propolis demonstrated in the post-operative Tarlov scores, biochemical parameters such as interleukin-6 (IL-6), tumor necrosis factor (TNF)-α, myeloperoxidase activity, ischemia-modified albumin (IMA), intercellular adhesion molecule-1 (ICAM-1), total oxidant status, and histopathological examination. |
| [15] | Animal model (n = 72, mice) | Aqueous extract 30, 100 and 200 mg/kg BW | Iran | Propolis improved grasping ability and sensory–motor function following permanent middle cerebral artery occlusion. | |
| [16] | Animal model (rats, n = 33) | Hydroethanolic extract 200 mg/kg BW | Indonesia | Propolis induced the expression of the protective heat-shock protein (hsp)-70 and reduced the expression of inflammatory markers such as caspase-3 and apoptosis inducing factor in traumatic brain injury animal model. | |
| [17] | Animal model (rats, n = 36) | Caffeic acid phenethyl ester (CAPE) 10 µmol | Not specified | CAPE reduced the severity of elastase-induced aortic aneurysm by reducing the expression of metalloproteinases MMP-2 and MMP-9, monocyte chemotactic protein-1 (MCP-1), and inducible nitric oxide synthase (iNOS). CAPE also circumvented the loss of vascular smooth muscle cells (VSMCs) in aortic walls of treated rats. | |
| [18] | Animal model (mice, n = 72) | Aqueous extract 100 and 200 mg/kg BW | Iran | In cerebral ischemia-induced mice, propolis reduced the expression of malondialdehyde (MDA) and improved the antioxidant status (ratio of superoxide dismutase (SOD) to glutathione peroxidase GPx). Propolis also ameliorated the sensory–motor impairment and neurological deficits induced by cerebral ischemia. | |
| [19] | Cell culture (neuroblastoma N2) | Hydroethanolic extract | Not specified | Propolis reduced the extent of neuronal damage in oxygen-glucose deprivation/reoxygenation (OGD/R)- induced cells. | |
| [20] | Animal model (mice, n = 40) | Chrysin 50 mg/kg BW | Not specified | Chrysin improved glutathione (GSH) level and decreased thiobarbituric acid reactive substances (TBARS) level. Chrysin attenuated the development of neurodegenerative histopathologies associated with global cerebral ischemia/reperfusion in mice. | |
| [21] | Animal model (rats, n = 36) | Hydroethanolic extract1 and 10 mg/kg BW | Brazil | In sciatic nerve-injured rats, propolis improved the motor function and sciatic functional index. Propolis also significantly accelerated the motor recovery and increased the number of myelinated fibers. | |
| [22] | Animal model (rats, n =75) | Propolis powder dissolved in water 200-mg/kg BW | Turkey | Propolis improved motor function (walking track and electrophysiological analyses), following crush injury of the sciatic nerve in rats. | |
| Detoxification | [23] | Cell cultures (SH-SY5Y) | Pinocembrin 1–25 µM | Not specified | Pinocembrin prevented the Chlorpyrifos-induced mitochondrial membrane potential (MMP) loss and ATP synthesis inhibition in SH-SY5Y cells. Pinocembrin also induced the anti-inflammatory activity. |
| [24] | Cell cultures (SH-SY5Y) | Caffeic acid, chrysin, quercetin and ferulic acid 100, 200 and 400 µM | Not specified | The phenolic compounds inhibited the cyclophosphamide-induced apoptosis of SH-SY5Y cells. | |
| [25] | Cell cultures (SH-SY5Y) | Chrysin 0.05 mM | Not specified | Chrysin inhibited the diclofenac-induced apoptosis of SH-SY5Y cells. Chrysin inhibited the expression of Bax, cytochrome c, cas-3, cas-8 and p53 genes associated with diclofenac treatment. | |
| [26] | Animal model (rats, n = 40) | Hydroethanolic extract 200 mL/kg BW | Egypt | Propolis reduced the development of aluminum silicate-induced irregular euchromatic nucleus and significantly increased the invagination of the nuclear envelope of Purkinje cells in the cerebellar cortex of aluminum silicate-intoxicated rats. | |
