Dupilumab Leads to Clinical Improvements including the Acquisition of Tolerance to Causative Foods in Non-Eosinophilic Esophagitis Eosinophilic Gastrointestinal Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
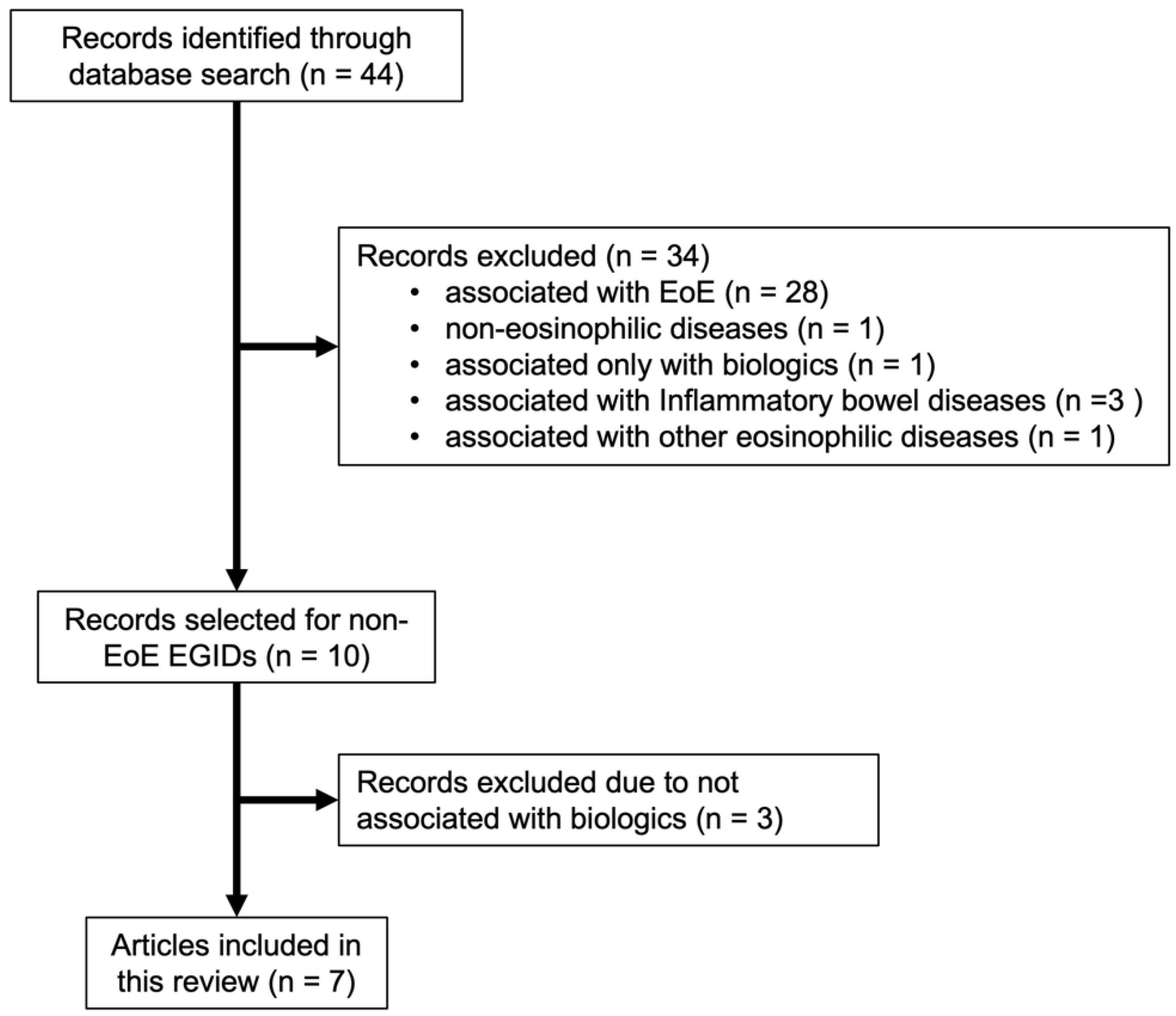
2.2. Literature Review of Biologics Use for Non-EoE EGIDs
3. Results
3.1. Literature Review
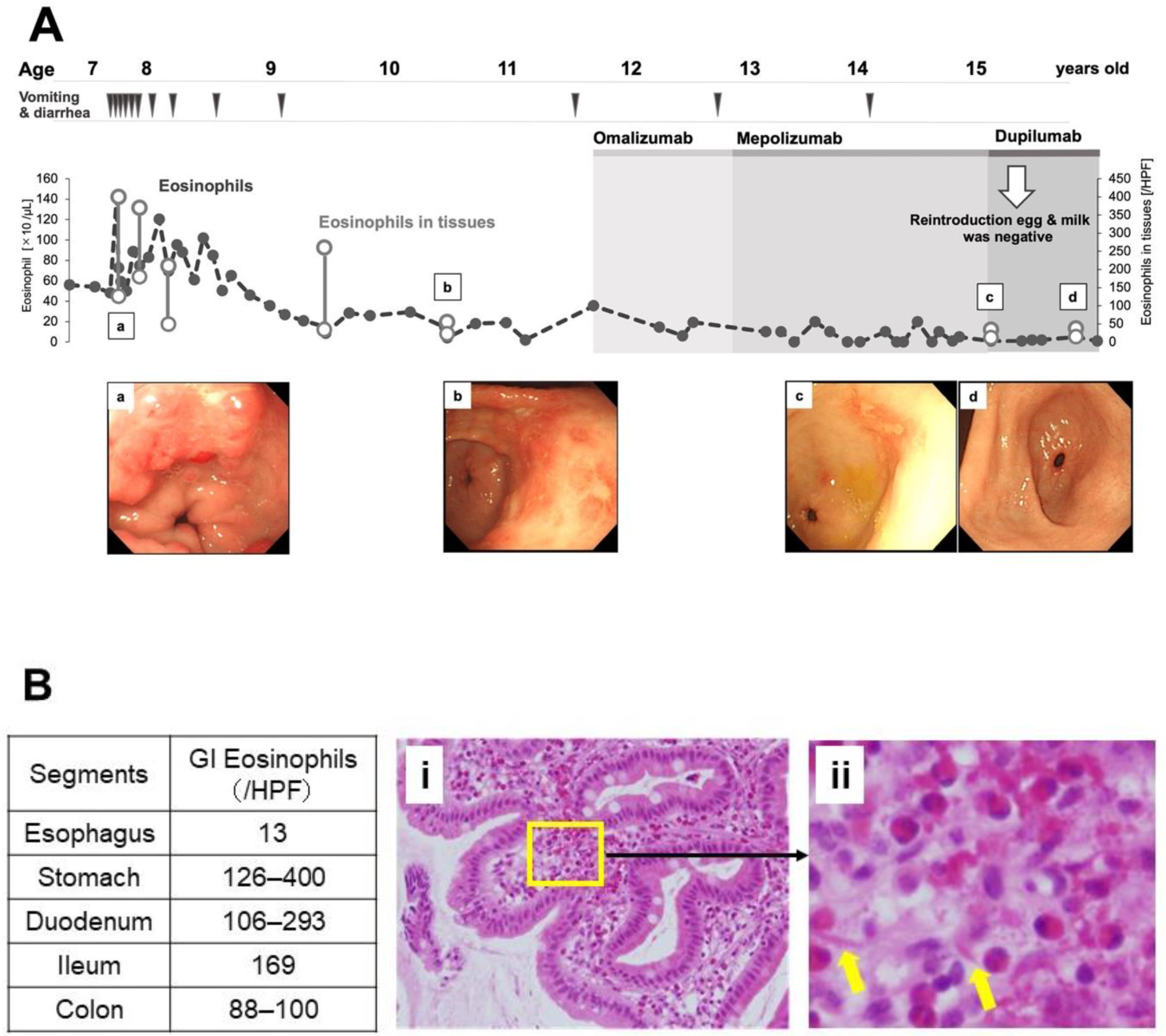
3.2. Dupilumab Led to the Acquisition of Tolerance to Causative Foods in Non-EoE EGID
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinoshita, Y.; Oouchi, S.; Fujisawa, T. Eosinophilic gastrointestinal diseases—Pathogenesis, diagnosis, and treatment. Allergol. Int. 2019, 68, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Gonsalves, N.; Abonia, J.P.; Alexander, J.A.; Arva, N.C.; Atkins, D.; Attwood, S.E.; Auth, M.K.H.; Bailey, D.D.; Biederman, L.; et al. International Consensus Recommendations for Eosinophilic Gastrointestinal Disease Nomenclature. Clin. Gastroenterol. Hepatol. 2022, 20, 2474–2484.e3. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.T.; Martin, C.F.; Kappelman, M.D.; Dellon, E.S. Prevalence of Eosinophilic Gastritis, Gastroenteritis, and Colitis: Estimates From a National Administrative Database. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, Y.; Furuta, K.; Ishimaura, N.; Ishihara, S.; Sato, S.; Maruyama, R.; Ohara, S.; Matsumoto, T.; Sakamoto, C.; Matsui, T.; et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J. Gastroenterol. 