Effects of Pitavastatin, Atorvastatin, and Rosuvastatin on the Risk of New-Onset Diabetes Mellitus: A Single-Center Cohort Study
Abstract
1. Introduction
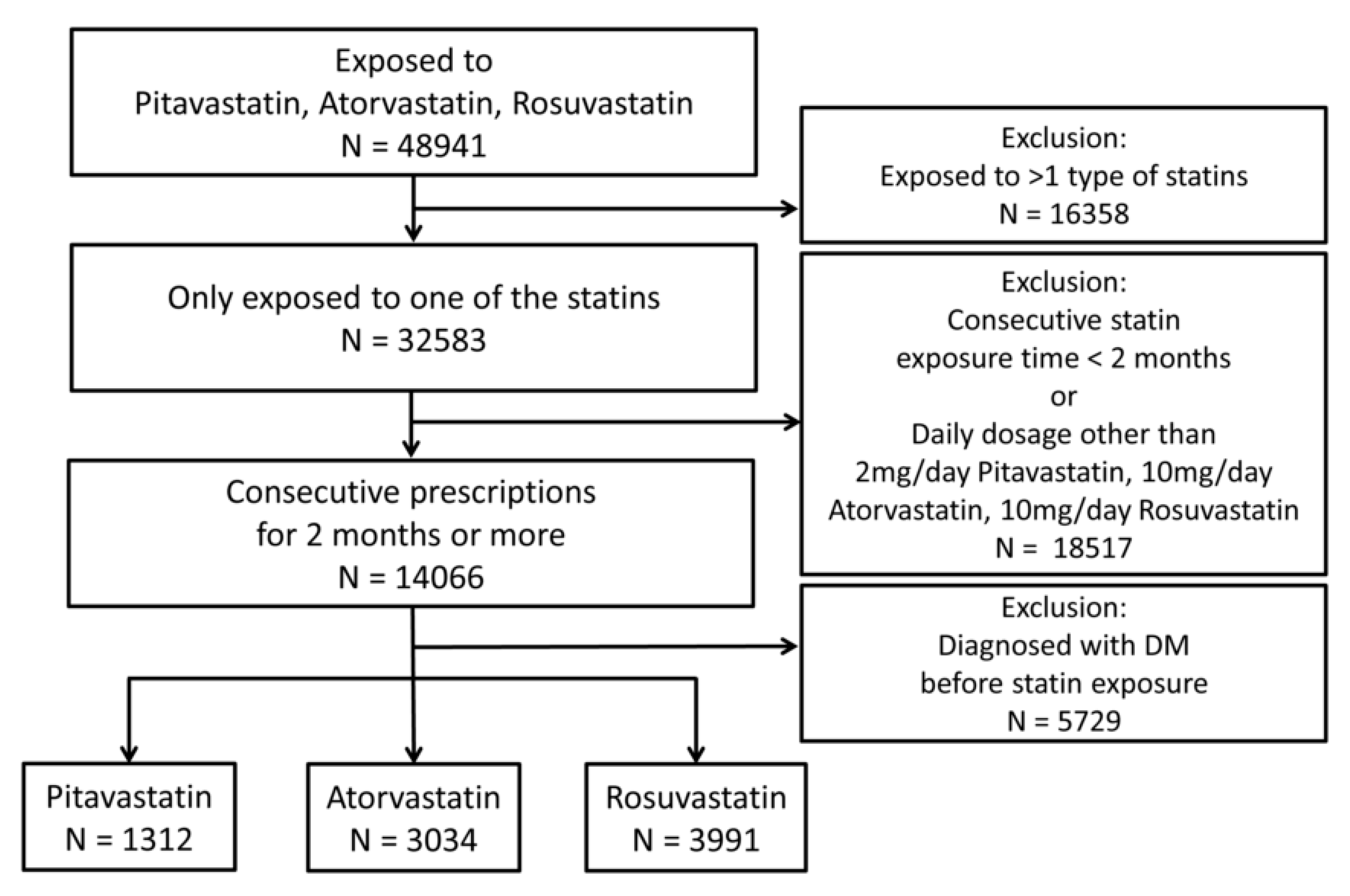
2. Material and Methods
2.1. Population
2.2. Observational Variables
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Adhyaru, B.B.; Jacobson, T.A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Carter, A.A.; Gomes, T.; Camacho, X.; Juurlink, D.N.; Shah, B.R.; Mamdani, M.M. Risk of incident diabetes among patients treated with statins: Population based study. BMJ 2013, 346, f2610. [Google Scholar] [CrossRef]
- Cho, Y.; Choe, E.; Lee, Y.H.; Seo, J.W.; Choi, Y.; Yun, Y.; Wang, H.J.; Ahn, C.W.; Cha, B.S.; Lee, H.C.; et al. Risk of diabetes in patients treated with HMG-CoA reductase inhibitors. Metabolism 2015, 64, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.S.; Lee, K.Y. Effect of statins on fasting glucose in non-diabetic individuals: Nationwide population-based health examination in Korea. Cardiovasc. Diabetol. 2018, 17, 155. [Google Scholar] [CrossRef]
- Cederberg, H.; Stancakova, A.; Yaluri, N.; Modi, S.; Kuusisto, J.; Laakso, M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: A 6 year follow-up study of the METSIM cohort. Diabetologia 2015, 58, 1109–1117. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kishimoto, J.; Ito, C.; Noda, M.; Odawara, M.; Terauchi, Y.; Shiba, T.; Kitazato, H.; Iwamoto, Y.; Akanuma, Y.; et al. Effect of pitavastatin on the incidence of diabetes in Japanese individuals with impaired glucose tolerance. Diabetologia 2013, 56 (Suppl. S1), S299. [Google Scholar] [CrossRef]
- Thakker, D.; Nair, S.; Pagada, A.; Jamdade, V.; Malik, A. Statin use and the risk of developing diabetes: A network meta-analysis. Pharmacoepidemiol. Drug Saf. 2016, 25, 1131–1149. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Sheen, S.S.; Lee, S.; Choi, Y.J.; Park, R.W.; Lim, H.S. Statins and risk for new-onset diabetes mellitus: A real-world cohort study using a clinical research database. Medicine (Baltimore) 2016, 95, e5429. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Sung, J.M.; Cho, I.J.; Kim, H.C.; Chang, H.J. Risk of new-onset diabetes among patients treated with statins according to hypertension and gender: Results from a nationwide health-screening cohort. PLoS ONE 2018, 13, e0195459. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Choi, C.U.; Hwang, S.Y.; Choi, B.G.; Jang, W.Y.; Kim, D.Y.; Kim, W.; Park, E.J.; Lee, S.; Na, J.O.; et al. Effect of Pitavastatin Compared with Atorvastatin andRosuvastatin on New-Onset Diabetes Mellitus in PatientsWith Acute Myocardial Infarction. Am. J. Cardiol. 2018, 122, 922–928. [Google Scholar] [CrossRef]
