Role of Angiotensin II in Cardiovascular Diseases: Introducing Bisartans as a Novel Therapy for Coronavirus 2019
Abstract
1. Introduction
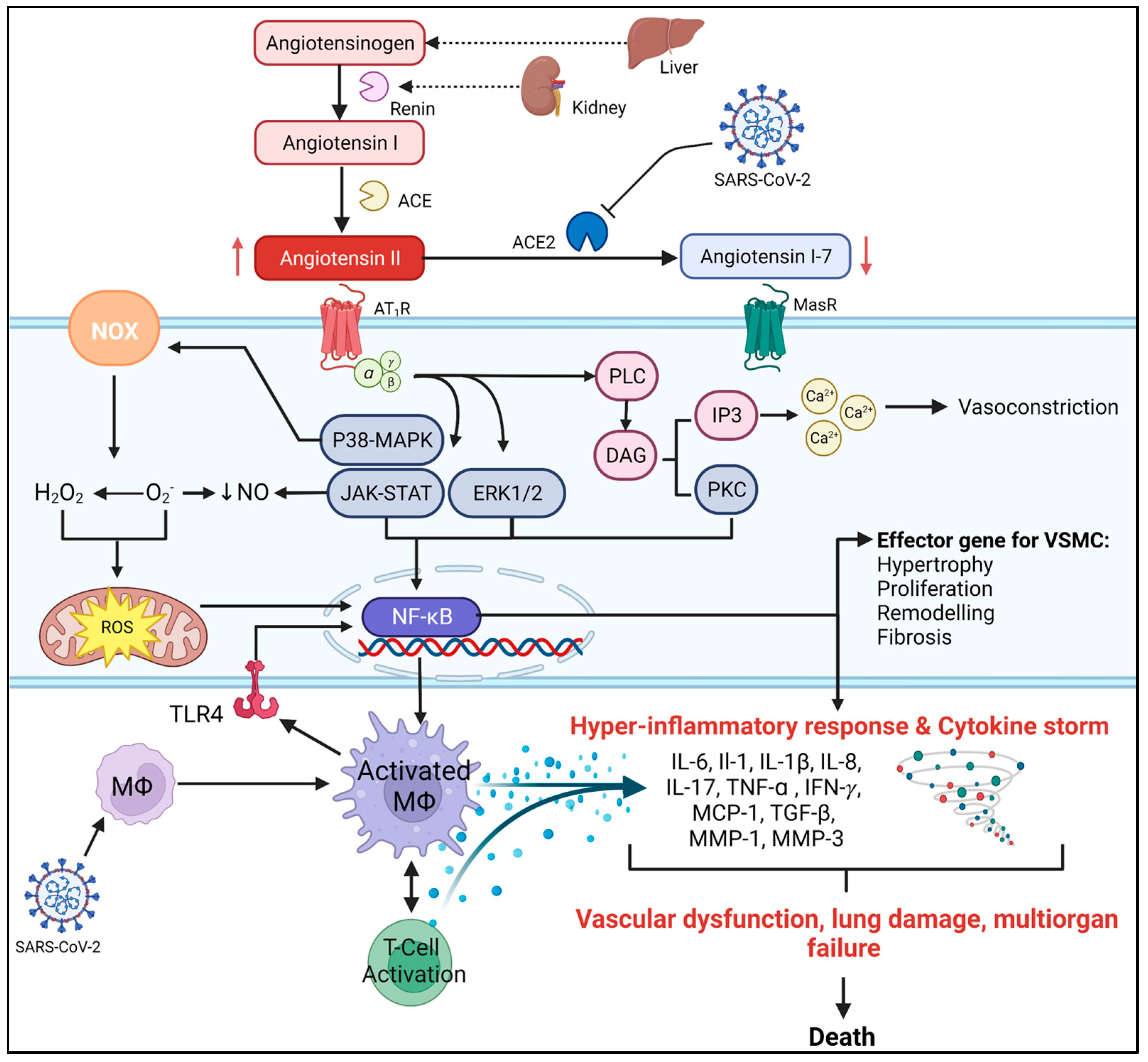
2. The Role of AngII in CVDs
2.1. AngII and Endothelial Dysfunction
2.2. AngII and Oxidative Stress
2.3. AngII and CVD Inflammation
3. The Relationship between CVDs and COVID-19
COVID-19 and RAS
4. ARBs as a Treatment for COVID-19
5. Bisartans, a New Generation of ARBs against SARS-CoV-2
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.; Aaron, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; CommodoreMensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- American Heart Association. Cardiovascular Disease: A Costly Burden for America. Projections through 2035; AHA: Dallas, TX, USA, 2017. [Google Scholar]
- Frak, W.; Wojtasinska, A.; Lisinska, W.; Mlynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef]
- Bitker, L.; Burrell, L.M. Classic and Nonclassic Renin-Angiotensin Systems in the Critically. Crit. Care Clin. 2019, 35, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; El-Arabey, A.A.; Gai, Z. Hypertension is still a moving target in the context of COVID-19 and post-acute COVID-19 syndrome. J. Med. Virol. 2023, 95, e28128. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, S.A.; Ugusman, A.; Kumar, J.; Skiba, D.; Hamid, A.A.; Aminuddin, A. COVID-19 and Hypertension: The What, the Why, and the How. Front. Physiol. 2021, 12, 665064. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Lorenzo, O.; Ruperez, M.; Esteban, V.; Suzuki, Y.; Mezzano, S.; Plaza, J.J.; Egido, J. Role of the renin-angiotensin system in vascular diseases: Expanding the field. Hypertension 2001, 38, 1382–1387. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. WHO Coronavirus (COVD-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 28 February 2023).
