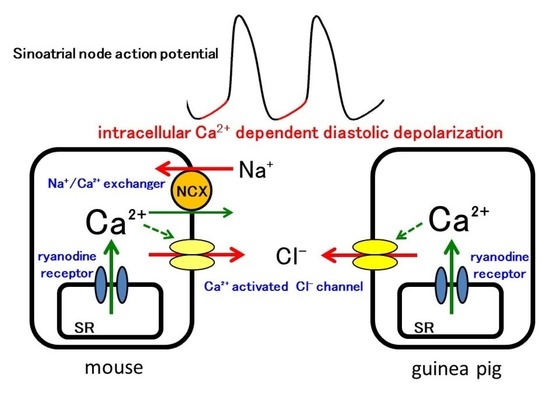
Intracellular Ca2+-Mediated Mechanisms for the Pacemaker Depolarization of the Mouse and Guinea Pig Sinus Node Tissue
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiFrancesco, D. The role of the funny current in pacemaker activity. Circ. Res. 2010, 106, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Komikado, C.; Namekata, I.; Nakamura, H.; Suzuki, M.; Tsuneoka, Y.; Shigenobu, K.; Takahara, A. Species difference in the contribution of T-type calcium current to cardiac pacemaking as revealed by R(−)-efonidipine. J. Pharmacol. Sci. 2008, 107, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Namekata, I.; Ogawa, T.; Tsuneoka, Y.; Komikado, C.; Takahara, A.; Iida-Tanaka, N.; Izumi-Nakaseko, H.; Tsuru, H.; Adachi-Akahane, S. Effects of S(+)-efonidipine on the rabbit sinus node action potential and calcium channel subunits CaV 1.2, CaV 1.3 and CaV 3.1. Eur. J. Pharmacol. 2010, 649, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Himeno, Y.; Toyoda, F.; Satoh, H.; Amano, A.; Cha, C.Y.; Matsuura, H.; Noma, A. Minor contribution of cytosolic Ca2+ transients to the pacemaker rhythm in guinea pig sinoatrial node cells. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, F.; Ding, W.G.; Matsuura, H. Heterogeneous functional expression of the sustained inward Na+ current in guinea pig sinoatrial node cells. Pflug. Arch. 2018, 470, 481–490. [Google Scholar] [CrossRef]
- Satoh, H. Sino-atrial nodal cells of mammalian hearts: Ionic currents and gene expression of pacemaker ionic channels. J. Smooth Muscle Res. 2003, 39, 175–193. [Google Scholar] [CrossRef]