- Carmena, R.; Betteridge, D.J. Diabetogenic Action of Statins: Mechanisms. Curr. Atheroscler. Rep. 2019, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, V.A.; Elvstam, O.; Eliasson, L.; Wendt, A. Rosuvastatin Treatment Affects Both Basal and Glucose-Induced Insulin Secretion in INS-1 832/13 Cells. PLoS ONE 2016, 11, e0151592. [Google Scholar] [CrossRef]
- Donath, M.Y.; Boni-Schnetzler, M.; Ellingsgaard, H.; Ehses, J.A. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009, 24, 325–331. [Google Scholar] [CrossRef]
- Supale, S.; Li, N.; Brun, T.; Maechler, P. Mitochondrial dysfunction in pancreatic beta cells. Trends Endocrinol. Metab. 2012, 23, 477–487. [Google Scholar] [CrossRef]
- Mabuchi, H.; Higashikata, T.; Kawashiri, M.; Katsuda, S.; Mizuno, M.; Nohara, A.; Inazu, A.; Koizumi, J.; Kobayashi, J. Reduction of serum ubiquinol-10 and ubiquinone-10 levels by atorvastatin in hypercholesterolemic patients. J. Atheroscler. Thromb. 2005, 12, 111–119. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Preiss, D.; Kuchenbaecker, K.B.; Holmes, M.V.; Engmann, J.E.; Shah, T.; Sofat, R.; Stender, S.; Johnson, P.C.; Scott, R.A.; et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: Evidence from genetic analysis and randomised trials. Lancet 2015, 385, 351–361. [Google Scholar] [CrossRef]
- Nakata, M.; Nagasaka, S.; Kusaka, I.; Matsuoka, H.; Ishibashi, S.; Yada, T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): Implications in glycaemic control. Diabetologia 2006, 49, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shin, H.J.; Ding, E.L.; van Dam, R.M. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2009, 302, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Tsugawa, Y.; Tseng, C.H.; Kobayashi, Y.; Shapiro, M.F. Different time trends of caloric and fat intake between statin users and nonusers among US adults: Gluttony in the time of statins? JAMA Intern. Med. 2014, 174, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Takaguri, A.; Satoh, K.; Itagaki, M.; Tokumitsu, Y.; Ichihara, K. Effects of atorvastatin and pravastatin on signal transduction related to glucose uptake in 3T3L1 adipocytes. J. Pharmacol. Sci. 2008, 107, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Arnaboldi, L.; Corsini, A. Could changes in adiponectin drive the effect of statins on the risk of new-onset diabetes? The case of pitavastatin. Atheroscler. Suppl. 2015, 16, 1–27. [Google Scholar] [CrossRef]