- Chung, M.K.; Zidar, D.A.; Bristow, M.R.; Cameron, S.J.; Chan, T.; Harding, C.V.; Kwon, D.H., 3rd; Singh, T.; Tilton, J.C.; Tsai, E.J.; et al. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ. Res. 2021, 128, 1214–1236. [Google Scholar] [CrossRef]
- Chavda, V.P.; Kapadia, C.; Soni, S.; Prajapati, R.; Chauhan, S.C.; Yallapu, M.M.; Apostolopoulos, V. A global picture: Therapeutic perspectives for COVID-19. Immunotherapy 2022, 14, 351–371. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Cooper, J.; Salih, A.; Raman, B.; Lee, A.M.; Neubauer, S.; Harvey, N.C.; Petersen, S.E. Cardiovascular disease and mortality sequelae of COVID-19 in the UK Biobank. Heart 2022, 109, 119–126. [Google Scholar] [CrossRef]
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochemistry 2020, 85, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Petitjean, S.J.L.; Koehler, M.; Zhang, Q.; Dumitru, A.C.; Chen, W.; Derclaye, S.; Vincent, S.P.; Soumillion, P.; Alsteens, D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020, 11, 4541. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, C.; Zhang, L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov. Today 2011, 16, 22–34. [Google Scholar] [CrossRef][Green Version]
- Wu, C.H.; Mohammadmoradi, S.; Chen, J.Z.; Sawada, H.; Daugherty, A.; Lu, H.S. Renin-Angiotensin System and Cardiovascular Functions. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e108–e116. [Google Scholar] [CrossRef][Green Version]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Costa, L.B.; Perez, L.G.; Palmeira, V.A.; Macedo, E.C.T.; Ribeiro, V.T.; Lanza, K.; Simoes, E.S.A.C. Insights on SARS-CoV-2 Molecular Interactions With the Renin-Angiotensin System. Front. Cell. Dev. Biol. 2020, 8, 559841. [Google Scholar] [CrossRef]
- Paz Ocaranza, M.; Riquelme, J.A.; Garcia, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef][Green Version]
- Triposkiadis, F.; Xanthopoulos, A.; Giamouzis, G.; Boudoulas, K.D.; Starling, R.C.; Skoularigis, J.; Boudoulas, H.; Iliodromitis, E. ACE2, the Counter-Regulatory Renin-Angiotensin System Axis and COVID-19 Severity. J. Clin. Med. 2021, 10, 3885. [Google Scholar] [CrossRef] [PubMed]
- Lautner, R.Q.; Villela, D.C.; Fraga-Silva, R.A.; Silva, N.; Verano-Braga, T.; Costa-Fraga, F.; Jankowski, J.; Jankowski, V.; Sousa, F.; Alzamora, A.; et al. Discovery and characterization of alamandine: A novel component of the renin-angiotensin system. Circ. Res. 2013, 112, 1104–1111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villela, D.C.; Passos-Silva, D.G.; Santos, R.A. Alamandine: A new member of the angiotensin family. Curr. Opin. Nephrol. Hypertens. 2014, 23, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Qaradakhi, T.; Apostolopoulos, V.; Zulli, A. Angiotensin (1–7) and Alamandine: Similarities and differences. Pharmacol. Res. 2016, 111, 820–826. [Google Scholar] [CrossRef][Green Version]
- De Souza-Neto, F.P.; Silva, M.M.E.; Santuchi, M.C.; de Alcantara-Leonidio, T.C.; Motta-Santos, D.; Oliveira, A.C.; Melo, M.B.; Canta, G.N.; de Souza, L.E.; Irigoyen, M.C.C.; et al. Alamandine attenuates arterial remodelling induced by transverse aortic constriction in mice. Clin. Sci. 2019, 133, 629–643. [Google Scholar] [CrossRef]
- Hekmat, A.S.; Navabi, Z.; Alipanah, H.; Javanmardi, K. Alamandine significantly reduces doxorubicin-induced cardiotoxicity in rats. Hum. Exp. Toxicol. 2021, 40, 1781–1795. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, B.; Zhang, Y.; Huang, W.; Hong, Q.; Meng, Y. Alamandine via MrgD receptor attenuates pulmonary fibrosis via NOX4 and autophagy pathway. Can. J. Physiol. Pharmacol. 2021, 99, 885–893. [Google Scholar] [CrossRef]
- Soltani Hekmat, A.; Chenari, A.; Alipanah, H.; Javanmardi, K. Protective effect of alamandine on doxorubicin-induced nephrotoxicity in rats. BMC Pharmacol. Toxicol. 2021, 22, 31. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Dias, H.B.; de Souza Jaques, W.A.; Becker, T.; Rigatto, K. Assessment of Alamandine in Pulmonary Fibrosis and Respiratory Mechanics in Rodents. J. Renin. Angiotensin. Aldosterone Syst. 2021, 2021, 9975315. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Esteban, V.; Egido, J. The regulation of the inflammatory response through nuclear factor-kappab pathway by angiotensin IV extends the role of the renin angiotensin system in cardiovascular diseases. Trends Cardiovasc. Med. 2007, 17, 19–25. [Google Scholar] [CrossRef]
- Murphy, T.J.; Alexander, R.W.; Griendling, K.K.; Runge, M.S.; Bernstein, K.E. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 1991, 351, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Reaux, A.; Fournie-Zaluski, M.C.; Llorens-Cortes, C. Angiotensin III: A central regulator of vasopressin release and blood pressure. Trends Endocrinol. Metab. 2001, 12, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zeng, X.J.; Wang, H.X.; Zhang, L.K.; Dong, X.L.; Guo, S.; Du, J.; Li, H.H.; Tang, C.S. Angiotensin IV protects against angiotensin II-induced cardiac injury via AT4 receptor. Peptides 2011, 32, 2108–2115. [Google Scholar] [CrossRef]
- Wang, Q.G.; Xue, X.; Yang, Y.; Gong, P.Y.; Jiang, T.; Zhang, Y.D. Angiotensin IV suppresses inflammation in the brains of rats with chronic cerebral hypoperfusion. J. Renin. Angiotensin. Aldosterone Syst. 2018, 19, 1470320318799587. [Google Scholar] [CrossRef][Green Version]
- Coleman, J.K.; Krebs, L.T.; Hamilton, T.A.; Ong, B.; Lawrence, K.A.; Sardinia, M.F.; Harding, J.W.; Wright, J.W. Autoradiographic identification of kidney angiotensin IV binding sites and angiotensin IV-induced renal cortical blood flow changes in rats. Peptides 1998, 19, 269–277. [Google Scholar] [CrossRef]