- Ono, K.; Iijima, T. Cardiac T-type Ca2+ channels in the heart. J. Mol. Cell. Cardiol. 2010, 48, 65–70. [Google Scholar] [CrossRef]
- Maltsev, V.A.; Vinogradova, T.M.; Lakatta, E.G. The emergence of a general theory of the initiation and strength of the heartbeat. J. Pharmacol. Sci. 2006, 100, 338–369. [Google Scholar] [CrossRef]
- Sirenko, S.G.; Yang, D.; Maltseva, L.A.; Kim, M.S.; Lakatta, E.G.; Maltsev, V.A. Spontaneous, local diastolic subsarcolemmal calcium releases in single, isolated guinea-pig sinoatrial nodal cells. PLoS ONE 2017, 12, e0185222. [Google Scholar] [CrossRef]
- Sanders, L.; Rakovic, S.; Lowe, M.; Mattick, P.A.; Terrar, D.A. Fundamental importance of Na+-Ca2+ exchange for the pacemaking mechanism in guinea-pig sino-atrial node. J. Physiol. 2006, 571, 639–649. [Google Scholar] [CrossRef]
- Mangoni, M.E.; Nargeot, J. Properties of the hyperpolarization-activated current (If) in isolated mouse sino-atrial cells. Cardiovasc. Res. 2001, 52, 51–64. [Google Scholar] [CrossRef]
- Tanaka, H.; Nishimaru, K.; Aikawa, T.; Hirayama, W.; Tanaka, Y.; Shigenobu, K. Effect of SEA0400, a novel inhibitor of sodium-calcium exchanger, on myocardial ionic currents. Br. J. Pharmacol. 2002, 135, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Namekata, I.; Takeda, K.; Kazama, A.; Shimizu, Y.; Moriwaki, R.; Hirayama, W.; Sato, A.; Kawanishi, T.; Shigenobu, K. Unique excitation-contraction characteristics of mouse myocardium as revealed by SEA0400, a specific inhibitor of Na+-Ca2+ exchanger. Naunyn Schmiedebergs Arch. Pharmacol. 