- Preiss, D.; Seshasai, S.R.; Welsh, P.; Murphy, S.A.; Ho, J.E.; Waters, D.D.; DeMicco, D.A.; Barter, P.; Cannon, C.P.; Sabatine, M.S.; et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA 2011, 305, 2556–2564. [Google Scholar] [CrossRef]
- Nakamura, H.; Arakawa, K.; Itakura, H.; Kitabatake, A.; Goto, Y.; Toyota, T.; Nakaya, N.; Nishimoto, S.; Muranaka, M.; Yamamoto, A.; et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): A prospective randomised controlled trial. Lancet 2006, 368, 1155–1163. [Google Scholar] [CrossRef]
- Kim, T.M.; Kim, H.; Jeong, Y.J.; Baik, S.J.; Yang, S.J.; Lee, S.H.; Cho, J.H.; Lee, H.; Yim, H.W.; Choi, I.Y.; et al. The differences in the incidence of diabetes mellitus and prediabetes according to the type of HMG-CoA reductase inhibitors prescribed in Korean patients. Pharmacoepidemiol. Drug Saf. 2017, 26, 1156–1163. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Kondapally Seshasai, S.R.; Kurogi, K.; Michishita, I.; Nozue, T.; Sugiyama, S.; Tsimikas, S.; Yoshida, H.; Ray, K.K. Effect of pitavastatin on glucose, HbA1c and incident diabetes: A meta-analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis 2015, 241, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.; Halim, A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab. Syndr. 2019, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.X.; Tarrio, M.; Grabie, N.; Zhang, Y.; Yamazaki, H.; Stavrakis, G.; Maganto-Garcia, E.; Pepper-Cunningham, Z.; Jarolim, P.; Aikawa, M.; et al. Statin-induced Kruppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. J. Clin. Investig. 2010, 120, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Lin, C.S.; Tsai, M.C.; Shih, S.F.; Lim, Z.W.; Chen, S.J.; Tsui, P.F.; Ho, L.J.; Lai, J.H.; Liou, J.T. Pitavastatin Exerts Potent Anti-Inflammatory and Immunomodulatory Effects via the Suppression of AP-1 Signal Transduction in Human T Cells. Int. J. Mol. Sci. 2019, 20, 3534. [Google Scholar] [CrossRef]
- Goldberg, R.B. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J. Clin. Endocrinol. Metab. 2009, 94, 3171–3182. [Google Scholar] [CrossRef]