| [27] | Animal model (rats, n = 24–32) | Not specified 600 mg/kg BW | Not specified | Propolis reduced the expression of inflammatory markers; malondealdehyde (MDA) and nitric oxide (NO). Propolis improved the antioxidant status by maintaining glutathione level and the activity of superoxide dismutase and catalase in monosodium glutamate-intoxicated rats. Propolis also prevented the accumulation of β-amyloid and oxidative-stress marker 8-hydroxy-2′-deoxyguanosine. | |
| [28] | Animal model (rats, n = 24) | Propolis capsules (extract not specified) 50 mg/kg BW | Not specified | Propolis attenuated the Paclitaxel-induced morphological deterioration of myelinated fibers of sciatic nerve. | |
| [29] | Animal model (rats, n = 60) | Not specified 100 and 200 mg/kg | Not specified | Propolis reduced the expression of inducible nitric oxide synthase (iNOS) gene in thioacetamide (TAA)-induced rats. | |
| [30] | Animal model (rats, n = 120) | Hydroethanolic extract 200 mg/kg BW | Egypt | Propolis reduced the adverse effect of methotrexate by reducing MDA and increasing the activity of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GR), and GSH. | |
| [31] | Animal model (rats, n = 40) | Propolis (extract not specified) and CAPE | Not specified | Propolis and CAPE prevented the increase in xanthine oxidase activity, nitric oxide synthase activity, nitric oxide (NO●) and peroxynitrite (ONOO−) levels in radiation-treated rats. | |
| [32] | Animal model (rats, n = 24) | Chrysin 50mg/kg BW | Not specified | In 3-Nitropropionic acid treated rats, chrysin improved the behavioral performance and attenuated the oxidative stress by maintaining the level of antioxidant parameters and reducing the oxidative stress parameters. | |
| [33] | Animal model (rats, n = not specified) | Aqeuous extract 100 mg/kg BW | Turkey | Propolis reversed the scopolamine-induced cognitive deterioration. | |
| [34] | Animal model (rats, n = 54) | Propolis (extract not specified) 80 mg/kg BW and CAPE 10 µmol/kg BW | Not specified | Propolis prevented the increase in MDA associated with radiation toxicity. | |
| [35] | Cell cultures (PC-12) | CAPE 1, 5 or 10μM | Not specified | CAPE induced the formation of synapses and neuritis, and prevented the MPP+ (1-methyl-4-phenylpyridinium) cytotoxicity by increasing the expression of increases the expression of GAP-43, synapsin and synaptophysin. | |
| [36] | Animal model (rats, n = 24) | Hydroethanolic 150 mg/kg BW | Malaysia | Propolis inhibited the expression of NOS, NO, TNF-α and caspase-3 in the cerebral cortex (CC), cerebellum (CB) and brain stem (BS) of kainic acid-induced rats. | |
| [37] | Animal model (rats, n = 40) | Not specified 50 mg/kg BW | Not specified | Propolis attenuated chlorpyrifos-induced toxicity. Propolis reduced the activity of serum and brain cholinesterase induced by chlorpyrifos. Propolis also inhibited the increase in glial fibrillary acidic protein-expression. | |
| [38] | Animal model (rats, n = 78) | Not specified 150mg/kg BW | Egypt | In endotoxin-treated rats, propolis attenuated the decrease in the level of norepinehrine (NE), dopamine (DA) and 5-hydroxytryptamine (serotonin, 5-HT) in both thalamus-hypothalamus and cerebellum. | |
| Alzheimer’s disease model | [39] | In vitro | Hydroethanolic extract 1:10 (w/v) | Iran | Propolis inhibited amyloid fibrillation. |
| [40] | In vitro | Essential oils of propolis | Cameroon | Components of propolis essential oil exhibited anti-cholinesterase activity. | |
| [41] | In vitro | Essential oils and methanolic extract IC50 value: 20–35 μg/mL | Algeria | Components of propolis essential oil and methanolic extract of propolis exhibited anti-cholinesterase activity against both acetylcholinesterase and butyrylcholinesterase. | |
| [42] | Animal model (rats, n = 56) | Ethanolic extract 100, 200, 300 mg/kg BW | India | Propolis reduced the severity of the cognitive impairment in the β-amyloid induced rats. Propolis improved the antioxidant status, brain monoamines, and brain-derived neurotrophic factor. Propolis inhibited the activity of acetylcholinesterase activity in a dose-dependent manner. | |
