Characterization of Stress Responses in a Drosophila Model of Werner Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Stocks and Maintenance
2.2. Larval Stress Assays
2.3. Adult Oxidative Stress Assays
2.4. Antioxidant Activity
2.5. Larval Buoyancy
2.6. Drosophila Activity Monitors (Thermal Stress, Starvation, and Sleep Analysis)
3. Results
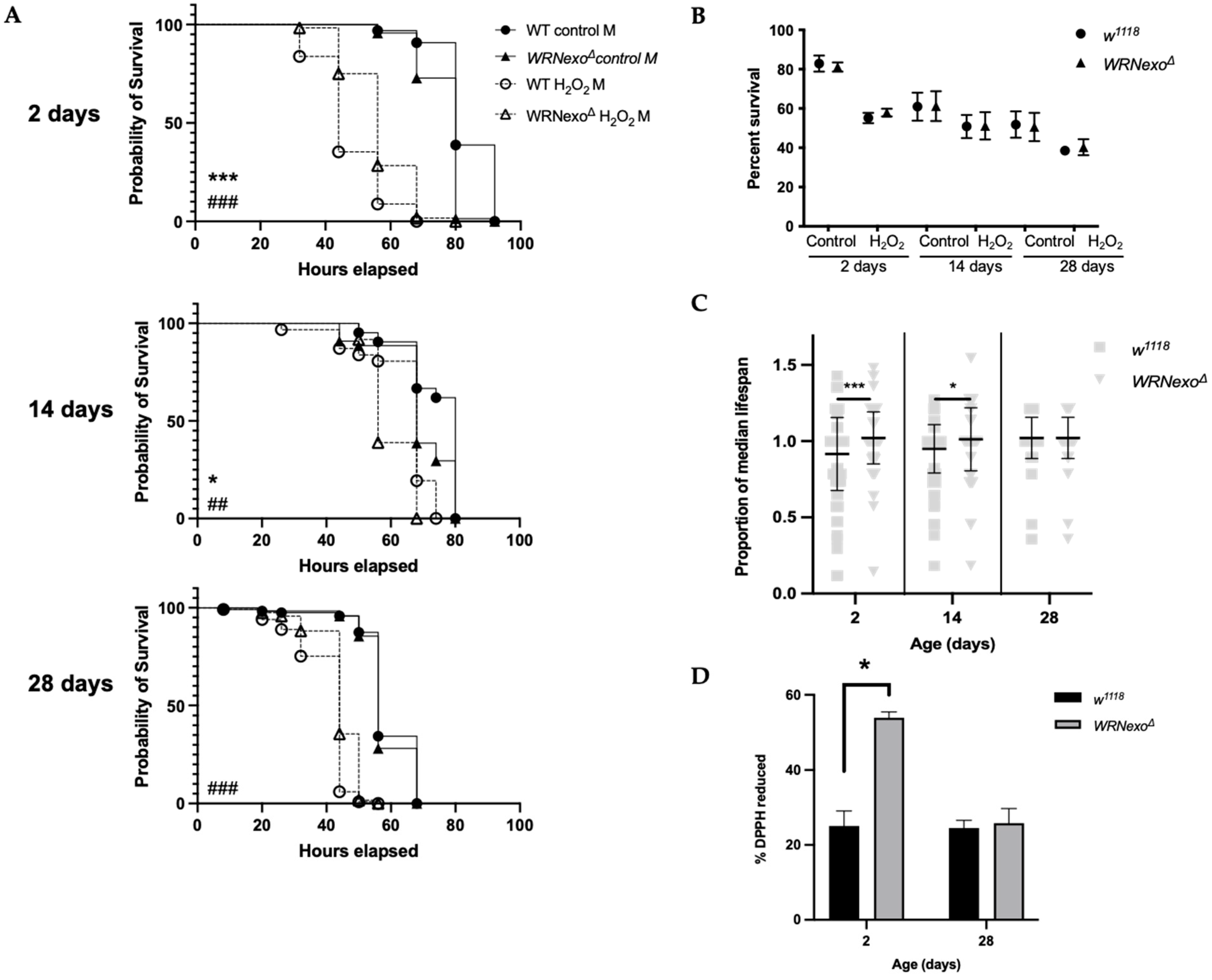
3.1. Exogenous Stressors
3.1.1. Oxidative Stress
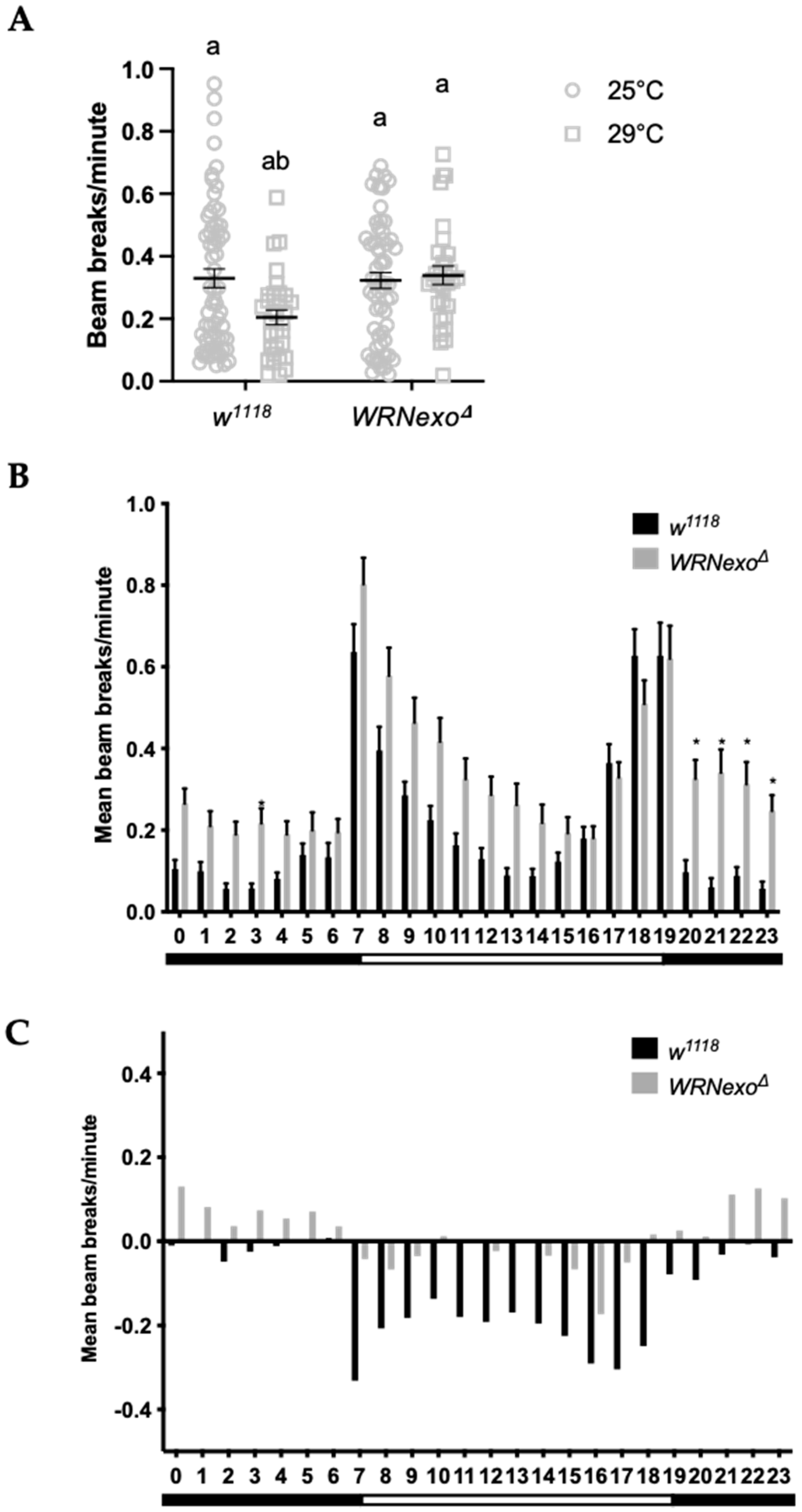
3.1.2. Response to Non-Optimal Ambient Temperature
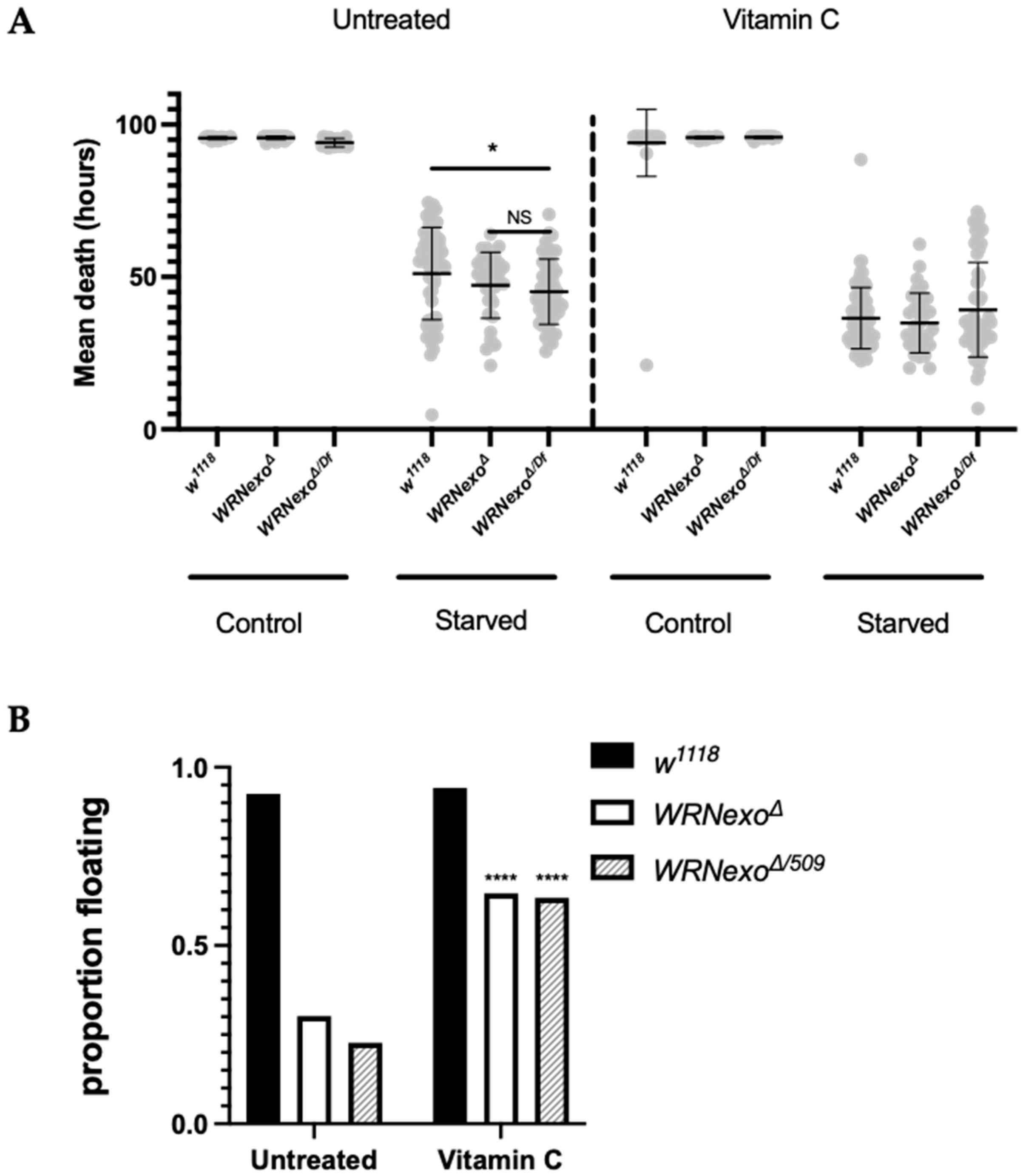
3.1.3. Starvation
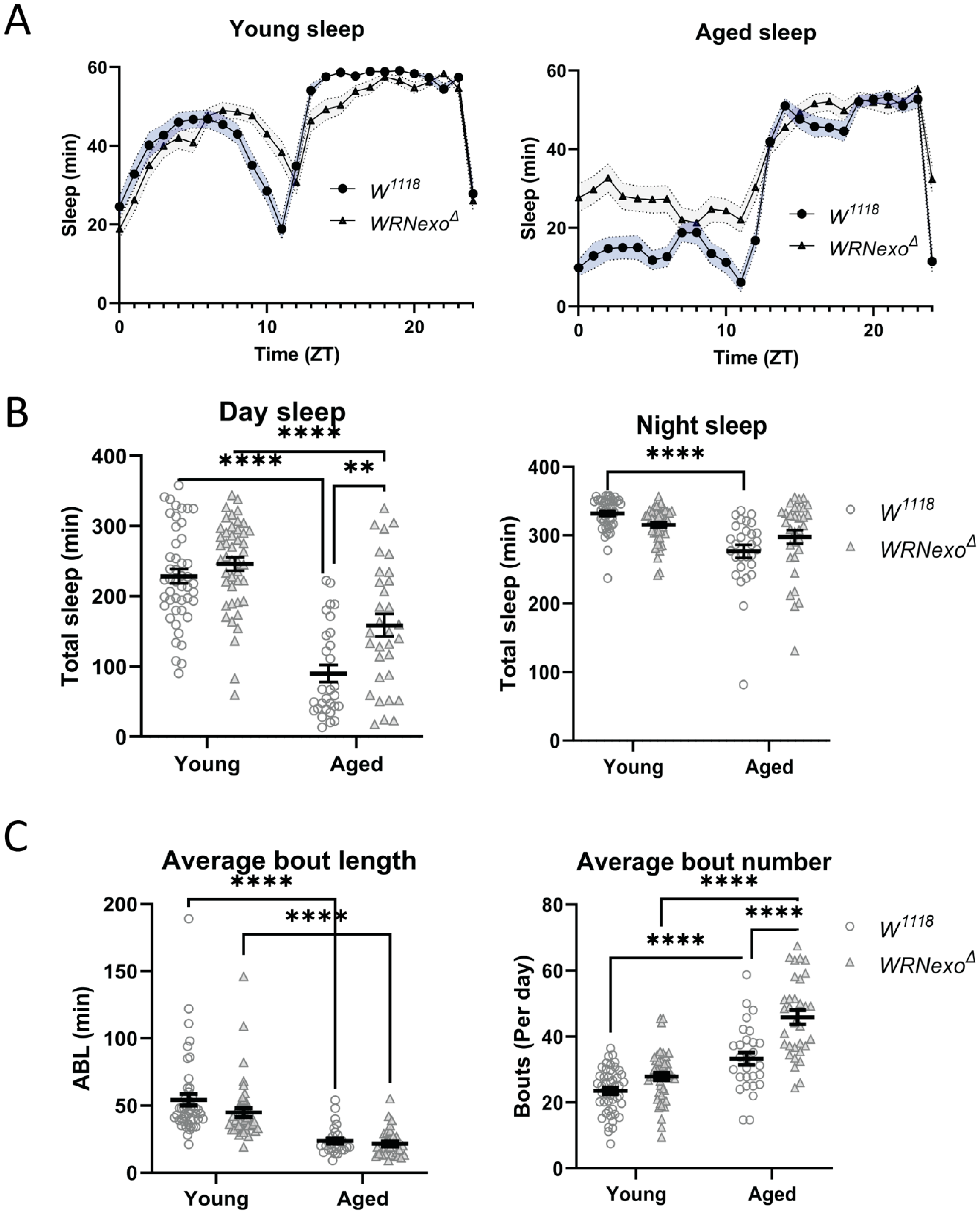
3.2. Sleep Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Berger, S.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking Aging to Chronic Disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Yankner, B.A. The Aging Stress Response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef]
- Hamilton, K.L.; Miller, B.F. What is the evidence for stress resistance and slowed aging? Exp. Gerontol. 2016, 82, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, M.M.; Solanki, P.; Singh, P. Oxidative stress, antioxidants, hormesis and calorie restriction: The current perspective in the biology of aging. Arch. Gerontol. Geriatr. 2021, 95, 104413. [Google Scholar] [CrossRef]
- Le Bourg, É. Characterisation of the positive effects of mild stress on ageing and resistance to stress. Biogerontology 2020, 21, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.D.W.; Partridge, L. Drosophila as a model for ageing. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2707–2717. [Google Scholar] [CrossRef]
