Concomitant High Apoptosis Inhibitor of Macrophage (AIM) and Low Prostate-Specific Antigen (PSA) Indicates Activated T Cell-Mediated Anticancer Immunity, Enhance Sensitivity to Pembrolizumab, and Elicit Good Prognosis in Prostate Cancer
Abstract
:1. Introduction
2. Material and Methods
2.1. Prostate Cancer Tissue Samples
2.2. Cell Culture and Chemicals
2.3. Drugs and Antibodies
2.4. Construction and Transfection of Plasmids Expressing AIM/CD5L
2.5. Cell Viability and Proliferation Colorimetric Assay
2.6. Immunohistochemical (IHC) Staining Assays
2.7. Scratch Wound-Healing Migration Assay
2.8. Invasion Assay
2.9. Public Cancer Dataset Access and Analysis
2.10. Statistical Analysis
3. Results
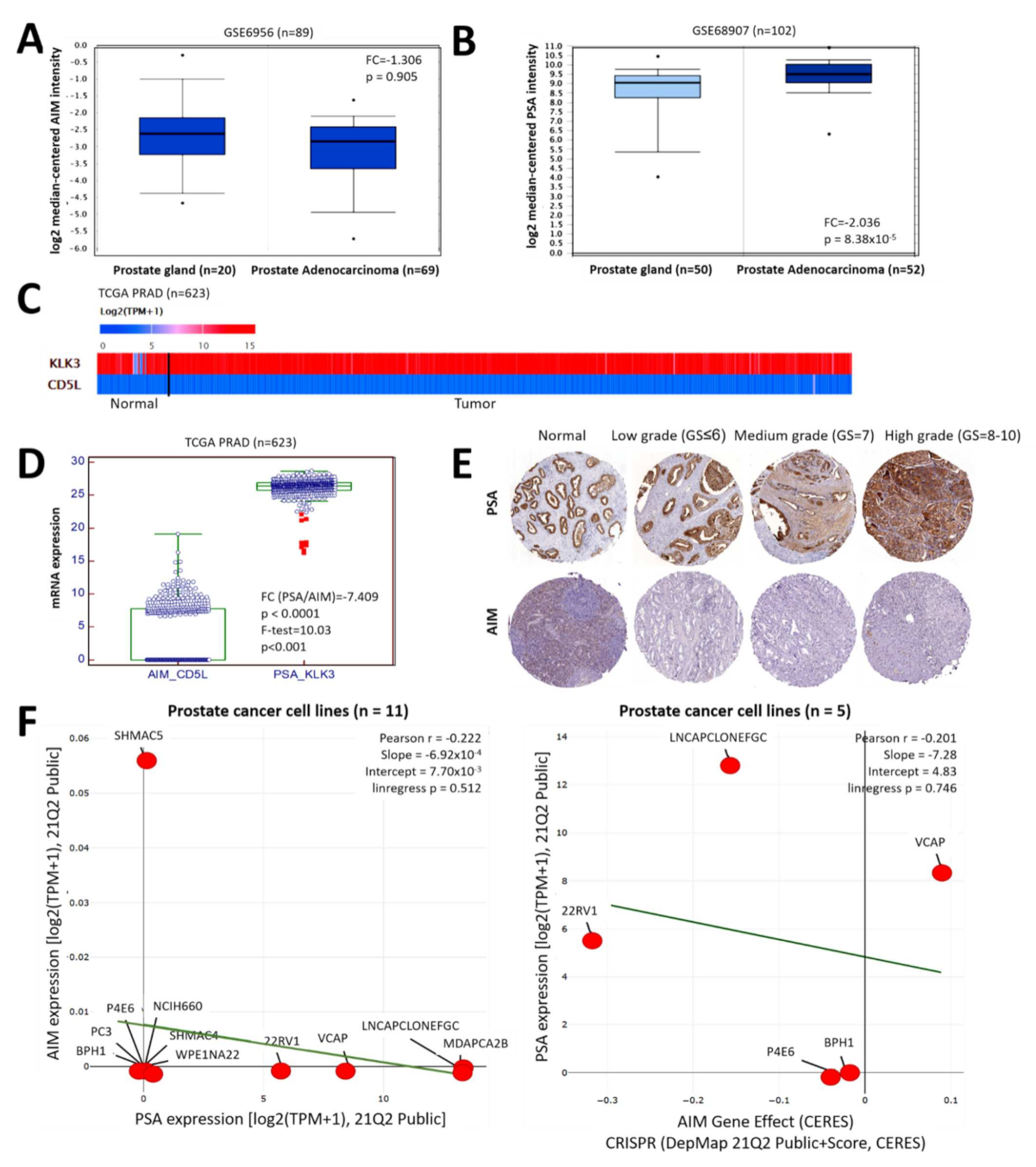
3.1. AIM and PSA Expression Levels Are Inversely Correlated in Patients with Prostate Cancer
3.2. Aberrantly Expressed PSA and Significantly Suppressed AIM Expression Characterize Advanced Stage Prostate Cancer, Regardless of Mutation Status
3.3. The Differential Expression of PSA and AIM Is Associated with Disease Recurrence in Patients with Prostate Cancer but Is Equivocal for Overall Survival
3.4. PSA-Associated Suppression of AIM Is Implicated in the Enhanced Metastability of Prostate Cancer and a High AIM/PSA Ratio Is Associated with Strong Castration-Induced Regression
3.5. The Functional Association between PSA and AIM Modulates Metastasis and Mirrors the Immunogenicity in Patients with Prostate Cancer
3.6. The Inversely Correlated Expression of PSA and AIM Differentially Modulate Treg, T cell, and Macrophage Activities in Patients with Prostate Cancer
3.7. AIM Binds Directly to PSA, and the AIM-PSA Interactome Reveals Complicity in Immune Landscape, Macrophage, EMT and CSC Regulation
3.8. Ectopic (Re)Expression of AIM Enhances the Anticancer Therapeutic Effect of Pembrolizumab against PCa Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow. International Agency for Research on Cancer: Lyon, France, 2020. Available online: https://gco.iarc.fr/tomorrow (accessed on 14 December 2020).
- Prostate Cancer Recurrence: Genomic Clues. Cancer Discov. 2017, 7, 240. [CrossRef] [Green Version]
- Mansinho, A.; Macedo, D.; Fernandes, I.; Costa, L. Castration-Resistant Prostate Cancer: Mechanisms, Targets and Treatment. Mol. Diagn. Imaging Prostate Cancer 2018, 1096, 117–133. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Zhao, S.G.; Lehrer, J.; Chang, S.L.; Das, R.; Erho, N.; Liu, Y.; Sjöström, M.; Den, R.B.; Freedland, S.J.; Klein, E.A.; et al. The Immune Landscape of Prostate Cancer and Nomination of PD-L2 as a Potential Therapeutic Target. J. Natl. Cancer Inst. 2019, 111, 301–310. [Google Scholar] [CrossRef]