2013, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nagashima, S.; Yamada, Y.; Murakoshi, T.; Shimoyama, Y.; Takahashi, S.; Seki, H.; Kobayashi, T.; Hara, Y.; Tadaki, H.; et al. Comparison of Nonesophageal Eosinophilic Gastrointestinal Disorders with Eosinophilic Esophagitis: A Nationwide Survey. J. Allergy Clin. Immunol. Pract. 2021, 9, 3339–3349.e3338. [Google Scholar] [CrossRef] [PubMed]
- Pineton de Chambrun, G.; Gonzalez, F.; Canva, J.Y.; Gonzalez, S.; Houssin, L.; Desreumaux, P.; Cortot, A.; Colombel, J.F. Natural history of eosinophilic gastroenteritis. Clin. Gastroenterol. Hepatol. 2011, 9, 950–956.e951. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, E.; Ishimura, N.; Okada, M.; Mikami, H.; Sonoyama, H.; Ishikawa, N.; Araki, A.; Oshima, N.; Hirai, J.; Ishihara, S.; et al. Successful Food-Elimination Diet in an Adult with Eosinophilic Gastroenteritis. ACG Case Rep. J. 2018, 5, e38. [Google Scholar] [CrossRef]
- Yamada, Y.; Kato, M.; Isoda, Y.; Nishi, A.; Jinbo, Y.; Hayashi, Y. Eosinophilic gastroenteritis treated with a multiple-food elimination diet. Allergol. Int. 2014, 63 (Suppl. S1), 53–56. [Google Scholar] [CrossRef] [Green Version]
- Justinich, C.; Katz, A.; Gurbindo, C.; Lepage, G.; Chad, Z.; Bouthillier, L.; Seidman, E. Elemental diet improves steroid-dependent eosinophilic gastroenteritis and reverses growth failure. J. Pediatr. Gastroenterol. Nutr. 1996, 23, 81–85. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Serrano-Montalban, B.; Arias, A.; Redondo, O.; Tenias, J.M. Efficacy of Dietary Treatment for Inducing Disease Remission in Eosinophilic Gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 56–64. [Google Scholar] [CrossRef]