2005, 371, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Shimada, H.; Namekata, I.; Kawanishi, T.; Iida-Tanaka, N.; Shigenobu, K. Involvement of the Na+/Ca2+ exchanger in ouabain-induced inotropy and arrhythmogenesis in guinea-pig myocardium as revealed by SEA0400. J. Pharmacol. Sci. 2007, 103, 241–246. [Google Scholar] [CrossRef]
- Namekata, I.; Nakamura, H.; Shimada, H.; Tanaka, H.; Shigenobu, K. Cardioprotection without cardiosuppression by SEA0400, a novel inhibitor of Na+-Ca2+ exchanger, during ischemia and reperfusion in guinea-pig myocardium. Life Sci. 2005, 77, 312–324. [Google Scholar] [CrossRef]
- Torrente, A.G.; Zhang, R.; Zaini, A.; Giani, J.F.; Kang, J.; Lamp, S.T.; Philipson, K.D.; Goldhaber, J.I. Burst pacemaker activity of the sinoatrial node in sodium-calcium exchanger knockout mice. Proc. Natl. Acad. Sci. USA 2015, 112, 9769–9774. [Google Scholar] [CrossRef]
- Gao, Z.; Rasmussen, T.P.; Li, Y.; Kutschke, W.; Koval, O.M.; Wu, Y.; Wu, Y.; Hall, D.D.; Joiner, M.L.; Wu, X.Q.; et al. Genetic inhibition of Na+-Ca2+ exchanger current disables fight or flight sinoatrial node activity without affecting resting heart rate. Circ. Res. 2013, 112, 309–317. [Google Scholar] [CrossRef]
- Kojima, A.; Ito, Y.; Kitagawa, H.; Matsuura, H.; Nosaka, S. Direct negative chronotropic action of desflurane on sinoatrial node pacemaker activity in the guinea pig heart. Anesthesiology 2014, 120, 1400–1413. [Google Scholar] [CrossRef]