- Neckameyer, W.S.; Bhatt, P. Protocols to Study Behavior in Drosophila. In Springer Protocols Handbooks; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1478, pp. 303–320. [Google Scholar]
- Neckameyer, W.S.; Nieto, A. Response to stress in Drosophila is mediated by gender, age and stress paradigm. Stress 2015, 18, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Johnmark, N.; Kinyi, H.W. Amaranth leaf extract protects against hydrogen peroxide induced oxidative stress in Drosophila melanogaster. BMC Res. Notes 2021, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Maruzs, T.; Simon-Vecsei, Z.; Kiss, V.; Csizmadia, T.; Juhász, G. On the Fly: Recent Progress on Autophagy and Aging in Drosophila. Front. Cell Dev. Biol. 2019, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Hosamani, R.; Muralidhara. Acute exposure of Drosophila melanogaster to paraquat causes oxidative stress and mitochondrial dysfunction. Arch. Insect Biochem. Physiol. 2013, 83, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Bahadorani, S.; Bahadorani, P.; Phillips, J.P.; Hilliker, A.J. The Effects of Vitamin Supplementation on Drosophila Life Span Under Normoxia and Under Oxidative Stress. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2008, 63, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, M.; Proshkina, E.; Shilova, L.; Zhavoronkov, A.; Moskalev, A. Lifespan and Stress Resistance in Drosophila with Overexpressed DNA Repair Genes. Sci. Rep. 2015, 5, 15299. [Google Scholar] [CrossRef]