- Kramar, E.A.; Krishnan, R.; Harding, J.W.; Wright, J.W. Role of nitric oxide in angiotensin IV-induced increases in cerebral blood flow. Regul. Pept. 1998, 74, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chansel, D.; Vandermeersch, S.; Oko, A.; Curat, C.; Ardaillou, R. Effects of angiotensin IV and angiotensin-(1-7) on basal and angiotensin II-stimulated cytosolic Ca2+ in mesangial cells. Eur. J. Pharmacol. 2001, 414, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Szczepanska-Sadowska, E.; Czarzasta, K.; Cudnoch-Jedrzejewska, A. Dysregulation of the Renin-Angiotensin System and the Vasopressinergic System Interactions in Cardiovascular Disorders. Curr. Hypertens. Rep. 2018, 20, 19. [Google Scholar] [CrossRef][Green Version]
- Kawai, T.; Forrester, S.J.; O’Brien, S.; Baggett, A.; Rizzo, V.; Eguchi, S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res. 2017, 125, 4–13. [Google Scholar] [CrossRef]
- Fellner, S.K.; Arendshorst, W.J. Angiotensin II Ca2+ signaling in rat afferent arterioles: Stimulation of cyclic ADP ribose and IP3 pathways. Am. J. Physiol. Renal. Physiol. 2005, 288, F785–F791. [Google Scholar] [CrossRef]
- Alexander, R.W.; Brock, T.A.; Gimbrone, M.A., Jr.; Rittenhouse, S.E. Angiotensin increases inositol trisphosphate and calcium in vascular smooth muscle. Hypertension 1985, 7, 447–451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simoes, S.C.; Balico-Silva, A.L.; Parreiras, E.S.L.T.; Bitencourt, A.L.B.; Bouvier, M.; Costa-Neto, C.M. Signal Transduction Profiling of Angiotensin II Type 1 Receptor With Mutations Associated to Atrial Fibrillation in Humans. Front. Pharmacol. 2020, 11, 600132. [Google Scholar] [CrossRef] [PubMed]
- Spat, A.; Hunyady, L. Control of aldosterone secretion: A model for convergence in cellular signaling pathways. Physiol. Rev. 2004, 84, 489–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verma, K.; Pant, M.; Paliwal, S.; Dwivedi, J.; Sharma, S. An Insight on Multicentric Signaling of Angiotensin II in Cardiovascular system: A Recent Update. Front. Pharmacol. 2021, 12, 734917. [Google Scholar] [CrossRef]
- Wei, H.; Ahn, S.; Shenoy, S.K.; Karnik, S.S.; Hunyady, L.; Luttrell, L.M.; Lefkowitz, R.J. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc. Natl. Acad. Sci. USA 2003, 100, 10782–10787. [Google Scholar] [CrossRef][Green Version]
- Kim, S.-M.; Huh, J.-W.; Kim, E.-Y.; Shin, M.-K.; Park, J.-E.; Kim, S.W.; Lee, W.; Choi, B.; Chang, E.-J. Endothelial dysfunction induces atherosclerosis: Increased aggrecan expression promotes apoptosis in vascular smooth muscle cells. BMB Rep. 2019, 52, 145. [Google Scholar] [CrossRef][Green Version]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308. [Google Scholar] [CrossRef]
- Ruhl, L.; Pink, I.; Kuhne, J.F.; Beushausen, K.; Keil, J.; Christoph, S.; Sauer, A.; Boblitz, L.; Schmidt, J.; David, S.; et al. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal. Transduct. Target Ther. 2021, 6, 418. [Google Scholar] [CrossRef]
- Vukelic, S.; Griendling, K.K. Angiotensin II, from vasoconstrictor to growth factor: A paradigm shift. Circ. Res. 2014, 114, 754–757. [Google Scholar] [CrossRef][Green Version]
- Welsh, D.G.; Tran, C.H.T.; Hald, B.O.; Sancho, M. The Conducted Vasomotor Response: Function, Biophysical Basis, and Pharmacological Control. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 391–410. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shatanawi, A.; Romero, M.J.; Iddings, J.A.; Chandra, S.; Umapathy, N.S.; Verin, A.D.; Caldwell, R.B.; Caldwell, R.W. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway. Am. J. Physiol. Cell Physiol. 2011, 300, C1181–C1192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Satoh, M.; Fujimoto, S.; Arakawa, S.; Yada, T.; Namikoshi, T.; Haruna, Y.; Horike, H.; Sasaki, T.; Kashihara, N. Angiotensin II type 1 receptor blocker ameliorates uncoupled endothelial nitric oxide synthase in rats with experimental diabetic nephropathy. Nephrol. Dial. Transplant. 2008, 23, 3806–3813. [Google Scholar] [CrossRef][Green Version]
- Prathapan, A.; Vineetha, V.P.; Abhilash, P.A.; Raghu, K.G. Boerhaavia diffusa L. attenuates angiotensin II-induced hypertrophy in H9c2 cardiac myoblast cells via modulating oxidative stress and down-regulating NF-kappabeta and transforming growth factor beta1. Br. J. Nutr. 2013, 110, 1201–1210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, K.; Chen, J.; Li, D.; Zhang, X.; Mehta, J.L. Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: Modulation by PPAR-gamma ligand pioglitazone. Hypertension 2004, 44, 655–661. [Google Scholar] [CrossRef][Green Version]
- Lee, C.Y.; Park, H.K.; Lee, B.S.; Jeong, S.; Hyun, S.A.; Choi, J.W.; Kim, S.W.; Lee, S.; Lim, S.; Hwang, K.C. Novel Therapeutic Effects of Pterosin B on Ang II-Induced Cardiomyocyte Hypertrophy. Molecules 2020, 25, 5279. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864C. [Google Scholar] [CrossRef]
- Wen, H.; Gwathmey, J.K.; Xie, L.H. Oxidative stress-mediated effects of angiotensin II in the cardiovascular system. World J. Hypertens. 2012, 2, 34–44. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Kurz, S.; Munzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef][Green Version]
- Sena, C.M.; Leandro, A.; Azul, L.; Seica, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef][Green Version]
- Sugden, P.H.; Clerk, A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid. Redox Signal. 2006, 8, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, S.; Otsu, K.; Nishida, K.; Higuchi, Y.; Morita, T.; Nakayama, H.; Yamaguchi, O.; Mano, T.; Matsumura, Y.; Ueno, H.; et al. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation 2002, 105, 509–515. [Google Scholar] [CrossRef] [PubMed]