- Chen, B.; Wu, Y.; Mohler, P.J.; Anderson, M.E.; Song, L.S. Local control of Ca2+-induced Ca2+ release in mouse sinoatrial node cells. J. Mol. Cell. Cardiol. 2009, 47, 706–715. [Google Scholar] [CrossRef][Green Version]
- Magee, W.P.; Deshmukh, G.; Deninno, M.P.; Sutt, J.C.; Chapman, J.G.; Tracey, W.R. Differing cardioprotective efficacy of the Na+/Ca2+ exchanger inhibitors SEA0400 and KB-R7943. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, 903–910. [Google Scholar] [CrossRef]
- Takahashi, T.; Takahashi, K.; Onishi, M.; Suzuki, T.; Tanaka, Y.; Ota, T.; Yoshida, S.; Nakaike, S.; Matsuda, T.; Baba, A. Effects of SEA0400, a novel inhibitor of the Na+/Ca2+ exchanger, on myocardial stunning in anesthetized dogs. Eur. J. Pharmacol. 2004, 505, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Namekata, I.; Tsuneoka, Y.; Takahara, A.; Shimada, H.; Sugimoto, T.; Takeda, K.; Nagaharu, M.; Shigenobu, K.; Kawanishi, T.; Tanaka, H. Involvement of the Na+/Ca2+ exchanger in the automaticity of guinea-pig pulmonary vein myocardium as revealed by SEA0400. J. Pharmacol. Sci. 2009, 110, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Namekata, I.; Tanaka, Y.; Ohmori, T.; Tsuneoka, Y.; Hamaguchi, S.; Tanaka, H.; Tanaka, H. Cell morphology and early-phase Ca2+ transients of guinea-pig pulmonary vein cardiomyocytes compared with atrial and ventricular cardiomyocytes. Bioimages 2019, 27, 1–12. [Google Scholar]