- Garcia, A.M.; Calder, R.B.; Dollé, M.E.T.; Lundell, M.; Kapahi, P.; Vijg, J. Age- and Temperature-Dependent Somatic Mutation Accumulation in Drosophila melanogaster. PLoS Genet. 2010, 6, e1000950. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, E. A cold stress applied at various ages can increase resistance to heat and fungal infection in aged Drosophila melanogaster flies. Biogerontology 2011, 12, 185–193. [Google Scholar] [CrossRef]
- Rubin, G.M.; Hong, L.; Brokstein, P.; Evans-Holm, M.; Frise, E.; Stapleton, M.; Harvey, D.A. A Drosophila Complementary DNA Resource. Science 2000, 287, 2222–2224. [Google Scholar] [CrossRef] [PubMed]
- Dung, V.M.; Thao, D.T.P. Parkinson’s Disease Model. In Drosophila Models for Human Diseases; Yamaguchi, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 41–61. [Google Scholar]
- Azuma, Y.; Mizuta, I.; Tokuda, T.; Mizuno, T. Amyotrophic Lateral Sclerosis Model. In Drosophila Models for Human Diseases; Yamaguchi, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 79–95. [Google Scholar]
- Yokote, K.; Chanprasert, S.; Lee, L.; Eirich, K.; Takemoto, M.; Watanabe, A.; Koizumi, N.; Lessel, D.; Mori, T.; Hisama, F.M.; et al. WRN Mutation Update: Mutation Spectrum, Patient Registries, and Translational Prospects. Hum. Mutat. 2016, 38, 7–15. [Google Scholar] [CrossRef]
- Croteau, D.L.; Popuri, V.; Opresko, P.L.; Bohr, V.A. Human RecQ Helicases in DNA Repair, Recombination, and Replication. Annu. Rev. Biochem. 2014, 83, 519–552. [Google Scholar] [CrossRef] [PubMed]
- Shamanna, R.A.; Croteau, D.L.; Lee, J.-H.; Bohr, V.A. Recent Advances in Understanding Werner Syndrome. F1000Research 2017, 6, 1779. [Google Scholar] [CrossRef]