- St. Paul, A.; Corbett, C.B.; Okune, R.; Autieri, M.V. Angiotensin II, Hypercholesterolemia, and Vascular Smooth Muscle Cells: A Perfect Trio for Vascular Pathology. Int. J. Mol. Sci. 2020, 21, 4525. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, Z.; Xu, Q.; Peng, H.; Chen, L.; Huang, X.; Yang, T.; Yu, Z.; Cheng, G.; Zhang, G.; et al. Involvement of vascular peroxidase 1 in angiotensin II-induced hypertrophy of H9c2 cells. J. Am. Soc. Hypertens. 2017, 11, 519–529.e511. [Google Scholar] [CrossRef] [PubMed]
- Canty, T.G., Jr.; Boyle, E.M., Jr.; Farr, A.; Morgan, E.N.; Verrier, E.D.; Pohlman, T.H. Oxidative stress induces NF-kappaB nuclear translocation without degradation of IkappaBalpha. Circulation 1999, 100, II361–II364. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef]
- Amin, M.N.; Siddiqui, S.A.; Ibrahim, M.; Hakim, M.L.; Ahammed, M.S.; Kabir, A.; Sultana, F. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020, 8, 2050312120965752. [Google Scholar] [CrossRef]
- Tian, R.; Hou, G.; Li, D.; Yuan, T.F. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. Sci. World J. 2014, 2014, 780616. [Google Scholar] [CrossRef][Green Version]
- Ekholm, M.; Kahan, T. The Impact of the Renin-Angiotensin-Aldosterone System on Inflammation, Coagulation, and Atherothrombotic Complications, and to Aggravated COVID-19. Front. Pharmacol. 2021, 12, 640185. [Google Scholar] [CrossRef]
- Yang, D.; Elner, S.G.; Bian, Z.M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef][Green Version]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 2009, 20, 604–612. [Google Scholar] [CrossRef][Green Version]
- Guo, F.; Chen, X.L.; Wang, F.; Liang, X.; Sun, Y.X.; Wang, Y.J. Role of angiotensin II type 1 receptor in angiotensin II-induced cytokine production in macrophages. J. Interferon. Cytokine Res. 2011, 31, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory cytokines in heart failure: Mediators and markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Porter, K.E.; Turner, N.A. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol. Ther. 2009, 123, 255–278. [Google Scholar] [CrossRef]
- Santos, A.G.; da Rocha, G.O.; de Andrade, J.B. Occurrence of the potent mutagens 2-nitrobenzanthrone and 3-nitrobenzanthrone in fine airborne particles. Sci. Rep. 2019, 9, 1. [Google Scholar] [CrossRef][Green Version]
- Gomolak, J.R.; Didion, S.P. Angiotensin II-induced endothelial dysfunction is temporally linked with increases in interleukin-6 and vascular macrophage accumulation. Front. Physiol. 2014, 5, 396. [Google Scholar] [CrossRef]
- Li, Y.; Kinzenbaw, D.A.; Modrick, M.L.; Pewe, L.L.; Faraci, F.M. Context-dependent effects of SOCS3 in angiotensin II-induced vascular dysfunction and hypertension in mice: Mechanisms and role of bone marrow-derived cells. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H146–H156. [Google Scholar] [CrossRef][Green Version]
- Satou, R.; Miyata, K.; Gonzalez-Villalobos, R.A.; Ingelfinger, J.R.; Navar, L.G.; Kobori, H. Interferon-gamma biphasically regulates angiotensinogen expression via a JAK-STAT pathway and suppressor of cytokine signaling 1 (SOCS1) in renal proximal tubular cells. FASEB J. 2012, 26, 1821–1830. [Google Scholar] [CrossRef][Green Version]
- Hessami, A.; Shamshirian, A.; Heydari, K.; Pourali, F.; Alizadeh-Navaei, R.; Moosazadeh, M.; Abrotan, S.; Shojaie, L.; Sedighi, S.; Shamshirian, D.; et al. Cardiovascular diseases burden in COVID-19: Systematic review and meta-analysis. Am. J. Emerg. Med. 2021, 46, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Malik, J.; Ahmed, S.; Shinde, M.; Almermesh, M.H.S.; Alghamdi, S.; Hussain, A.; Anwar, S. The Impact of COVID-19 on Comorbidities: A Review of Recent Updates for Combating It. Saudi J. Biol. Sci. 2022, 29, 3586–3599. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Xu, Y.; Wang, H.; Jia, Q.; Shou, X.; Zhang, X.; Zhang, N.; Li, Y.; Zhai, H.; Hu, Y. Bibliometric and visual analysis of cardiovascular diseases and COVID-19 research. Front. Public Health 2022, 10, 1022810. [Google Scholar] [CrossRef]
- Roshdy, A.; Zaher, S.; Fayed, H.; Coghlan, J.G. COVID-19 and the Heart: A Systematic Review of Cardiac Autopsies. Front. Cardiovasc. Med. 2020, 7, 626975. [Google Scholar] [CrossRef]
- Salazar, M.R. Is hypertension without any other comorbidities an independent predictor for COVID-19 severity and mortality? J. Clin. Hypertens. 2021, 23, 232–234. [Google Scholar] [CrossRef]
- Healy, E.F.; Lilic, M. A model for COVID-19-induced dysregulation of ACE2 shedding by ADAM17. Biochem. Biophys. Res. Commun. 2021, 573, 158–163. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef]
- Reddy, R.; Asante, I.; Liu, S.; Parikh, P.; Liebler, J.; Borok, Z.; Rodgers, K.; Baydur, A.; Louie, S.G. Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: A pilot study. PLoS ONE 2019, 14, e0213096. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Melo, M.B.; Motta-Santos, D.; Peluso, A.A.; Souza-Neto, F.; da Silva, R.F.; Almeida, J.F.Q.; Canta, G.; Reis, A.M.; Goncalves, G.; et al. Genetic deletion of the alamandine receptor MRGD leads to dilated cardiomyopathy in mice. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H123–H133. [Google Scholar] [CrossRef][Green Version]
- Qi, Y.F.; Zhang, J.; Wang, L.; Shenoy, V.; Krause, E.; Oh, S.P.; Pepine, C.J.; Katovich, M.J.; Raizada, M.K. Angiotensin-converting enzyme 2 inhibits high-mobility group box 1 and attenuates cardiac dysfunction post-myocardial ischemia. J. Mol. Med. 2016, 94, 37–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Qian, C.; Roks, A.J.; Westermann, D.; Schumacher, S.M.; Escher, F.; Schoemaker, R.G.; Reudelhuber, T.L.; van Gilst, W.H.; Schultheiss, H.P.; et al. Circulating rather than cardiac angiotensin-(1-7) stimulates cardioprotection after myocardial infarction. Circ. Heart Fail. 2010, 3, 286–293. [Google Scholar] [CrossRef][Green Version]