- Verkerk, A.O.; Wilders, R.; Zegers, J.G.; van Borren, M.M.; Ravesloot, J.H.; Verheijck, E.E. Ca2+-activated Cl− current in rabbit sinoatrial node cells. J. Physiol. 2002, 540, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Wang, Y.; Peng, H.; He, F.; Zhu, L.; Huang, H.; Huang, X.; Lu, X.; Tan, X. A newly identified missense mutation in CLCA2 is associated with autosomal dominant cardiac conduction block. Gene 2019, 714, 143990. [Google Scholar] [CrossRef]
- Turner, D.; Kang, C.; Mesirca, P.; Hong, J.; Mangoni, M.E.; Glukhov, A.V.; Sah, R. Electrophysiological and Molecular Mechanisms of Sinoatrial node mechanosensitivity. Front. Cardiovasc. Med. 2021, 8, 662410. [Google Scholar] [CrossRef]
- Lei, M.; Jones, S.A.; Liu, J.; Lancaster, M.K.; Fung, S.S.; Dobrzynski, H.; Camelliti, P.; Maier, S.K.; Noble, D.; Boyett, M.R. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J. Physiol. 2004, 559, 835–843. [Google Scholar] [CrossRef]
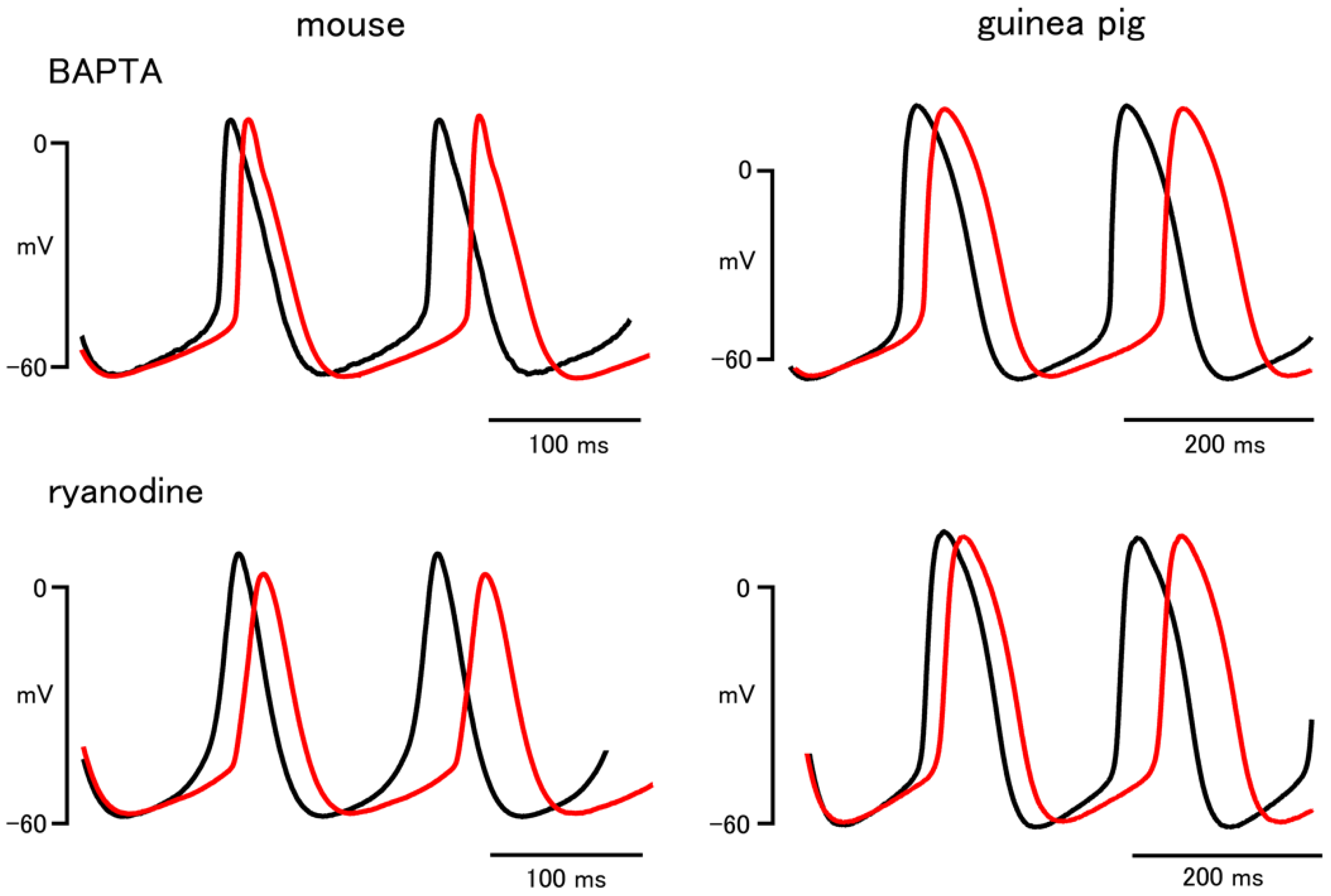

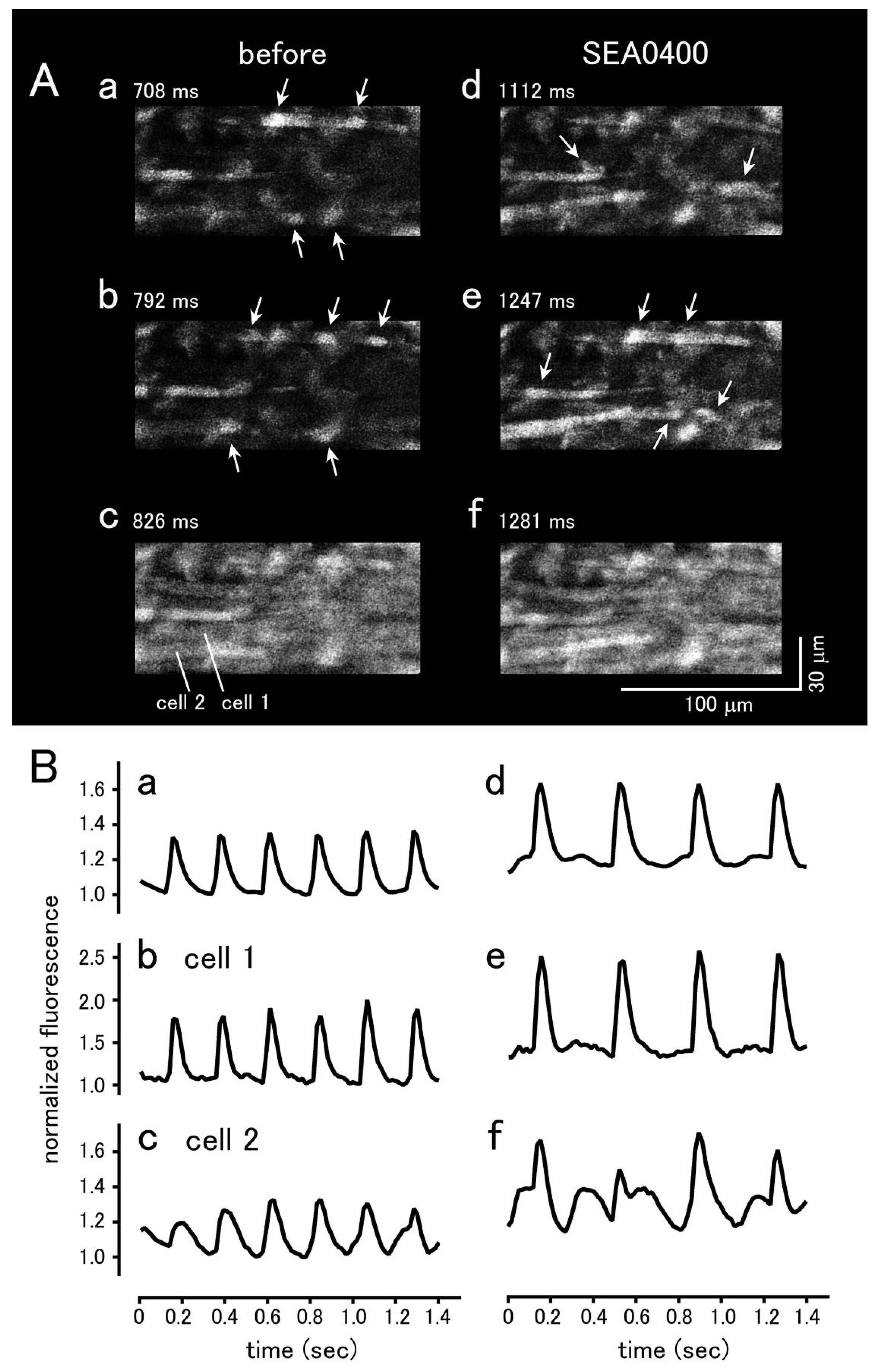
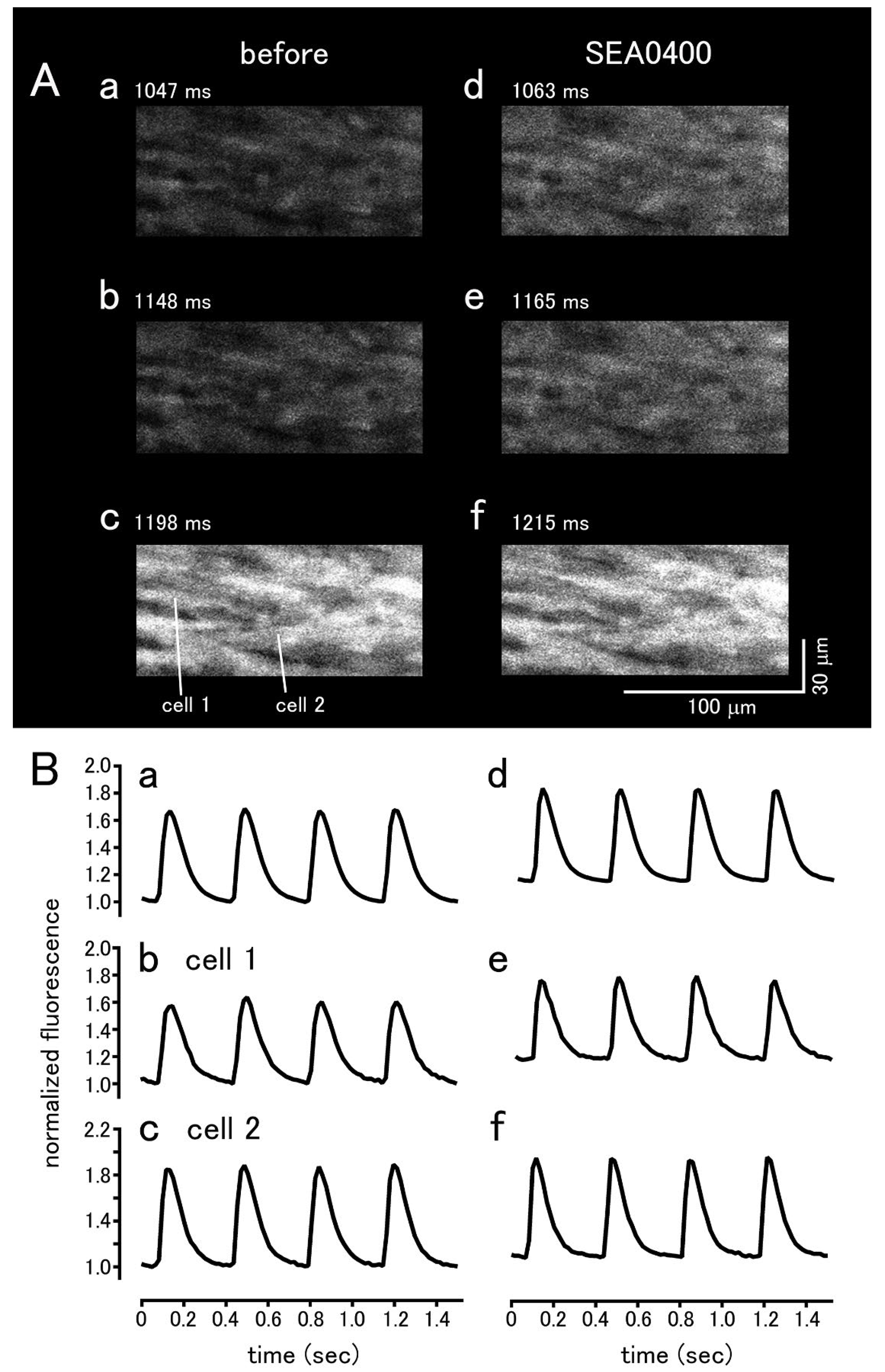
| BAPTA | Ryanodine | |||
|---|---|---|---|---|
| Mouse | Guinea Pig | Mouse | Guinea Pig | |
| Firing rate (bpm) | 423.7 ± 10.1 | 241.4 ± 16.1 | 453.6 ± 10.0 | 248.7 ± 11.7 |
| 396.0 ± 6.4 * | 213.1 ± 12.2 * | 414.3 ± 13.9 * | 237.4 ± 12.6 * | |
| Cycle length (ms) | 141.9 ± 3.4 | 254.2 ± 17.0 | 132.5 ± 2.9 | 241.3 ± 13.4 |
| 151.7 ± 2.4 * | 286.7 ± 17.2 * | 145.5 ± 4.7 * | 256.7 ± 13.6 * | |
| Maximum diastolic potential (mV) | −60.6 ± 1.4 | −62.9 ± 1.2 | −58.8 ± 1.2 | −63.2 ± 1.3 |
| −60.0 ± 1.7 | −58.9 ± 2.1 * | −55.9 ± 1.7 | −60.9 ± 1.7 | |
| Slope of pacemaker depolarization (mV/s) | 192.0 ± 26.2 | 147.4 ± 14.7 | 223.5 ± 25.8 | 138.2 ± 10.4 |
| 130.0 ± 17.7 * | 104.8 ± 9.8 * | 110.0 ± 24.5 * | 119.2 ± 9.1 * | |