- Chen, L.; Huang, S.; Lee, L.; Davalos, A.; Schiestl, R.H.; Campisi, J.; Oshima, J. WRN, the protein deficient in Werner syndrome, plays a critical structural role in optimizing DNA repair. Aging Cell 2003, 2, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kamath-Loeb, A.; Loeb, L.A.; Fry, M. The Werner Syndrome Protein Is Distinguished from the Bloom Syndrome Protein by Its Capacity to Tightly Bind Diverse DNA Structures. PLoS ONE 2012, 7, e30189. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sinha, D.; Bhattacharya, S.; Srinivasan, K.; Abdisalaam, S.; Asaithamby, A. Werner Syndrome Protein and DNA Replication. Int. J. Mol. Sci. 2018, 19, 3442. [Google Scholar] [CrossRef] [PubMed]
- Li, B. Functional Interaction between Ku and the Werner Syndrome Protein in DNA End Processing. J. Biol. Chem. 2000, 275, 28349–28352. [Google Scholar] [CrossRef]
- Shamanna, R.A.; Lu, H.; De Freitas, J.K.; Tian, J.; Croteau, D.L.; Bohr, V.A. WRN regulates pathway choice between classical and alternative non-homologous end joining. Nat. Commun. 2016, 7, 13785. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, R.; Dawut, L.; Marchetti, C.; Lee, J.W.; Vindigni, A.; Ramsden, D.; Bohr, V.A. Werner Protein Cooperates with the XRCC4-DNA Ligase IV Complex in End-Processing. Biochemistry 2008, 47, 7548–7556. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Navarro, S.; Kasahara, N.; Comai, L. Identification and Biochemical Characterization of a Werner’s Syndrome Protein Complex with Ku70/80 and Poly(ADP-ribose) Polymerase-1. J. Biol. Chem. 2004, 279, 13659–13667. [Google Scholar] [CrossRef] [PubMed]