- Zhao, K.; Xu, T.; Mao, Y.; Wu, X.; Hua, D.; Sheng, Y.; Li, P. Alamandine alleviated heart failure and fibrosis in myocardial infarction mice. Biol. Direct 2022, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Zangrillo, A.; Landoni, G.; Beretta, L.; Morselli, F.; Serpa Neto, A.; Bellomo, R.; the COVID-BioB Study Group. Angiotensin II infusion in COVID-19-associated vasodilatory shock: A case series. Crit. Care 2020, 24, 227. [Google Scholar] [CrossRef] [PubMed]
- Alhenc-Gelas, F.; Drueke, T.B. Blockade of SARS-CoV-2 infection by recombinant soluble ACE2. Kidney Int. 2020, 97, 1091–1093. [Google Scholar] [CrossRef]
- Pang, X.; Cui, Y.; Zhu, Y. Recombinant human ACE2: Potential therapeutics of SARS-CoV-2 infection and its complication. Acta Pharmacol. Sin. 2020, 41, 1255–1257. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagunduz, P.; Tabak, F.; Atagunduz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Gudowska-Sawczuk, M.; Mroczko, B. The Role of Nuclear Factor Kappa B (NF-kappaB) in Development and Treatment of COVID-19: Review. Int. J. Mol. Sci. 2022, 23, 5283. [Google Scholar] [CrossRef]
- Gadanec, L.K.; McSweeney, K.R.; Qaradakhi, T.; Ali, B.; Zulli, A.; Apostolopoulos, V. Can SARS-CoV-2 virus use multiple receptors to enter host cells? Int. J. Mol. Sci. 2021, 22, 992. [Google Scholar] [CrossRef]
- Kosyreva, A.; Dzhalilova, D.; Lokhonina, A.; Vishnyakova, P.; Fatkhudinov, T. The Role of Macrophages in the Pathogenesis of SARS-CoV-2-Associated Acute Respiratory Distress Syndrome. Front. Immunol. 2021, 12, 682871. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, J.L.; Caruso-Neves, C.; Pinheiro, A.A. Angiotensin II type-1 receptor (AT(1)R) regulates expansion, differentiation, and functional capacity of antigen-specific CD8(+) T cells. Sci. Rep. 2016, 6, 35997. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Yancey, P.G.; Zuo, Y.; Ma, L.J.; Kaseda, R.; Fogo, A.B.; Ichikawa, I.; Linton, M.F.; Fazio, S.; Kon, V. Macrophage polarization by angiotensin II-type 1 receptor aggravates renal injury-acceleration of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2856–2864. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kate Gadanec, L.; Qaradakhi, T.; Renee McSweeney, K.; Ashiana Ali, B.; Zulli, A.; Apostolopoulos, V. Dual targeting of Toll-like receptor 4 and angiotensin-converting enzyme 2: A proposed approach to SARS-CoV-2 treatment. Future Microbiol. 2021, 16, 205–209. [Google Scholar] [CrossRef]
- Chen, X.S.; Wang, S.H.; Liu, C.Y.; Gao, Y.L.; Meng, X.L.; Wei, W.; Shou, S.T.; Liu, Y.C.; Chai, Y.F. Losartan attenuates sepsis-induced cardiomyopathy by regulating macrophage polarization via TLR4-mediated NF-kappaB and MAPK signaling. Pharmacol. Res. 2022, 185, 106473. [Google Scholar] [CrossRef]
- Mehrabadi, M.E.; Hemmati, R.; Tashakor, A.; Homaei, A.; Yousefzadeh, M.; Hemati, K.; Hosseinkhani, S. Induced dysregulation of ACE2 by SARS-CoV-2 plays a key role in COVID-19 severity. Biomed. Pharmacother. 2021, 137, 111363. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Heterogeneity of CD4+ memory T cells: Functional modules for tailored immunity. Eur. J. Immunol. 2009, 39, 2076–2082. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Lane, N.; Robins, R.A.; Corne, J.; Fairclough, L. Regulation in chronic obstructive pulmonary disease: The role of regulatory T-cells and Th17 cells. Clin. Sci. 2010, 119, 75–86. [Google Scholar] [CrossRef][Green Version]
- Almutlaq, M.; Mansour, F.A.; Alghamdi, J.; Alhendi, Y.; Alamro, A.A.; Alghamdi, A.A.; Alamri, H.S.; Alroqi, F.; Barhoumi, T. Angiotensin II Exaggerates SARS-CoV-2 Specific T-Cell Response in Convalescent Individuals following COVID-19. Int. J. Mol. Sci. 2022, 23, 8669. [Google Scholar] [CrossRef] [PubMed]
- Parackova, Z.; Bloomfield, M.; Klocperk, A.; Sediva, A. Neutrophils mediate Th17 promotion in COVID-19 patients. J. Leukoc. Biol. 2021, 109, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Itani, H.A.; McMaster, W.G., Jr.; Saleh, M.A.; Nazarewicz, R.R.; Mikolajczyk, T.P.; Kaszuba, A.M.; Konior, A.; Prejbisz, A.; Januszewicz, A.; Norlander, A.E.; et al. Activation of Human T Cells in Hypertension: Studies of Humanized Mice and Hypertensive Humans. Hypertension 2016, 68, 123–132. [Google Scholar] [CrossRef][Green Version]
- Schwartz, D.M.; Burma, A.M.; Kitakule, M.M.; Luo, Y.; Mehta, N.N. T Cells in Autoimmunity-Associated Cardiovascular Diseases. Front. Immunol. 2020, 11, 588776. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Behm, D.J.; Nerurkar, S.; Eybye, M.E.; Haimbach, R.E.; Olzinski, A.R.; Douglas, S.A.; Willette, R.N. p38 MAPK inhibitors ameliorate target organ damage in hypertension: Part 1. p38 MAPK-dependent endothelial dysfunction and hypertension. J. Pharmacol. Exp. Ther. 2003, 307, 932–938. [Google Scholar] [CrossRef][Green Version]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef][Green Version]
- Kuba, K.; Imai, Y.; Penninger, J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006, 6, 271–276. [Google Scholar] [CrossRef]
- Tan, W.S.D.; Liao, W.; Zhou, S.; Mei, D.; Wong, W.F. Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr. Opin. Pharmacol. 2018, 40, 9–17. [Google Scholar] [CrossRef]
- Rossi, G.A.; Sacco, O.; Mancino, E.; Cristiani, L.; Midulla, F. Differences and similarities between SARS-CoV and SARS-CoV-2: Spike receptor-binding domain recognition and host cell infection with support of cellular serine proteases. Infection 2020, 48, 665–669. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Patra, T.; Meyer, K.; Geerling, L.; Isbell, T.S.; Hoft, D.F.; Brien, J.; Pinto, A.K.; Ray, R.B.; Ray, R. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog. 2020, 16, e1009128. [Google Scholar] [CrossRef]