| Threshold potential (mV) | −50.4 ± 1.6 | −49.0 ± 1.6 | −46.9 ± 1.9 | −52.6 ± 1.9 |
| −51.6 ± 1.5 | −46.4 ± 2.1 * | −45.8 ± 3.0 | −49.1 ± 2.3 | |
| Maximum rate of rise (V/s) | 10.3 ± 2.6 | 9.0 ± 2.2 | 8.7 ± 2.7 | 18.7 ± 9.4 |
| 9.0 ± 2.2 | 8.1 ± 1.9 | 7.8 ± 2.2 | 15.6 ± 7.8 | |
| Peak potential (mV) | 0.9 ± 2.5 | 18.9 ± 1.1 | 4.6 ± 1.2 | 18.3 ± 1.7 |
| −0.9 ± 3.2 | 17.7 ± 1.5 | 4.2 ± 1.0 | 17.8 ± 1.6 | |
| Duration at 50% repolarization (ms) | 30.3 ± 1.7 | 81.8 ± 2.0 | 28.3 ± 0.9 | 75.4 ± 5.9 |
| 30.2 ± 1.4 | 85.6 ± 6.3 | 28.9 ± 1.4 | 77.4 ± 6.4 | |
| SEA0400 (1 μM) | SEA0400 (10 μM) | |||
|---|---|---|---|---|
| Mouse | Guinea Pig | Mouse | Guinea Pig | |
| Firing rate (bpm) | 449.7 ± 24.9 | 239.0 ± 9.2 | 428.2 ± 27.2 | 239.4 ± 8.9 |
| 396.8 ± 29.0 * | 240.1 ± 9.2 | 363.8 ± 17.7 * | 237.4 ± 11.6 | |
| Cycle length (ms) | 135.2 ± 8.1 | 253.0 ± 10.1 | 142.1 ± 8.0 | 252.0 ± 9.0 |
| 155.0 ± 12.2 * | 251.8 ± 9.7 | 166.4 ± 7.6 * | 255.1 ± 12.3 | |
| Maximum diastolic potential (mV) | −55.8 ± 1.8 | −62.6 ± 2.5 | −59.2 ± 1.0 | −61.7 ± 2.1 |
| −51.7 ± 1.9 * | −62.1 ± 2.4 | −55.9 ± 1.3 * | −59.1 ± 3.0 | |
| Slope of pacemaker depolarization (mV/s) | 217.0 ± 22.2 | 148.3 ± 16.2 | 215.4 ± 26.6 | 172.5 ± 6.0 |
| 173.2 ± 22.9 * | 150.8 ± 13.8 | 161.0 ± 20.9 * | 177.0 ± 16.3 | |
| Threshold potential (mV) | −46.0 ± 1.2 | −49.8 ± 2.7 | −48.6 ± 1.2 | −48.3 ± 2.5 |
| −41.9 ± 1.4 * | −48.7 ± 2.5 | −45.5 ± 1.3 * | −43.9 ± 3.1 | |
| Maximum rate of rise (V/s) | 6.7 ± 0.6 | 7.0 ± 1.5 | 7.9 ± 1.6 | 5.8 ± 1.5 |
| 5.6 ± 0.7 * | 7.7 ± 2.2 | 4.5 ± 0.8 * | 4.8 ± 1.1 | |