- Friesen, C.A.; Kearns, G.L.; Andre, L.; Neustrom, M.; Roberts, C.C.; Abdel-Rahman, S.M. Clinical efficacy and pharmacokinetics of montelukast in dyspeptic children with duodenal eosinophilia. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.R.; Bochner, B.S.; Peters, A.T. Mepolizumab use: Post-approval academic practice experience. Ann. Allergy Asthma Immunol. 2018, 121, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Lee, J.K. Severe asthma with eosinophilic gastroenteritis effectively managed by mepolizumab and omalizumab. Ann. Allergy Asthma Immunol. 2018, 121, 742–743. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Spergel, J.M. Biologics in eosinophilic gastrointestinal diseases. Ann. Allergy Asthma Immunol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, S.; Foster, B.; Kim, N.; Bernardino, L.B.; Scott, L.M.; Hamilton, R.G.; Metcalfe, D.D.; Mannon, P.J.; Prussin, C. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J. Allergy Clin. Immunol. 2007, 120, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.; Goyal, A.; Thaker, A.; Troendle, D.; Parrish, C. A Case Series on the Use of Dupilumab for Treatment of Refractory Eosinophilic Gastrointestinal Disorders. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 192–195. [Google Scholar] [CrossRef]
- Dellon, E.S.; Peterson, K.A.; Murray, J.A.; Falk, G.W.; Gonsalves, N.; Chehade, M.; Genta, R.M.; Leung, J.; Khoury, P.; Klion, A.D.; et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N. Engl. J. Med. 2020, 383, 1624–1634. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Lopez-Sanchez, P. Targeted Therapies for Eosinophilic Gastrointestinal Disorders. BioDrugs 2020, 34, 477–493. [Google Scholar] [CrossRef]