- Harrigan, J.A.; Wilson, D.M.; Prasad, R.; Opresko, P.L.; Beck, G.; May, A.; Wilson, S.H.; Bohr, V.A. The Werner syndrome protein operates in base excision repair and cooperates with DNA polymerase. Nucleic Acids Res. 2006, 34, 745–754. [Google Scholar] [CrossRef] [PubMed]
- von Kobbe, C.; Thoma, N.H.; Czyzewski, B.K.; Pavletich, N.P.; Bohr, V.A. Werner Syndrome Protein Contains Three Structure-specific DNA Binding Domains. J. Biol. Chem. 2003, 278, 52997–53006. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Boldogh, I.; Lee, J.W.; Harrigan, J.A.; Hegde, M.L.; Piotrowski, J.; Souza-Pinto, N.; Ramos, W.; Greenberg, M.M.; Hazra, T.K.; et al. The Human Werner Syndrome Protein Stimulates Repair of Oxidative DNA Base Damage by the DNA Glycosylase NEIL1. J. Biol. Chem. 2007, 282, 26591–26602. [Google Scholar] [CrossRef] [PubMed]
- Brosh, R.M.; von Kobbe, C.; Sommers, J.A.; Karmakar, P.; Opresko, P.L.; Piotrowski, J.; Dianova, I.; Dianov, G.L.; Bohr, V.A. Werner syndrome protein interacts with human flap endonuclease 1 and stimulates its cleavage activity. EMBO J. 2001, 20, 5791–5801. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.D.C.; Boubriak, I.; Clancy, D.J.; Cox, L.S. Identification and characterization of a Drosophila ortholog of WRN exonuclease that is required to maintain genome integrity. Aging Cell 2008, 7, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Boubriak, I.; Mason, P.A.; Clancy, D.J.; Dockray, J.; Saunders, R.D.C.; Cox, L.S. DmWRNexo is a 3′–5′ exonuclease: Phenotypic and biochemical characterization of mutants of the Drosophila orthologue of human WRN exonuclease. Biogerontology 