| Peak potential (mV) | 4.8 ± 0.7 | 12.0 ± 2.1 | 4.7 ± 1.6 | 11.9 ± 1.6 |
| −0.5 ± 1.3 * | 12.8 ± 1.8 | −11.2 ± 3.0 * | 12.0 ± 1.6 | |
| Duration at 50% repolarization (ms) | 33.5 ± 2.1 | 80.4 ± 5.4 | 31.3 ± 2.2 | 85.4 ± 6.2 |
| 35.5 ± 1.5 | 79.8 ± 4.4 | 36.2 ± 2.2 | 83.6 ± 5.3 | |
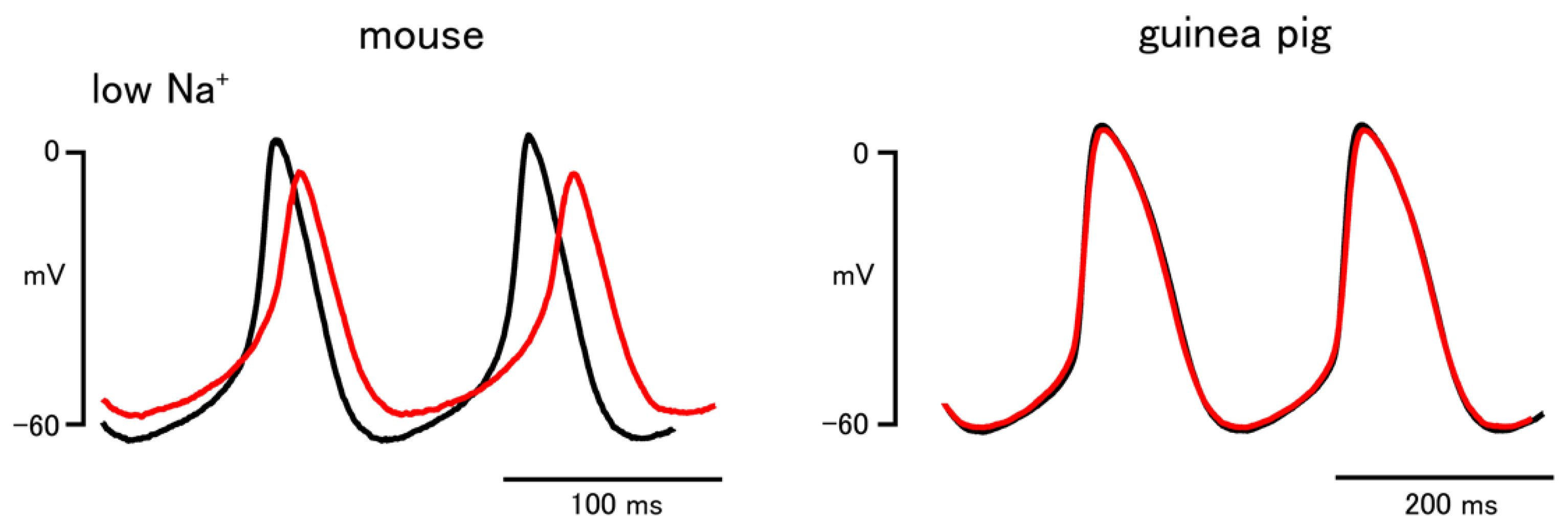
| Low Na+ | ||
|---|---|---|
| Mouse | Guinea Pig | |
| Firing rate (bpm) | 498.4 ± 17.1 | 232.7 ± 9.5 |
| 455.5 ± 20.5 * | 232.7 ± 9.9 | |
| Cycle length (ms) | 121.1 ± 4.0 | 260.2 ± 12.0 |
| 133.1 ± 5.9 * | 260.5 ± 12.6 | |
| Maximum diastolic potential (mV) | −60.4 ± 1.1 | −63.2 ± 0.9 |
| −56.9 ± 0.9 * | −64.0 ± 1.6 | |
| Slope of pacemaker depolarization (mV/s) | 251.4 ± 25.3 | 166.3 ± 17.4 |
| 206.7 ± 30.7 * | 187.3 ± 20.4 | |
| Threshold potential (mV) | −49.8 ± 1.1 | −48.2 ± 1.1 |
| −47.6 ± 1.0 * | −48.1 ± 1.8 | |
| Maximum rate of rise (V/s) | 8.6 ± 1.1 | 4.0 ± 0.7 |
| 5.6 ± 0.6 * | 3.6 ± 0.6 * | |
| Peak potential (mV) | 1.9 ± 1.3 | 11.0 ± 1.2 |
| −5.1 ± 2.4 * | 8.3 ± 2.4 | |
| Duration at 50% repolarization (ms) | 28.7 ± 1.3 | 89.3 ± 3.5 |
| 32.2 ± 1.3 * | 88.8 ± 4.2 | |
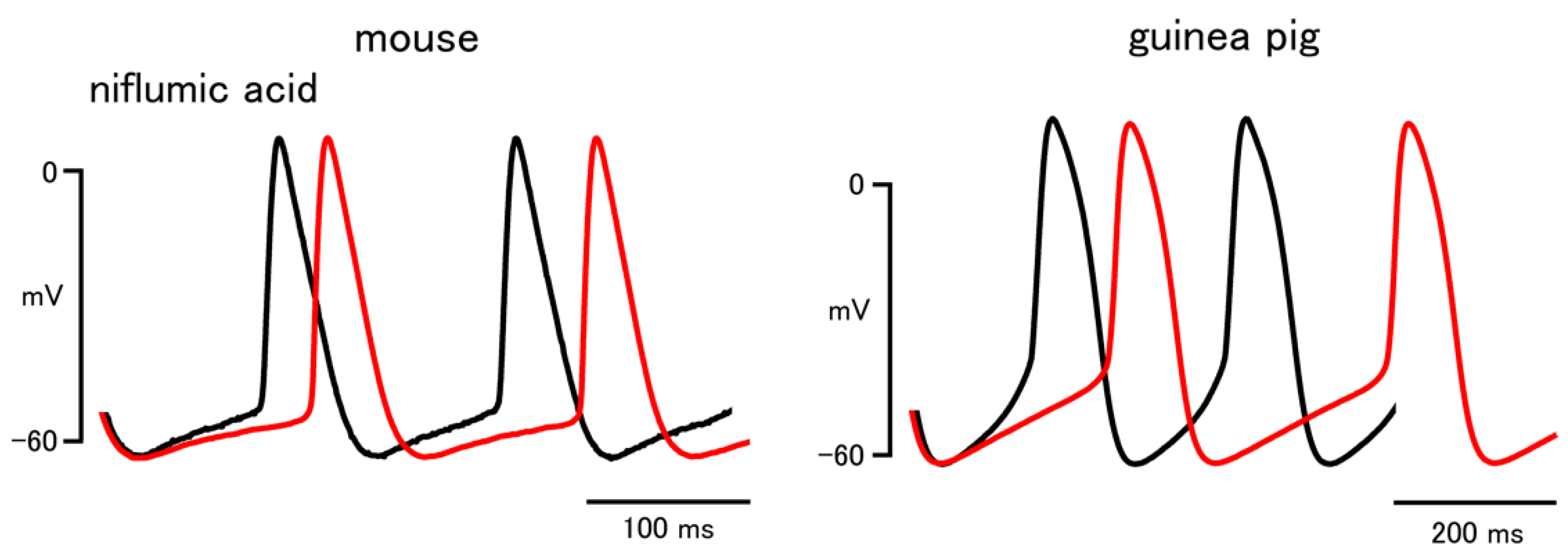
| Niflumic Acid | ||
|---|---|---|
| Mouse | Guinea Pig | |
| Firing rate (bpm) | 444.5 ± 18.1 | 236.6 ± 14.1 |
| 418.4 ± 21.0 * | 205.0 ± 12.5 * | |
| Cycle length (ms) | 136.1 ± 5.5 | 258.4 ± 15.9 |
| 145.3 ± 7.6 * | 298.0 ± 17.7 * | |
| Maximum diastolic potential (mV) | −61.0 ± 1.1 | −62.7 ± 3.1 |
| −60.6 ± 1.8 | −61.8 ± 2.8 | |
| Slope of pacemaker depolarization (mV/s) | 221.5 ± 40.9 | 152.2 ± 20.2 |