| [43] | In vitro | CAPE Ki = 322.02 pM to 4.467 μM | Not specified | Inhibition of the activity of acetylcholinesterase and butyrylcholinesterase. | |
| [44] | Animal model (mice, n = 48) | Pinocembrin 20 and 40 mg/kg BW | Not specified | Pinocembrin inhibited the expression of receptor for advanced glycation end-products (RAGE) and its downstream inflammatory signaling pathway markers such as p38 mitogen-activated protein kinase (MAPK), protein kinase (SAPK)/c-Jun N-terminal kinase (JNK), and NF-κB. Pinocembrin also exhibited mitochondrial-protective properties. | |
| Parkinson’s disease model | [45] | Animal model (Drosophila melanogaster, n = not specified) | Ethanolic extract 250 and 500 mg/mL | Not specified | Propolis improved motor activity, antioxidant status, and lifespan. |
| [46] | Animal model (rats, n = 21) | Not specified 200 mg/kg BW | Brazil | Propolis inhibited neuronal loss in the substantia nigra and attenuated striatal fiber degeneration in 6-hydroxydopamine (6-OHDA)-induced rats. | |
| [47] | Animal model (rats, n = 48) | Hydroethanolic extract of propolis (10 and 50 mg/kg BW) and Formononetin (10 and 20 mg/kg BW) | Brazil | Propolis and one of its bioactive compound, formononetin reduced the neuron loss and motor impairment in 6-OHDA-induced rats. | |
| [48] | Animal model (rats, n = 70) | Aqueous extract 1:5 (w/v) | Iran | Propolis improved the antioxidant status in terms of SOD and GPx activities and ferric reducing ability of plasma in 6-OHDA-induced rats. Propolis also appeared to protect tyrosine hydroxylase neurons. | |
| [49] | Animal model (rats, n = 18) | CAPE 10μmol/kg BW | Not determined | CAPE prevented the dopaminergic neuronal loss induced by 6-OHDA in rats. CAPE also prevented mitochondrial permeability transition (neuronal death mediator) | |
| Cytotoxicity | [50] | Cell cultures (glioblastoma) | Hydroethanolic extract | Turkey | Propolis reduced the expression of oncogenic miRNAs associated with glioblastoma. |
| [51] | Cell cultures (glioblastoma) | Propolis extracted in Lavender oil 1:10 (w/v) | Turkey | Cytotoxic activity against glioblastoma cells. | |
| [52] | Cell cultures (rat primary astrocytes) | Hydroethanolic extract 10, 25, or 100 µg/ml | Turkey | Dose-dependent cytotoxicity on astrocytes was observed. Propolis inducedcytoskeletonrearrangements and pro-apoptotic signaling pathways; NF-kB and poly (ADP-ribose) polymerase (PARP). | |
| [53] | Animal model (mice, n = not specified) | Ethanolic extract up to 1000 mg/kg BW | India | High concentration of propolis extract up to 1000 mg/kg BW did not negatively affect the histological appearance of organs, including the brain. | |
| [54] | Cell cultures (astroglia cell line/SVGp12) | Ethanolic extract 10–100 µg/mL CAPE and Chrysin 5–50 µM | Poland | Propolis and its bioactive compounds reduced the viability of astroglial cells. | |
| Cognitive Improvement | [55] | Randomized, placebo-controlled trial (n = 79) | Dietary supplement containing propolis extract. Types of extract not specified 6 capsules of propolis extract containing artepillin C, 57.68 mg; culifolin, 0.95 mg | Not specified | Propolis significantly improved verbal memory and information processing speed (Cognitrax). Propolis also improved serum total cholesterol, LDL cholesterol, urea nitrogen, creatinine, and uric acid. |
| [56] | Animal model (rats, n = 40) | Aqueous extract 100 mg/kg BW | Turkey | Propolis reversed the transfer latency parameter associated with physiological aging in rats. Transfer latency is defined as the time taken by the animals to move from the open arm to the enclosed arm of an experimental compartment. | |