- Santa Cruz, A.; Mendes-Frias, A.; Oliveira, A.I.; Dias, L.; Matos, A.R.; Carvalho, A.; Capela, C.; Pedrosa, J.; Castro, A.G.; Silvestre, R. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front. Immunol. 2021, 12, 613422. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, R.; Zhang, C.; Ren, W.; Yu, A.; Zhou, X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit. Care 2020, 24, 290. [Google Scholar] [CrossRef] [PubMed]
- Osman, I.O.; Melenotte, C.; Brouqui, P.; Million, M.; Lagier, J.C.; Parola, P.; Stein, A.; La Scola, B.; Meddeb, L.; Mege, J.L.; et al. Expression of ACE2, Soluble ACE2, Angiotensin I, Angiotensin II and Angiotensin-(1-7) Is Modulated in COVID-19 Patients. Front. Immunol. 2021, 12, 625732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Camargo, R.L.; Bombassaro, B.; Monfort-Pires, M.; Mansour, E.; Palma, A.C.; Ribeiro, L.C.; Ulaf, R.G.; Bernardes, A.F.; Nunes, T.A.; Agrela, M.V.; et al. Plasma Angiotensin II Is Increased in Critical Coronavirus Disease 2019. Front. Cardiovasc. Med. 2022, 9, 847809. [Google Scholar] [CrossRef] [PubMed]
- Caputo, I.; Caroccia, B.; Frasson, I.; Poggio, E.; Zamberlan, S.; Morpurgo, M.; Seccia, T.M.; Cali, T.; Brini, M.; Richter, S.N.; et al. Angiotensin II Promotes SARS-CoV-2 Infection via Upregulation of ACE2 in Human Bronchial Cells. Int. J. Mol. Sci. 2022, 23, 5125. [Google Scholar] [CrossRef]
- Huertas, A.; Montani, D.; Savale, L.; Pichon, J.; Tu, L.; Parent, F.; Guignabert, C.; Humbert, M. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020, 56, 2001634. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Taylor, A.A.; Siragy, H.; Nesbitt, S. Angiotensin receptor blockers: Pharmacology, efficacy, and safety. J. Clin. Hypertens. 2011, 13, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Abraham, H.M.; White, C.M.; White, W.B. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015, 38, 33–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siragy, H.M. A current evaluation of the safety of angiotensin receptor blockers and direct renin inhibitors. Vasc. Health Risk Manag. 2011, 7, 297–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oparil, S.; Williams, D.; Chrysant, S.G.; Marbury, T.C.; Neutel, J. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J. Clin. Hypertens. 2001, 3, 283–291, 318. [Google Scholar] [CrossRef]
- Kassler-Taub, K.; Littlejohn, T.; Elliott, W.; Ruddy, T.; Adler, E. Comparative efficacy of two angiotensin II receptor antagonists, irbesartan and losartan in mild-to-moderate hypertension. Irbesartan/Losartan Study Investigators. Am. J. Hypertens. 1998, 11, 445–453. [Google Scholar] [CrossRef]
- Giles, T.D.; Oparil, S.; Silfani, T.N.; Wang, A.; Walker, J.F. Comparison of increasing doses of olmesartan medoxomil, losartan potassium, and valsartan in patients with essential hypertension. J. Clin. Hypertens. 2007, 9, 187–195. [Google Scholar] [CrossRef][Green Version]
- The ONTARGET Investigators; Yusuf, S.; Teo, K.K.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P.; Anderson, C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar] [CrossRef]
- Oparil, S.; Melino, M.; Lee, J.; Fernandez, V.; Heyrman, R. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: The TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin. Ther. 2010, 32, 1252–1269. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.F.; Emanuele, N.; Zhang, J.H.; Brophy, M.; Conner, T.A.; Duckworth, W.; Leehey, D.J.; McCullough, P.A.; O’Connor, T.; Palevsky, P.M.; et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 2013, 369, 1892–1903. [Google Scholar] [CrossRef][Green Version]
- Matsoukas, J.; Apostolopoulos, V.; Zulli, A.; Moore, G.; Kelaidonis, K.; Moschovou, K.; Mavromoustakos, T. From angiotensin II to cyclic peptides and angiotensin receptor blockers (ARBs): Perspectives of ARBs in COVID-19 therapy. Molecules 2021, 26, 618. [Google Scholar] [CrossRef]
- Ridgway, H.; Ntallis, C.; Chasapis, C.T.; Kelaidonis, K.; Matsoukas, M.-T.; Plotas, P.; Apostolopoulos, V.; Moore, G.; Tsiodras, S.; Paraskevis, D. Molecular Epidemiology of SARS-CoV-2: The Dominant Role of Arginine in Mutations and Infectivity. Viruses 2023, 15, 309. [Google Scholar] [CrossRef]
- Benicky, J.; Sanchez-Lemus, E.; Pavel, J.; Saavedra, J.M. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell. Mol. Neurobiol. 2009, 29, 781–792. [Google Scholar] [CrossRef][Green Version]
- Takagi, H.; Mizuno, Y.; Yamamoto, H.; Goto, S.N.; Umemoto, T. All-Literature Investigation of Cardiovascular Evidence G. Effects of telmisartan therapy on interleukin-6 and tumor necrosis factor-alpha levels: A meta-analysis of randomized controlled trials. Hypertens. Res. 2013, 36, 368–373. [Google Scholar] [CrossRef][Green Version]
- Ararat, E.; Brozovich, F.V. Losartan decreases p42/44 MAPK signaling and preserves LZ+ MYPT1 expression. PLoS ONE 2009, 4, e5144. [Google Scholar] [CrossRef][Green Version]
- Nejat, R.; Sadr, A.S. Are losartan and imatinib effective against SARS-CoV2 pathogenesis? A pathophysiologic-based in silico study. In Silico Pharmacol. 2021, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Gurwitz, D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020, 81, 537–540. [Google Scholar] [CrossRef][Green Version]
- Ma, J.; Shi, X.; Yu, J.; Lv, F.; Wu, J.; Sheng, X.; Pan, Q.; Yang, J.; Cao, H.; Li, L. Association of ACEi/ARB Use and Clinical Outcomes of COVID-19 Patients With Hypertension. Front. Cardiovasc. Med. 2021, 8, 577398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.J.; Xie, J.; Liu, Y.M.; Zhao, Y.C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Genet, B.; Vidal, J.S.; Cohen, A.; Boully, C.; Beunardeau, M.; Marine Harle, L.; Goncalves, A.; Boudali, Y.; Hernandorena, I.; Bailly, H.; et al. COVID-19 In-Hospital Mortality and Use of Renin-Angiotensin System Blockers in Geriatrics Patients. J. Am. Med. Dir. Assoc. 2020, 21, 1539–1545. [Google Scholar] [CrossRef]