2008, 10, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.A.; Boubriak, I.; Robbins, T.; Lasala, R.; Saunders, R.; Cox, L.S. The Drosophila orthologue of progeroid human WRN exonuclease, DmWRNexo, cleaves replication substrates but is inhibited by uracil or abasic sites: Analysis of DmWRNexo activity in vitro. Age 2013, 35, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, D.; Epiney, D.G.; Salameh, C.; Zhou, L.T.; Salomon, R.N.; Schirmer, A.E.; McVey, M.; Bolterstein, E. Evidence for premature aging in a Drosophila model of Werner syndrome. Exp. Gerontol. 2019, 127, 110733. [Google Scholar] [CrossRef]
- Bolterstein, E.; Rivero, R.; Marquez, M.; McVey, M. The Drosophila Werner Exonuclease Participates in an Exonuclease-Independent Response to Replication Stress. Genetics 2014, 197, 643–652. [Google Scholar] [CrossRef]
- Sekelsky, J. DNA Repair in Drosophila: Mutagens, Models, and Missing Genes. Genetics 2017, 205, 471–490. [Google Scholar] [CrossRef]
- Emery, P. Protein Extraction From Drosophila Heads. In Circadian Rhythms: Methods and Protocols; Rosato, E., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 375–377. [Google Scholar]
- Reis, T.; Van Gilst, M.R.; Hariharan, I.K. A Buoyancy-Based Screen of Drosophila Larvae for Fat-Storage Mutants Reveals a Role for Sir2 in Coupling Fat Storage to Nutrient Availability. PLoS Genet. 2010, 6, e1001206. [Google Scholar] [CrossRef]
- Pfeiffenberger, C.; Lear, B.C.; Keegan, K.P.; Allada, R. Processing Sleep Data Created with the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb. Protoc. 2010, 11, pdb-prot5520. [Google Scholar] [CrossRef]