- Nakagomi, A.; Shibui, T.; Kohashi, K.; Kosugi, M.; Kusama, Y.; Atarashi, H.; Shimizu, W. Differential Effects of Atorvastatin and Pitavastatin on Inflammation, Insulin Resistance, and the Carotid Intima-Media Thickness in Patients with Dyslipidemia. J. Atheroscler. Thromb. 2015, 22, 1158–1171. [Google Scholar] [CrossRef]
- Tsai, A.C.; Lee, S.H. Determinants of new-onset diabetes in older adults-Results of a national cohort study. Clin. Nutr. 2015, 34, 937–942. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C. Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: Cohort study. BMJ 2017, 359, j5019. [Google Scholar] [CrossRef]
- Lin, C.S.; Liu, C.C.; Yeh, C.C.; Chang, Y.C.; Chung, C.L.; Lane, H.L.; Shih, C.C.; Chen, T.L.; Liao, C.C. Diabetes risks and outcomes in chronic obstructive pulmonary disease patients: Two nationwide population-based retrospective cohort studies. PLoS ONE 2017, 12, e0181815. [Google Scholar] [CrossRef]
- Fathallah, N.; Slim, R.; Larif, S.; Hmouda, H.; Ben Salem, C. Drug-Induced Hyperglycaemia and Diabetes. Drug Saf. 2015, 38, 1153–1168. [Google Scholar] [CrossRef] [PubMed]

| Variables | Pitavastatin (N = 1312, 15.7%) | Atorvastatin (N = 3034, 36.4%) | Rosuvastatin (N = 3991, 47.9%) | p-Value |
|---|---|---|---|---|
| Follow up (days) | 468.31 ± 446.18 | 558.30 ± 666.85 | 648.30 ± 796.06 | <0.001 |
| NODM | 167 (12.7%) | 555 (18.3%) | 864 (21.6%) | <0.001 |
| Gender (male) | 728 (55.5%) | 1546 (51.0%) | 2236 (56.0%) | <0.001 |
| Age | 60.42 ± 12.44 | 61.53 ± 13.54 | 58.21 ± 13.45 | <0.001 |
| BMI | 25.71 ± 4.18 | 24.75 ± 4.08 | 25.11 ± 4.38 | 0.017 |
| Comorbidities | ||||
| CAD | 551 (42.0%) | 798 (26.3%) | 931 (23.3%) | <0.001 |
| Hypertension | 796 (60.7%) | 1416 (46.7%) | 1864 (46.7%) | <0.001 |
| COPD | 195 (14.9%) | 363 (12.0%) | 419 (10.5%) | <0.001 |
| CKD | 29 (2.2%) | 154 (5.1%) | 90 (2.3%) | <0.001 |
| Cancer | 82 (6.2%) | 313 (10.3%) | 266 (6.7%) | <0.001 |
| Ischemic stroke | 160 (12.2%) | 508 (16.7%) | 736 (18.4%) | <0.001 |
| Hemorrhagic stroke | 24 (1.8%) | 76 (2.5%) | 71 (1.8%) | 0.086 |
| Heart failure | 100 (7.6%) | 213 (7.0%) | 256 (6.4%) | 0.279 |
| Biochemistry | ||||
| LDL (mg/dL) | 131.52 ± 28.51 | 122.94 ± 37.57 | 141.30 ± 45.86 | <0.001 |
| TC (mg/dL) | 202.91 ± 35.03 | 198.53 ± 46.67 | 218.96 ± 54.14 | <0.001 |
| TG (mg/dL) | 138.95 ± 74.01 | 138.80 ± 84.14 | 164.14 ± 133.90 | <0.001 |
| Creatinine (mg/dL) | 0.89 ± 0.27 | 1.03 ± 0.98 | 0.94 ± 0.64 | <0.001 |