- Albertsen, P.C. Prostate cancer screening with prostate-specific antigen: Where are we going? Cancer 2018, 124, 453–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertsen, P.C. Prostate cancer screening and treatment: Where have we come from and where are we going? BJU Int. 2020, 126, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Danila, D.C.; Fleisher, M.; Scher, H.I. Circulating Tumor Cells as Biomarkers in Prostate Cancer. Clin. Cancer Res. 2011, 17, 3903–3912. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Cheng, C.; Wu, Y.; Guo, L.; Kong, D.; Zhang, Z.; Wang, Y.; Zheng, E.; Liu, Y.; He, Y. Interactions of ferritin with scavenger receptor class A members. J. Biol. Chem. 2020, 295, 15727–15741. [Google Scholar] [CrossRef]
- Martinez, V.G.; Escoda-Ferran, C.; Simoes, I.T.; Arai, S.; Mascaró, M.O.; Carreras, E.; Martínez-Florensa, M.; Yélamos, J.; Miyazaki, T.; Lozano, F. The macrophage soluble receptor AIM/Api6/CD5L displays a broad pathogen recognition spectrum and is involved in early response to microbial aggression. Cell. Mol. Immunol. 2014, 11, 343–354. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hirokami, Y.; Matsuhashi, N.; Takatsuka, H.; Naito, M. Increased Susceptibility of Thymocytes to Apoptosis in Mice Lacking AIM, a Novel Murine Macrophage-derived Soluble Factor Belonging to the Scavenger Receptor Cysteine-rich Domain Superfamily. J. Exp. Med. 1999, 189, 413–422. [Google Scholar] [CrossRef]
- Tym, J.E.; Mitsopoulos, C.; Coker, E.A.; Razaz, P.; Schierz, A.C.; Antolin, A.A.; Al-Lazikani, B. canSAR: An updated cancer research and drug discovery knowledgebase. Nucleic Acids Res. 2016, 44, D938–D943. [Google Scholar] [CrossRef] [Green Version]
- Fuller, Z.L.; Berg, J.J.; Mostafavi, H.; Sella, G.; Przeworski, M. Measuring intolerance to mutation in human genetics. Nat. Genet. 2019, 51, 772–776. [Google Scholar] [CrossRef]
- Robinson, D.R.; Wu, Y.-M.; Lonigro, R.J.; Vats, P.; Cobain, E.; Everett, J.; Cao, X.; Rabban, E.; Kumar, C.; Raymond, V.; et al. Integrative clinical genomics of metastatic cancer. Nature 2017, 548, 297–303. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Owji, H.; Nezafat, N.; Negahdaripour, M.; Hajiebrahimi, A.; Ghasemi, Y. A comprehensive review of signal peptides: Structure, roles, and applications. Eur. J. Cell Biol. 2018, 97, 422–441. [Google Scholar] [CrossRef]
- Mohanty, S.; Chaudhary, B.P.; Zoetewey, D. Structural Insight into the Mechanism of N-Linked Glycosylation by Oligosaccharyltransferase. Biomolecules 2020, 10, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostova, M.B.; Brennen, W.N.; Lopez, D.; Anthony, L.; Wang, H.; Platz, E.; Denmeade, S.R. PSA-alpha-2-macroglobulin complex is enzymatically active in the serum of patients with advanced prostate cancer and can degrade circulating peptide hormones. Prostate 2018, 78, 819–829. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Huang, C.-Y.; Huang, K.-H.; Chow, P.-M.; Chang, Y.-K.; Hung, F.-C.; Chen, C.-H.; Jaw, F.-S.; Hong, J.-H. Association between low prostate-specific antigen levels and greater disease progression in high-grade locally-advanced prostate cancer. J. Formos. Med Assoc. 