- Soleimani, A.; Kazemian, S.; Karbalai Saleh, S.; Aminorroaya, A.; Shajari, Z.; Hadadi, A.; Talebpour, M.; Sadeghian, H.; Payandemehr, P.; Sotoodehnia, M.; et al. Effects of Angiotensin Receptor Blockers (ARBs) on In-Hospital Outcomes of Patients With Hypertension and Confirmed or Clinically Suspected COVID-19. Am. J. Hypertens. 2020, 33, 1102–1111. [Google Scholar] [CrossRef]
- Meng, J.; Xiao, G.; Zhang, J.; He, X.; Ou, M.; Bi, J.; Yang, R.; Di, W.; Wang, Z.; Li, Z.; et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020, 9, 757–760. [Google Scholar] [CrossRef]
- Xie, Q.; Tang, S.; Li, Y. The divergent protective effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on clinical outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Ann. Palliat. Med. 2022, 11, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Rothlin, R.P.; Duarte, M.; Pelorosso, F.G.; Nicolosi, L.; Salgado, M.V.; Vetulli, H.M.; Spitzer, E. Angiotensin Receptor Blockers for COVID-19: Pathophysiological and Pharmacological Considerations About Ongoing and Future Prospective Clinical Trials. Front. Pharmacol. 2021, 12, 603736. [Google Scholar] [CrossRef] [PubMed]
- Rothlin, R.P.; Vetulli, H.M.; Duarte, M.; Pelorosso, F.G. Telmisartan as tentative angiotensin receptor blocker therapeutic for COVID-19. Drug Dev. Res. 2020, 81, 768–770. [Google Scholar] [CrossRef]
- Lukito, A.A.; Widysanto, A.; Lemuel, T.A.Y.; Prasetya, I.B.; Massie, B.; Yuniarti, M.; Lumbuun, N.; Pranata, R.; Meidy, C.; Wahjoepramono, E.J.; et al. Candesartan as a tentative treatment for COVID-19: A prospective non-randomized open-label study. Int. J. Infect. Dis. 2021, 108, 159–166. [Google Scholar] [CrossRef]
- Duarte, M.; Pelorosso, F.; Nicolosi, L.N.; Salgado, M.V.; Vetulli, H.; Aquieri, A.; Azzato, F.; Castro, M.; Coyle, J.; Davolos, I.; et al. Telmisartan for treatment of Covid-19 patients: An open multicenter randomized clinical trial. eClinicalMedicine 2021, 37, 100962. [Google Scholar] [CrossRef]
- Kakuta, H.; Sudoh, K.; Sasamata, M.; Yamagishi, S. Telmisartan has the strongest binding affinity to angiotensin II type 1 receptor: Comparison with other angiotensin II type 1 receptor blockers. Int. J. Clin. Pharmacol. Res. 2005, 25, 41–46. [Google Scholar] [PubMed]
- Michel, M.C.; Foster, C.; Brunner, H.R.; Liu, L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol. Rev. 2013, 65, 809–848. [Google Scholar] [CrossRef][Green Version]
- Elkahloun, A.G.; Saavedra, J.M. Candesartan could ameliorate the COVID-19 cytokine storm. Biomed. Pharmacother. 2020, 131, 110653. [Google Scholar] [CrossRef]
- Liu, D.; Wu, P.; Gu, W.; Yang, C.; Yang, X.; Deng, X.; Xu, J.; Jiang, J.; Jiang, C. Potential of angiotensin II receptor blocker telmisartan in reducing mortality among hospitalized patients with COVID-19 compared with recommended drugs. Cell Discov. 2022, 8, 91. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Young, D.; Coupland, C.; Channon, K.M.; Tan, P.S.; Harrison, D.A.; Rowan, K.; Aveyard, P.; Pavord, I.D.; Watkinson, P.J. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart 2020, 106, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Verdecchia, P.; Balestrino, A.; Bruschi, C.; Ceriana, P.; Chiovato, L.; Dalla Vecchia, L.A.; Fanfulla, F.; La Rovere, M.T.; Perego, F.; et al. Renin Angiotensin System Blockers and Risk of Mortality in Hypertensive Patients Hospitalized for COVID-19: An Italian Registry. J. Cardiovasc. Dev. Dis. 2022, 9, 15. [Google Scholar] [CrossRef]
- Cremer, S.; Pilgram, L.; Berkowitsch, A.; Stecher, M.; Rieg, S.; Shumliakivska, M.; Bojkova, D.; Wagner, J.U.G.; Aslan, G.S.; Spinner, C.; et al. Angiotensin II receptor blocker intake associates with reduced markers of inflammatory activation and decreased mortality in patients with cardiovascular comorbidities and COVID-19 disease. PLoS ONE 2021, 16, e0258684. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Mancone, M.; De Ferrari, G.M.; Forleo, G.; Secco, G.G.; Ruocco, G.M.; D’Ascenzo, F.; Monticone, S.; Paggi, A.; Vicenzi, M.; et al. Antecedent Administration of Angiotensin-Converting Enzyme Inhibitors or Angiotensin II Receptor Antagonists and Survival After Hospitalization for COVID-19 Syndrome. J. Am. Heart Assoc. 2020, 9, e017364. [Google Scholar] [CrossRef] [PubMed]
- Koka, V.; Huang, X.R.; Chung, A.C.; Wang, W.; Truong, L.D.; Lan, H.Y. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am. J. Pathol. 2008, 172, 1174–1183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burrell, L.M.; Risvanis, J.; Kubota, E.; Dean, R.G.; MacDonald, P.S.; Lu, S.; Tikellis, C.; Grant, S.L.; Lew, R.A.; Smith, A.I.; et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005, 26, 369–375, discussion 322–364. [Google Scholar] [CrossRef][Green Version]
- Ishiyama, Y.; Gallagher, P.E.; Averill, D.B.; Tallant, E.A.; Brosnihan, K.B.; Ferrario, C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 2004, 43, 970–976. [Google Scholar] [CrossRef][Green Version]
- Pires de Souza, G.A.; Osman, I.O.; Le Bideau, M.; Baudoin, J.P.; Jaafar, R.; Devaux, C.; La Scola, B. Angiotensin II Receptor Blockers (ARBs Antihypertensive Agents) Increase Replication of SARS-CoV-2 in Vero E6 Cells. Front. Cell. Infect. Microbiol. 2021, 11, 639177. [Google Scholar] [CrossRef]
- Essaidi-Laziosi, M.; Perez Rodriguez, F.J.; Hulo, N.; Jacquerioz, F.; Kaiser, L.; Eckerle, I. Estimating clinical SARS-CoV-2 infectiousness in Vero E6 and primary airway epithelial cells. Lancet Microbe 2021, 2, e571. [Google Scholar] [CrossRef]