- Kim, E.M.; Randall, C.; Betancourt, R.; Keene, S.; Lilly, A.; Fowler, M.; Dellon, E.S.; Herfarth, H.H. Mucosal Eosinophilia Is an Independent Predictor of Vedolizumab Efficacy in Inflammatory Bowel Diseases. Inflamm. Bowel. Dis. 2020, 26, 1232–1238. [Google Scholar] [CrossRef]
- Grandinetti, T.; Biedermann, L.; Bussmann, C.; Straumann, A.; Hruz, P. Eosinophilic Gastroenteritis: Clinical Manifestation, Natural Course, and Evaluation of Treatment with Corticosteroids and Vedolizumab. Dig. Dis. Sci. 2019, 64, 2231–2241. [Google Scholar] [CrossRef]
- Kim, H.P.; Reed, C.C.; Herfarth, H.H.; Dellon, E.S. Vedolizumab Treatment May Reduce Steroid Burden and Improve Histology in Patients with Eosinophilic Gastroenteritis. Clin. Gastroenterol. Hepatol. 2018, 16, 1992–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakawa, H.; Adachi, Y.; Ebisawa, M.; Fujisawa, T.; Committee for Japanese Pediatric Guideline for Childhood Asthma; The Japanese Society of Pediatric Allergy and Clinical Immunology; The Japanese Society of Allergology. Japanese guidelines for childhood asthma 2020. Allergol. Int. 2020, 69, 314–330. [Google Scholar] [CrossRef]
- Eosinophilic Gastrointestinal Disorder Research Group of the the Ministry of Health, Labour and Welfare of Japan. Japanese Clinical Practice Guidelines for Eosinophilic Gastrointestinal Disorders in Preschool Children to Adults. Available online: https://minds.jcqhc.or.jp/n/med/4/med0445/G0001228 (accessed on 19 August 2022).