| [57] | Randomized, placebo-controlled trial (n = 80) | Propolis dietary supplement (types of extract not specified) 0.33 g | Brazil | Propolis improved cognitive function measured by Mini-Mental State Examination (MMSE) and Alzheimer Disease Assessment Scale-cognitive subscale (ADAS-cog). Propolis reduced serum level of IL-6 and TGFβ1. | |
| [58] | Randomized, placebo-controlled trial (n = 87) | Propolis dietary supplement (Extract not specified) | Brazil | Propolis improved cognitive function measured by MMSE and reduced the serum level of hs-CRP and LDL. | |
| Regenerative medicine | [59] | Animal model (mice, n = 24) | Implantation of an artificial guidance channel containing whole propolis combined with Gum Arabic. | Iran | Propolis–gum Arabic graft increased the mean number of muscle fiber diameters and myelinated axons. |
| [60] | Oligodendrocyte progenitor cell (OPC) cultures Animal model (mice, n = 30) | Pinocembrin 10 mM | Not specified | Pinocembrin induced the OPC differentiation and remyelination through the phosphorylated mTOR pathway in multiple sclerosis disease model. | |
| Neuroinflammation, Pain, and Oxidative stress | [61] | Cell cultures (BV2 cells and primary microglia cells) Animal model (mice, n = 16) | Chrysin 5, 10, and 20 µM | Not specified | Chrysin inhibited the inflammation of LPS-induced BV2, primary microglial cells, and mice by upregulating the expression of zinc-finger protein A20. |
| [62] | Animal model (rats, n = 18) | Aqueous extract 100 and 200 mg/kg BW | Not specified | Propolis decreased the expression of Bax and reduced the number of neurons in the hippocampal CA1 area of sodium nitrite-induced rats. | |
| [63] | Cell cultures (bEnd.3) | Chrysin 10, 30, and 100 µM | Not specified | Chrysin reduced the expression of vascular cell adhesion molecule-1 (VCAM-1), Nuclear factor-κB (NF-κB), p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase in the LPS-induced bEnd.3 cells. Chrysin also prevented the adhesion of monocytes to the LPS-induced bEnd.3 cells. | |
| [64] | Cell culture (microglia) and animal model (mice, n = not specified) | Not specified 50 μg/mL | Brazil | Propolis inhibit the cytotoxicity and the expression of pro-inflammatory biomarkers; IL-1β, TNF-α, IL-6, and 8-oxo-deoxyguanosine following hypoxia exposure. Propolis also significantly reduced the hypoxia-induced generation of reactive oxygen species (ROS) in the mitochondria and downregulated the expression of nuclear factor-κB (NF-κB) in microglia. | |
| [65] | Animal model (mice, n = 180; rats, n = 36) | Hydroethanolic extract of propolis 3, 10, and 30 mg/kg BW and formononetin 10 mg/kg BW | Brazil | Propolis and formononetin demonstrated anti-inflammatory activity. Propolis and formononetin inhibited oedema response and carrageenan-induced leukocyte migration during inflammatory process. Propolis was also shown to have antinoceptive properties on inflammatory and neurogenic pain. | |
| [66] | Animal model (rats and mice, n = 20) | Aqueous extract 1, 2.5, abd 5% (w/v) | Morocco | Propolis exhibited both central and peripheral antinociceptive activities. | |
| [67] | Cell cultures (microglia) and mice, n = 9) | Ethanolic extract 5, 50, and 500 µg/mL | Brazil | Propolis reduced the expression of oxidative markers IL-1β, TNF-α, IL-6, and 8-oxo-deoxyguanosine. Propolis also reduced the production of ROS in mitochondria. | |
| [68] | Animal model (mice, n = 36) | Ethanolic extract 1 mM | Brazil | Propolis exhibited antinoceptive properties by modulating the expression of IL-1β and TNF-α. | |
| Epilepsy model | [69] | Animal model (rats, n = 50) | Aqueous extract 0.012 mg/kg BW | Turkey | Propolis increased the histopathological score of the hippocampus and motor/cognitive score in the lithium and pilocarpine-induced rats. |
| [70] | Animal model (rats, n = 21) | CAPE 30 mg/kg BW | Not determined | CAPE significantly protected the number of neurons in the CA1, CA3, and dentate gyrus regions of the hippocampus and the prefrontal cortex. Apoptosis in the hippocampus and the prefrontal cortex was also inhibited by CAPE. | |