- Matsoukas, J.M.; Gadanec, L.K.; Zulli, A.; Apostolopoulos, V.; Kelaidonis, K.; Ligielli, I.; Moschovou, K.; Georgiou, N.; Plotas, P.; Chasapis, C.T. Diminazene aceturate reduces angiotensin II constriction and interacts with the spike protein of severe acute respiratory syndrome coronavirus 2. Biomedicines 2022, 10, 1731. [Google Scholar] [CrossRef]
- Qaradakhi, T.; Gadanec, L.; Matsoukas, J.; Apostolopoulos, V.; Zulli, A. Could DIZE be the answer to COVID-19? Maturitas 2020, 140, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Rico-Mesa, J.S.; White, A.; Anderson, A.S. Outcomes in Patients with COVID-19 Infection Taking ACEI/ARB. Curr. Cardiol. Rep. 2020, 22, 31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ridgway, H.; Moore, G.J.; Mavromoustakos, T.; Tsiodras, S.; Ligielli, I.; Kelaidonis, K.; Chasapis, C.T.; Gadanec, L.K.; Zulli, A.; Apostolopoulos, V.; et al. Discovery of a new generation of angiotensin receptor blocking drugs: Receptor mechanisms and in silico binding to enzymes relevant to SARS-CoV-2. Comput. Struct. Biotechnol. J. 2022, 20, 2091–2111. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.J.; Pires, J.M.; Kelaidonis, K.; Gadanec, L.K.; Zulli, A.; Apostolopoulos, V.; Matsoukas, J.M. Receptor Interactions of Angiotensin II and Angiotensin Receptor Blockers-Relevance to COVID-19. Biomolecules 2021, 11, 979c. [Google Scholar] [CrossRef]
- Takezako, T.; Unal, H.; Karnik, S.S.; Node, K. Current topics in angiotensin II type 1 receptor research: Focus on inverse agonism, receptor dimerization and biased agonism. Pharmacol. Res. 2017, 123, 40–50. [Google Scholar] [CrossRef]
- Moore, G.J. Designing peptide mimetics. Trends Pharmacol. Sci. 1994, 15, 124–129. [Google Scholar] [CrossRef]
- Moore, G.J.; Ridgway, H.; Kelaidonis, K.; Chasapis, C.T.; Ligielli, I.; Mavromoustakos, T.; Bojarska, J.; Matsoukas, J.M. Actions of Novel Angiotensin Receptor Blocking Drugs, Bisartans, Relevant for COVID-19 Therapy: Biased Agonism at Angiotensin Receptors and the Beneficial Effects of Neprilysin in the Renin Angiotensin System. Molecules 2022, 27, 4854. [Google Scholar] [CrossRef]
- Agelis, G.; Resvani, A.; Koukoulitsa, C.; Tumova, T.; Slaninova, J.; Kalavrizioti, D.; Spyridaki, K.; Afantitis, A.; Melagraki, G.; Siafaka, A.; et al. Rational design, efficient syntheses and biological evaluation of N,N′-symmetrically bis-substituted butylimidazole analogs as a new class of potent Angiotensin II receptor blockers. Eur. J. Med. Chem. 2013, 62, 352–370. [Google Scholar] [CrossRef][Green Version]

| Subject | ARB/Dose | Outcome | Ref |
|---|---|---|---|
| 103 patients with hypertension and COVID-19 receiving ARB therapy | NA | Pre-administered ARBs reduced the need for mechanical ventilation, intensive care admission, and death. | [149] |
| 157 patients diagnosed with COVID-19 and hypertension on ARBs | NA | ARB group is associated with decreased mortality. | [150] |
| 201 hospitalized patients diagnosed with COVID019 | NA | Pre-administered ARB had a lower mortality rate compared to other antihypertensive medications. | [151] |
| 636 COVID-19 patients,1 of which 22 receiving ARBs | NA | Discontinuing ARB therapy during COVID-19 infection resulted in greater mortality, ventilation, and increased risk of acute kidney injury. | [152] |
| 19 586 patients with COVID-19 | NA | ARBs associated with reduced risk of COVID-19 in patients with hypertension. | [163] |
| 566 hypertensive patients with COVID-19, 147 on ARBs. | NA | ARB therapy resulted in a lower risk of mortality in patients with a high prognostic factor, low oxygen saturation, and high lymphocyte count than other RAS inhibitors. | [164] |
| 63,969 hospitalized participants with COVID-19 | Telmisartan | Telmisartan showed a reduction in mortality risks greater than standard care. | [162] |
| 52 COVID-19-diagnosed patients not receiving antihypertensive medication | Telmisartan 160 mg/day, 14 days | Telmisartan reduced morbidity and mortality of COVID-19 patients through anti-inflammatory effects. | [158] |
| 1946 patients with COVID019 and cardiovascular comorbidities of which 493 on ARB | NA | ARBs reduced mortality, leukocyte count, inflammatory markers, and IL-6. No changes in ACE2 expression were observed. | [165] |
| 178 patients with COVID-19 of which 133 used ARBs. | NA | ARBs reduced mortality in patients hospitalized with COVID-19. | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swiderski, J.; Gadanec, L.K.; Apostolopoulos, V.; Moore, G.J.; Kelaidonis, K.; Matsoukas, J.M.; Zulli, A. Role of Angiotensin II in Cardiovascular Diseases: Introducing Bisartans as a Novel Therapy for Coronavirus 2019. Biomolecules 2023, 13, 787. https://doi.org/10.3390/biom13050787
Swiderski J, Gadanec LK, Apostolopoulos V, Moore GJ, Kelaidonis K, Matsoukas JM, Zulli A. Role of Angiotensin II in Cardiovascular Diseases: Introducing Bisartans as a Novel Therapy for Coronavirus 2019. Biomolecules. 2023; 13(5):787. https://doi.org/10.3390/biom13050787
Chicago/Turabian StyleSwiderski, Jordan, Laura Kate Gadanec, Vasso Apostolopoulos, Graham J. Moore, Konstantinos Kelaidonis, John M. Matsoukas, and Anthony Zulli. 2023. "Role of Angiotensin II in Cardiovascular Diseases: Introducing Bisartans as a Novel Therapy for Coronavirus 2019" Biomolecules 13, no. 5: 787. https://doi.org/10.3390/biom13050787
APA StyleSwiderski, J., Gadanec, L. K., Apostolopoulos, V., Moore, G. J., Kelaidonis, K., Matsoukas, J. M., & Zulli, A. (2023). Role of Angiotensin II in Cardiovascular Diseases: Introducing Bisartans as a Novel Therapy for Coronavirus 2019. Biomolecules, 13(5), 787. https://doi.org/10.3390/biom13050787