- Kim, Y.J.; Prussin, C.; Martin, B.; Law, M.A.; Haverty, T.P.; Nutman, T.B.; Klion, A.D. Rebound eosinophilia after treatment of hypereosinophilic syndrome and eosinophilic gastroenteritis with monoclonal anti-IL-5 antibody SCH55700. J. Allergy Clin. Immunol. 2004, 114, 1449–1455. [Google Scholar] [CrossRef]
- Kuang, F.L.; De Melo, M.S.; Makiya, M.; Kumar, S.; Brown, T.; Wetzler, L.; Ware, J.M.; Khoury, P.; Collins, M.H.; Quezado, M.; et al. Benralizumab Completely Depletes Gastrointestinal Tissue Eosinophils and Improves Symptoms in Eosinophilic Gastrointestinal Disease. J. Allergy Clin. Immunol. Pract. 2022, 10, 1598–1605.e1592. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kephart, G.M.; Talley, N.J.; Wagner, J.M.; Sarr, M.G.; Bonno, M.; McGovern, T.W.; Gleich, G.J. Eosinophil infiltration and degranulation in normal human tissue. Anat. Rec. 1998, 252, 418–425. [Google Scholar] [CrossRef]
- Assa’ad, A.H.; Gupta, S.K.; Collins, M.H.; Thomson, M.; Heath, A.T.; Smith, D.A.; Perschy, T.L.; Jurgensen, C.H.; Ortega, H.G.; Aceves, S.S. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 2011, 141, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Conus, S.; Grzonka, P.; Kita, H.; Kephart, G.; Bussmann, C.; Beglinger, C.; Smith, D.A.; Patel, J.; Byrne, M.; et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: A randomised, placebo-controlled, double-blind trial. Gut 2010, 59, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.A. FDA Approves First Treatment for Eosinophilic Esophagitis, a Chronic Immune Disorder. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-eosinophilic-esophagitis-chronic-immune-disorder (accessed on 29 August 2022).
- Yamada, Y. Unique features of non-IgE-mediated gastrointestinal food allergy during infancy in Japan. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 299–304. [Google Scholar] [CrossRef]
- Caldwell, J.M.; Collins, M.H.; Stucke, E.M.; Putnam, P.E.; Franciosi, J.P.; Kushner, J.P.; Abonia, J.P.; Rothenberg, M.E. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J. Allergy Clin. Immunol. 2014, 134, 1114–1124. [Google Scholar] [CrossRef] [Green Version]
- Spekhorst, L.S.; van der Rijst, L.P.; de Graaf, M.; van Megen, M.; Zuithoff, N.P.A.; Knulst, A.C.; de Bruin-Weller, M.S.; Le, T.M. Dupilumab has a profound effect on specific-IgE levels of several food allergens in atopic dermatitis patients. Allergy, 2022; ahead of print. [Google Scholar] [CrossRef]
- Zdanowicz, K.; Kucharska, M.; Reszec, J.; Lebensztejn, D.M.; Daniluk, U. Immunohistochemical markers for eosinophilic esophagitis. Scand J. Gastroenterol. 2020, 55, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
| Search Number | Query | Results |
|---|---|---|
| #1 | ((“Eosinophilic enteropathy”[TW] OR “Eosinophilic gastrointestinal disorders”[TW] OR “Eosinophilic gastroenteritis”[TW] OR “Eosinophilic enteritis”[TW] OR “Eosinophilic duodenitis”[TW] OR “Eosinophilic colitis”[TW] OR “Gastrointestinal eosinophilia”[TW] OR “Intestinal eosinophilia”[TW] OR “Gastric eosinophilia”[TW] OR “Colonic eosinophilia”[TW] OR “Intestinal eosinophil infiltration”[TW] OR “Gastric eosinophil infiltration”[TW]) OR (“Eosinophilic enteropathy”[Supplementary Concept]) OR ((eosinophilia[Mesh]) AND (Gastroenteritis[MH] OR “Intestinal Diseases”[MH] OR “Stomach Diseases”[MH]))) AND “humans”[MeSH Terms] AND (“child”[MeSH Terms] OR “adolescent”[MeSH Terms] OR “adult”[MeSH Terms]) AND 1970[PDAT]: 2022[PDAT] AND (English[LA]) AND (“Treatment Outcome”[MH] OR “therapeutics”[MeSH Terms] OR “therapy”[Subheading]) | 1337 |