| Depression and Stress models | [71] | Animal model (rats, n = 40) | Aqueous extract 100 mg/kg BW | Turkey | In chronic unpredictable mild stress (CUMS)-induced rats, propolis exhibited anti-depressant properties. Propolis also reduced the level of corticosterone level and reversed cognitive impairments. |
| [72] | Animal model (rats, n = 24) | Not specified 100 and 200 mg/kg BW | Not specified | Propolis prevented the hippocampal areaneuronal loss associated with stress. | |
| [73] | Animal model (mice, n = 42) | Chrysin 5 and 20 mg/kg BW | Not specified | Chrysin reduced the elevation of corticotropin-releasing and adrenocorticotropic hormones, and tumor necrosis factor-α, interleukin-1β, interleukin-6 and kynurenine levels in the prefrontal cortex (PFC) and hippocampus (HP) in mice exposed to unpredictable chronic stress. | |
| [74] | Animal model (mice, n = 40) | Chrysin 5 or 20 mg/kg BW | Not specified | Chrysin alleviated behavioral modification following olfactory bulbectomy. Chrysin attenuated the alterations of biochemical markers associated with depressive behavior, namely tumor necrosis factor-α, interferon-γ, interleukin-1β, interleukin-6, kynurenine (KYN) levels, indoleamine-2,3-dioxygenase activity,5-hydroxytryptamine (5-HT), brain-derived neurotrophic factor (BDNF), KYN/tryptophan and 5-hydroxyindoleacetic acid/5-HT ratio. | |
| [75] | Animal model (mice, n = 28) | CAPE 5, 10, and 20 μmol/kg | Not specified | CAPE exhibited anti-depressant activity on the animals. CAPE also reduced CAPE significantly decreased glucocorticoid receptor (GR) phosphorylation at S234 (pGR(S234)). | |
| [76] | Animal model (mice, n = 50) | Hydroethanolic extract 50, 100, and 200 mg/kg BW | Korea | Propolis exhibited anti-depressant activity by increasing the expression of hippocampal glucocorticoid receptor. Propolis also increased pGR(S220)/(S234) ratio. Propolis upregulated the cAMP-responsive element binding protein phosphorylation at S133 (pCREB). | |
| Adverse effects | [77] | Case report | Not specified 50 g/day for 3 days | Turkey | Propolis appeared to induce psychotic episodes in a thirty four year old male. |
| Others | [78] | Cell cultures (rat brain microvascular endothelial cells) | Pinocembrin 5, 20, and 40 µg/mL | Not specified | Pinocembrin appeared to cross blood–brain barrier cell model without affecting the function and expression of p-glycoprotein. |
| Geographical Sources of Propolis and/or Bioactive Compounds | Percentage (%) |
|---|---|
| Bioactive compounds | 30 |
| Unspecified | 19 |
| Turkey | 14 |
| Brazil | 10 |
| Iran | 7 |
| Egypt | 6 |
| India | 3 |
| Algeria | 1 |
| Morocco | 1 |
| Cameroon | 1 |
| Poland | 1 |
| Korea | 1 |
| Indonesia | 1 |
| Malaysia | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulhendri, F.; Perera, C.O.; Tandean, S. Can Propolis Be a Useful Adjuvant in Brain and Neurological Disorders and Injuries? A Systematic Scoping Review of the Latest Experimental Evidence. Biomedicines 2021, 9, 1227. https://doi.org/10.3390/biomedicines9091227
Zulhendri F, Perera CO, Tandean S. Can Propolis Be a Useful Adjuvant in Brain and Neurological Disorders and Injuries? A Systematic Scoping Review of the Latest Experimental Evidence. Biomedicines. 2021; 9(9):1227. https://doi.org/10.3390/biomedicines9091227
Chicago/Turabian StyleZulhendri, Felix, Conrad O. Perera, and Steven Tandean. 2021. "Can Propolis Be a Useful Adjuvant in Brain and Neurological Disorders and Injuries? A Systematic Scoping Review of the Latest Experimental Evidence" Biomedicines 9, no. 9: 1227. https://doi.org/10.3390/biomedicines9091227
APA StyleZulhendri, F., Perera, C. O., & Tandean, S. (2021). Can Propolis Be a Useful Adjuvant in Brain and Neurological Disorders and Injuries? A Systematic Scoping Review of the Latest Experimental Evidence. Biomedicines, 9(9), 1227. https://doi.org/10.3390/biomedicines9091227