| ALT (U/L) | 23.59 ± 16.43 | 23.64 ± 20.52 | 25.78 ± 19.71 | <0.001 |
| Uric Acid (mg/dL) | 6.07 ± 1.56 | 6.05 ± 1.66 | 6.13 ± 1.79 | 0.376 |
| FG (mg/dL) | 99.94 ± 15.74 | 99.27 ± 17.31 | 99.57 ± 17.98 | 0.581 |
| HbA1c (%) | 6.05 ± 0.98 | 6.08 ± 1.17 | 6.09 ± 1.23 | 0.872 |
| Variables | Crude-HR (95% CI) | p-Value | Adj-HR (95% CI) # | p-Value |
|---|---|---|---|---|
| Gender (male) | 1.00 (0.91–1.10) | 0.987 | 1.17 (1.05–1.29) | 0.003 |
| Age | 1.01 (1.01–1.02) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| Comorbidities | ||||
| CAD | 0.64 (0.57–0.73) | <0.001 | 0.63 (0.55–0.72) | <0.001 |
| Hypertension | 0.81 (0.73–0.89) | <0.001 | 0.78 (0.70–0.87) | <0.001 |
| COPD | 0.73 (0.61–0.88) | 0.001 | 0.71 (0.59–0.86) | <0.001 |
| CKD | 1.58 (1.22–2.04) | 0.001 | 1.56 (1.20–2.02) | 0.001 |
| cancer | 0.93 (0.76–1.12) | 0.433 | 0.83 (0.69–1.01) | 0.067 |
| Ischemic stroke | 1.16 (1.02–1.32) | 0.028 | 1.00 (0.87–1.16) | 0.964 |
| Hemorrhagic stroke | 1.06 (0.73–1.54) | 0.761 | 0.99 (0.68–1.45) | 0.969 |
| Heart failure | 1.16 (0.96–1.40) | 0.113 | 1.17 (0.96–1.42) | 0.121 |
| Biochemistry | ||||
| LDL (per 10 mg/dL) | 0.97 (0.95–0.99) | <0.001 | 0.97 (0.96–0.99) | 0.006 |
| TC (per 10 mg/dL) | 0.97 (0.95–0.98) | <0.001 | 0.97 (0.96–0.99) | 0.001 |
| TG (per 10 mg/dL) | 1.01 (1.01–1.02) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| Cr (per 1 mg/dL) | 1.18 (1.12–1.25) | <0.001 | 1.14 (1.07–1.23) | <0.001 |
| ALT (per 1 U/L) | 1.01 (1.00–1.01) | <0.001 | 1.01 (1.01–1.01) | <0.001 |
| UA (per 1 mg/dL) | 1.06 (1.02–1.11) | 0.006 | 1.05 (1.00–1.09) | 0.043 |
| FG (per 1 mg/dL) | 1.03 (1.03–1.03) | <0.001 | 1.03 (1.03–1.03) | <0.001 |
| HbA1c (per 1%) | 1.63 (1.55–1.70) | <0.001 | 1.64 (1.57–1.73) | <0.001 |
| Independent Variables | Crude-HR (95% CI) | p-Value | Adj-HR (95% CI) # | p-Value |
|---|---|---|---|---|
| Comparison 1 | 0.038 | 0.230 | ||
| Pitavastatin | 1.00 | 1.00 | ||
| Atorvastatin | 1.21 (1.02–1.44) | 0.032 | 1.04 (0.87–1.25) | 0.677 |
| Rosuvastatin | 1.24 (1.05–1.47) | 0.011 | 1.13 (0.94–1.35) | 0.196 |
| Comparison 2 | ||||
| Pitavastatin | 1.00 | 1.00 | ||
| Atorvastatin/Rosuvastatin | 1.23 (1.05–1.45) | 0.012 | 1.09 (0.91–1.29) | 0.356 |
| Stratified Variables | Drugs | Crude-HR (95% CI) | p-Value | Adj-HR (95% CI) # | p-Value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | Pitavastatin | 1.00 | 1.00 | ||
| (N = 3827) | Atorvastatin | 1.25 (0.96–1.64) | 0.103 | 1.07 (0.81–1.43) | 0.627 |