| #2 | mepolizumab OR benralizumab OR omalizumab OR reslizumab OR dupilumab OR lirentelimab OR vedolizumab OR cendakimab | 7604 |
| #3 | #1 AND #2 | 44 |
| Articles | Biologics | Study Population | Age Group | Histological Change | Symptomatic Improvement | Segments | Study Design | Year |
|---|---|---|---|---|---|---|---|---|
| Dellon et al. [17] | Lirentelimab | n = 65 | 18–80 | change in GI Eos † (mean): placebo (−9%), active drug (−86%) 1 | change in total symptom score †: placebo (−22%), active drug (−48%) 1 | EoG | randomized, double-blind, placebo-control study | 2020 |
| Foroughi et al. [15] | Omalizumab | n = 9 | 12–76 | change in GI Eos † (mean): gastric antrum (−69%) and duodenum (−59%) | change in total symptom score † (−70%) 1 | EoG, EoD | single-center open-label study | 2007 |
| Patel et al. [16] | Dupilumab | n = 3 2 | 7, 14, and 9 | change in GI Eos †: stomach (−88%) (n = 2), duodenum (−81%), and jejunum (−15%) (n = 1) | symptomatic improvement (3/3 patients) | EoG, EoD, EoJ | case reports | 2022 |
| Grandinetti et al. [20] | Vedolizumab | n = 4 3 | 13–78 | change in GI Eos † (−66%) (n = 4) 4 | symptomatic improvement 3/4 patients 5 | EoD + EoEEGEEoCEoE + EoN + EoC | retrospective cohort analysis | 2019 |
| Kim et al. [21] | Vedolizumab | n = 4 3 | 22.7–53.7 | changes in GI Eos †: stomach (n = 0), duodenum (−100%) (n = 2), and jejunum (−29%) (n = 1) | symptomatic improvement 3/5 patients | EoG + EGE EoE + EoG + EoN (n = 2) EoG + EoN | retrospective study | 2018 |
| Benjamin et al. [12] | Mepolizumab | n = 2 | 29.5 (mean) | change in GI Eos: stomach (−100% and 125%) (increased) (n = 2) | clinical improvement (overall) 2/2 patients | EoE + EGE (n = 2) | retrospective study | 2018 |
| Han and Lee [13] | Mepolizumab(+ omalizumab) 6 | n = 1 | 67 | no evaluation after treatment | clinical improvement: mepolizumab alone | EoE + EGE | case report | 2018 |
| Arakawa et al. (our case) 7 | Omalizumab, mepolizumab and dupilumab | n = 1 | 15 | maintenance of historical remission: all three drugs reduced GI Eos: omalizumab and mepolizumab endoscopic improvement: dupilumab | maintenance of clinical remission: all three drugs acquisition of tolerance to causative foods: dupilumab | EoG + EGE + EoC | this study | 202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arakawa, N.; Yagi, H.; Shimizu, M.; Shigeta, D.; Shimizu, A.; Nomura, S.; Takizawa, T.; Yamada, Y. Dupilumab Leads to Clinical Improvements including the Acquisition of Tolerance to Causative Foods in Non-Eosinophilic Esophagitis Eosinophilic Gastrointestinal Disorders. Biomolecules 2023, 13, 112. https://doi.org/10.3390/biom13010112
Arakawa N, Yagi H, Shimizu M, Shigeta D, Shimizu A, Nomura S, Takizawa T, Yamada Y. Dupilumab Leads to Clinical Improvements including the Acquisition of Tolerance to Causative Foods in Non-Eosinophilic Esophagitis Eosinophilic Gastrointestinal Disorders. Biomolecules. 2023; 13(1):112. https://doi.org/10.3390/biom13010112
Chicago/Turabian StyleArakawa, Naoya, Hisako Yagi, Mariko Shimizu, Daisuke Shigeta, Akihiko Shimizu, Shigeru Nomura, Takumi Takizawa, and Yoshiyuki Yamada. 2023. "Dupilumab Leads to Clinical Improvements including the Acquisition of Tolerance to Causative Foods in Non-Eosinophilic Esophagitis Eosinophilic Gastrointestinal Disorders" Biomolecules 13, no. 1: 112. https://doi.org/10.3390/biom13010112