2021, 120, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Maehara, N.; Arai, S.; Mori, M.; Iwamura, Y.; Kurokawa, J.; Kai, T.; Kusunoki, S.; Taniguchi, K.; Ikeda, K.; Ohara, O.; et al. Circulating AIM Prevents Hepatocellular Carcinoma through Complement Activation. Cell Rep. 2014, 9, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunger, L.; Retz, M.; Bandur, M.; Souchay, M.; Vitzthum, E.; Jäger, M.; Weirich, G.; Schuster, T.; Autenrieth, M.; Kübler, H.; et al. KLK3 and TMPRSS2 for molecular lymph-node staging in prostate cancer patients undergoing radical prostatectomy. Prostate Cancer Prostatic Dis. 2021, 24, 362–369. [Google Scholar] [CrossRef]
- Heck, M.M.; Retz, M.; Bandur, M.; Souchay, M.; Vitzthum, E.; Weirich, G.; Schuster, T.; Autenrieth, M.; Kübler, H.; Maurer, T.; et al. Molecular Lymph Node Status for Prognostic Stratification of Prostate Cancer Patients Undergoing Radical Prostatectomy with Extended Pelvic Lymph Node Dissection. Clin. Cancer Res. 2018, 24, 2342–2349. [Google Scholar] [CrossRef] [Green Version]
- Boyukozer, F.B.; Tanoglu, E.G.; Ozen, M.; Ittmann, M.; Aslan, E.S. Kallikrein gene family as biomarkers for recurrent prostate cancer. Croat. Med. J. 2020, 61, 450–456. [Google Scholar] [CrossRef]
- Sugisawa, R.; Komatsu, G.; Hiramoto, E.; Takeda, N.; Yamamura, K.-I.; Arai, S.; Miyazaki, T. Independent modes of disease repair by AIM protein distinguished in AIM-felinized mice. Sci. Rep. 2018, 8, 13157. [Google Scholar] [CrossRef]
- Sanjurjo, L.; Aran, G.; Roher, N.; Valledor, A.F.; Sarrias, M.-R. AIM/CD5L: A key protein in the control of immune homeostasis and inflammatory disease. J. Leukoc. Biol. 2015, 98, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Barach, Y.S.; Lee, J.S.; Zang, X. T cell coinhibition in prostate cancer: New immune evasion pathways and emerging therapeutics. Trends Mol. Med. 2011, 17, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsenio, J.; Kakaradov, B.; Metz, P.J.; Kim, S.H.; Yeo, G.W.; Chang, J.T. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat. Immunol. 2014, 15, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Yao, D.; Tan, J.; He, Z.; Yu, Z.; Chen, J.; Luo, G.; Wang, C.; Zhou, F.; Zha, X.; et al. Memory T cells skew toward terminal differentiation in the CD8+ T cell population in patients with acute myeloid leukemia. J. Hematol. Oncol. 2018, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef]
- Xu, J.-L.; Guo, Y. FCGR1A Serves as a Novel Biomarker and Correlates With Immune Infiltration in Four Cancer Types. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef]
- Pahl, J.H.W.; Koch, J.; Götz, J.J.; Arnold, A.; Reusch, U.; Gantke, T.; Rajkovic, E.; Treder, M.; Cerwenka, A. CD16A Activation of NK Cells Promotes NK Cell Proliferation and Memory-Like Cytotoxicity against Cancer Cells. Cancer Immunol. Res. 2018, 6, 517–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panni, R.Z.; Herndon, J.M.; Zuo, C.; Hegde, S.; Hogg, G.D.; Knolhoff, B.L.; Breden, M.A.; Li, X.; Krisnawan, V.E.; Khan, S.Q.; et al. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci. Transl. Med. 2019, 11, eaau9240. [Google Scholar] [CrossRef]