| Rosuvastatin | 1.43 (1.10–1.86) | 0.007 | 1.31 (0.99–1.73) | 0.063 | |
| Male | Pitavastatin | 1.00 | 1.00 | ||
| (N = 4510) | Atorvastatin | 1.19 (0.95–1.50) | 0.131 | 1.03 (0.81–1.31) | 0.792 |
| Rosuvastatin | 1.12 (0.90–1.39) | 0.317 | 1.02 (0.80–1.29) | 0.899 | |
| Comorbidities | |||||
| No CAD | Pitavastatin | 1.00 | 1.00 | ||
| (N = 6057) | Atorvastatin | 1.09 (0.88–1.34) | 0.431 | 0.93 (0.75–1.16) | 0.535 |
| Rosuvastatin | 1.12 (0.91–1.37) | 0.279 | 1.01 (0.81–1.25) | 0.926 | |
| CAD | Pitavastatin | 1.00 | 1.00 | ||
| (N = 2280) | Atorvastatin | 1.17 (0.84–1.62) | 0.365 | 1.25 (0.89–1.74) | 0.202 |
| Rosuvastatin | 1.16 (0.85–1.59) | 0.357 | 1.47 (1.05–2.05) | 0.025 | |
| No HTN | Pitavastatin | 1.00 | 1.00 | ||
| (N = 4261) | Atorvastatin | 1.37 (1.03–1.82) | 0.031 | 0.87 (0.64–1.19) | 0.379 |
| Rosuvastatin | 1.44 (1.09–1.90) | 0.011 | 0.99 (0.73–1.34) | 0.934 | |
| HTN | Pitavastatin | 1.00 | 1.00 | ||
| (N = 4076) | Atorvastatin | 1.03 (0.82–1.30) | 0.778 | 1.13 (0.90–1.43) | 0.295 |
| Rosuvastatin | 1.05 (0.84–1.30) | 0.688 | 1.26 (1.00–1.59) | 0.047 | |
| No COPD | Pitavastatin | 1.00 | 1.00 | ||
| (N = 7360) | Atorvastatin | 1.23 (1.02–1.48) | 0.028 | 1.04 (0.86–1.27) | 0.691 |
| Rosuvastatin | 1.22 (1.02–1.46) | 0.026 | 1.09 (0.90–1.32) | 0.381 | |
| COPD | Pitavastatin | 1.00 | 1.00 | ||
| (N = 977) | Atorvastatin | 0.90 (0.52–1.55) | 0.698 | 0.99 (0.57–1.72) | 0.965 |
| Rosuvastatin | 1.31 (0.79–2.16) | 0.292 | 1.74 (1.02–2.94) | 0.040 | |
| No CKD | Pitavastatin | 1.00 | 1.00 | ||
| (N = 8064) | Atorvastatin | 1.17 (0.98–1.40) | 0.077 | 1.00 (0.83–1.21) | 0.999 |
| Rosuvastatin | 1.22 (1.03–1.44) | 0.024 | 1.08 (0.90–1.30) | 0.385 | |
| CKD | Pitavastatin | 1.00 | 1.00 | ||
| (N = 273) | Atorvastatin | 2.54 (0.78–8.32) | 0.123 | 2.67 (0.79–8.99) | 0.114 |
| Rosuvastatin | 3.08 (0.92–10.3) | 0.068 | 3.33 (0.93–11.9) | 0.064 | |
| No cancer | Pitavastatin | 1.00 | 1.00 | ||
| (N = 7676) | Atorvastatin | 1.28 (1.07–1.54) | 0.007 | 1.06 (0.88–1.29) | 0.549 |
| Rosuvastatin | 1.25 (1.05–1.49) | 0.012 | 1.09 (0.90–1.32) | 0.361 | |
| Cancer | Pitavastatin | 1.00 | 1.00 | ||
| (N = 661) | Atorvastatin | 0.65 (0.35–1.21) | 0.176 | 0.92 (0.48–1.75) | 0.797 |
| Rosuvastatin | 1.21 (0.66–2.22) | 0.534 | 1.62 (0.85–3.08) | 0.145 | |
| No IS | Pitavastatin | 1.00 | 1.00 | ||
| (N = 6933) | Atorvastatin | 1.28 (1.06–1.54) | 0.011 | 1.06 (0.86–1.29) | 0.601 |