| 173.8 ± 35.7 * | 104.8 ± 12.4 * | |
| Threshold potential (mV) | −50.5 ± 1.7 | −48.8 ± 3.2 |
| −51.7 ± 2.5 | −49.0 ± 3.5 | |
| Maximum rate of rise (V/s) | 14.5 ± 6.5 | 8.4 ± 2.1 |
| 14.7 ± 6.8 | 6.9 ± 1.3 | |
| Peak potential (mV) | 5.8 ± 0.8 | 19.4 ± 4.0 |
| 5.2 ± 1.6 | 17.8 ± 3.4 | |
| Duration at 50% repolarization (ms) | 31.3 ± 1.2 | 78.5 ± 2.6 |
| 32.1 ± 1.0 | 74.3 ± 3.2 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namekata, I.; Jitsukata, K.; Fukuda, A.; Odaka, R.; Hamaguchi, S.; Tanaka, H. Intracellular Ca2+-Mediated Mechanisms for the Pacemaker Depolarization of the Mouse and Guinea Pig Sinus Node Tissue. Biomolecules 2022, 12, 377. https://doi.org/10.3390/biom12030377
Namekata I, Jitsukata K, Fukuda A, Odaka R, Hamaguchi S, Tanaka H. Intracellular Ca2+-Mediated Mechanisms for the Pacemaker Depolarization of the Mouse and Guinea Pig Sinus Node Tissue. Biomolecules. 2022; 12(3):377. https://doi.org/10.3390/biom12030377
Chicago/Turabian StyleNamekata, Iyuki, Kento Jitsukata, Ayumi Fukuda, Ryosuke Odaka, Shogo Hamaguchi, and Hikaru Tanaka. 2022. "Intracellular Ca2+-Mediated Mechanisms for the Pacemaker Depolarization of the Mouse and Guinea Pig Sinus Node Tissue" Biomolecules 12, no. 3: 377. https://doi.org/10.3390/biom12030377
APA StyleNamekata, I., Jitsukata, K., Fukuda, A., Odaka, R., Hamaguchi, S., & Tanaka, H. (2022). Intracellular Ca2+-Mediated Mechanisms for the Pacemaker Depolarization of the Mouse and Guinea Pig Sinus Node Tissue. Biomolecules, 12(3), 377. https://doi.org/10.3390/biom12030377