- Schmid, M.C.; Khan, S.Q.; Kaneda, M.M.; Pathria, P.; Shepard, R.; Louis, T.L.; Anand, S.; Woo, G.; Leem, C.; Faridi, M.H.; et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat. Commun. 2018, 9, 5379. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Horner, J.W.; Paul, E.; Shang, X.; Troncoso, P.; Deng, P.; Jiang, S.; Chang, Q.; Spring, D.J.; Sharma, P.; et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017, 543, 728–732. [Google Scholar] [CrossRef] [Green Version]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [CrossRef] [Green Version]
- Sinha, M.; Zhang, L.; Subudhi, S.; Chen, B.; Marquez, J.; Liu, E.V.; Allaire, K.; Cheung, A.; Ng, S.; Nguyen, C.; et al. Pre-existing immune status associated with response to combination of sipuleucel-T and ipilimumab in patients with metastatic castration-resistant prostate cancer. J. Immunother. Cancer 2021, 9, e002254. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamodu, O.A.; Wang, Y.-H.; Yeh, C.-T.; Ho, C.-H.; Chiang, Y.-T.; Kao, W.-T.; Liu, C.-H.; Wu, C.-C. Concomitant High Apoptosis Inhibitor of Macrophage (AIM) and Low Prostate-Specific Antigen (PSA) Indicates Activated T Cell-Mediated Anticancer Immunity, Enhance Sensitivity to Pembrolizumab, and Elicit Good Prognosis in Prostate Cancer. Biomedicines 2021, 9, 1225. https://doi.org/10.3390/biomedicines9091225
Bamodu OA, Wang Y-H, Yeh C-T, Ho C-H, Chiang Y-T, Kao W-T, Liu C-H, Wu C-C. Concomitant High Apoptosis Inhibitor of Macrophage (AIM) and Low Prostate-Specific Antigen (PSA) Indicates Activated T Cell-Mediated Anticancer Immunity, Enhance Sensitivity to Pembrolizumab, and Elicit Good Prognosis in Prostate Cancer. Biomedicines. 2021; 9(9):1225. https://doi.org/10.3390/biomedicines9091225
Chicago/Turabian StyleBamodu, Oluwaseun Adebayo, Yuan-Hung Wang, Chi-Tai Yeh, Chen-Hsun Ho, Yi-Te Chiang, Wei-Tang Kao, Chia-Hung Liu, and Chia-Chang Wu. 2021. "Concomitant High Apoptosis Inhibitor of Macrophage (AIM) and Low Prostate-Specific Antigen (PSA) Indicates Activated T Cell-Mediated Anticancer Immunity, Enhance Sensitivity to Pembrolizumab, and Elicit Good Prognosis in Prostate Cancer" Biomedicines 9, no. 9: 1225. https://doi.org/10.3390/biomedicines9091225
APA StyleBamodu, O. A., Wang, Y.-H., Yeh, C.-T., Ho, C.-H., Chiang, Y.-T., Kao, W.-T., Liu, C.-H., & Wu, C.-C. (2021). Concomitant High Apoptosis Inhibitor of Macrophage (AIM) and Low Prostate-Specific Antigen (PSA) Indicates Activated T Cell-Mediated Anticancer Immunity, Enhance Sensitivity to Pembrolizumab, and Elicit Good Prognosis in Prostate Cancer. Biomedicines, 9(9), 1225. https://doi.org/10.3390/biomedicines9091225