| Rosuvastatin | 1.31 (1.09–1.58) | 0.004 | 1.16 (0.95–1.42) | 0.149 | |
| IS | Pitavastatin | 1.00 | 1.00 | ||
| (N = 1404) | Atorvastatin | 0.82 (0.53–1.28) | 0.384 | 0.89 (0.57–1.40) | 0.617 |
| Rosuvastatin | 0.87 (0.57–1.33) | 0.520 | 1.01 (0.65–1.56) | 0.972 | |
| No HS | Pitavastatin | 1.00 | 1.00 | ||
| (N = 8166) | Atorvastatin | 1.22 (1.03–1.46) | 0.024 | 1.05 (0.87–1.27) | 0.582 |
| Rosuvastatin | 1.26 (1.07–1.50) | 0.007 | 1.15 (0.96–1.38) | 0.142 | |
| HS | Pitavastatin | 1.00 | 1.00 | ||
| (N = 171) | Atorvastatin | 0.56 (0.18–1.79) | 0.328 | 0.43 (0.12–1.49) | 0.183 |
| Rosuvastatin | 0.50 (0.16–1.63) | 0.251 | 0.38 (0.10–1.42) | 0.151 | |
| No HF | Pitavastatin | 1.00 | 1.00 | ||
| (N = 7768) | Atorvastatin | 1.20 (1.00–1.44) | 0.045 | 1.00 (0.82–1.21) | 0.960 |
| Rosuvastatin | 1.24 (1.04–1.48) | 0.015 | 1.08 (0.90–1.31) | 0.407 | |
| HF | Pitavastatin | 1.00 | 1.00 | ||
| (N = 569) | Atorvastatin | 1.29 (0.70–2.36) | 0.411 | 1.54 (0.83–2.87) | 0.173 |
| Rosuvastatin | 1.30 (0.72–2.34) | 0.384 | 1.82 (0.96–3.43) | 0.066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-T.; Lin, C.; Tsai, M.-C.; Cheng, C.-C.; Chen, S.-J.; Liou, J.-T.; Lin, W.-S.; Cheng, S.-M.; Lin, C.-S.; Tsao, T.-P. Effects of Pitavastatin, Atorvastatin, and Rosuvastatin on the Risk of New-Onset Diabetes Mellitus: A Single-Center Cohort Study. Biomedicines 2020, 8, 499. https://doi.org/10.3390/biomedicines8110499
Liu W-T, Lin C, Tsai M-C, Cheng C-C, Chen S-J, Liou J-T, Lin W-S, Cheng S-M, Lin C-S, Tsao T-P. Effects of Pitavastatin, Atorvastatin, and Rosuvastatin on the Risk of New-Onset Diabetes Mellitus: A Single-Center Cohort Study. Biomedicines. 2020; 8(11):499. https://doi.org/10.3390/biomedicines8110499
Chicago/Turabian StyleLiu, Wei-Ting, Chin Lin, Min-Chien Tsai, Cheng-Chung Cheng, Sy-Jou Chen, Jun-Ting Liou, Wei-Shiang Lin, Shu-Meng Cheng, Chin-Sheng Lin, and Tien-Ping Tsao. 2020. "Effects of Pitavastatin, Atorvastatin, and Rosuvastatin on the Risk of New-Onset Diabetes Mellitus: A Single-Center Cohort Study" Biomedicines 8, no. 11: 499. https://doi.org/10.3390/biomedicines8110499
APA StyleLiu, W.-T., Lin, C., Tsai, M.-C., Cheng, C.-C., Chen, S.-J., Liou, J.-T., Lin, W.-S., Cheng, S.-M., Lin, C.-S., & Tsao, T.-P. (2020). Effects of Pitavastatin, Atorvastatin, and Rosuvastatin on the Risk of New-Onset Diabetes Mellitus: A Single-Center Cohort Study. Biomedicines, 8(11), 499. https://doi.org/10.3390/biomedicines8110499