- Shaw, P.J.; Cirelli, C.; Greenspan, R.J.; Tononi, G. Correlates of Sleep and Waking in Drosophila melanogaster. Science 2000, 287, 1834–1837. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Jeppesen, D.K.; Bohr, V.A.; Stevnsner, T. DNA repair deficiency in neurodegeneration. Prog. Neurobiol. 2011, 94, 166–200. [Google Scholar] [CrossRef]
- Wang, X.-G.; Johnson, M.W.; Daane, K.M.; Nadel, H. High Summer Temperatures Affect the Survival and Reproduction of Olive Fruit Fly (Diptera: Tephritidae). Environ. Entomol. 2009, 38, 1496–1504. [Google Scholar] [CrossRef]
- Patton, Z.J.; Krebs, R.A. The Effect of Thermal Stress on the Mating Behavior ofThreeDrosophilaSpecies. Physiol. Biochem. Zool. 2001, 74, 783–788. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Siggens, L.; Figg, N.; Bennett, M.; Foo, R. Nutrient deprivation regulates DNA damage repair in cardiomyocytes via loss of the base-excision repair enzyme OGG1. FASEB J. 2012, 26, 2117–2124. [Google Scholar] [CrossRef]
- Ishimoto, H.; Lark, A.R.; Kitamoto, T. Factors that Differentially Affect Daytime and Nighttime Sleep in Drosophila melanogaster. Front. Neurol. 2012, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Petronek, M.; Stolwijk, J.; Murray, S.; Steinbach, E.; Zakharia, Y.; Buettner, G.; Spitz, D.; Allen, B. Utilization of redox modulating small molecules that selectively act as pro-oxidants in cancer cells to open a therapeutic window for improving cancer therapy. Redox Biol. 2021, 42, 101864. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. J. Neurosci. 2017, 94, 19–36. [Google Scholar] [CrossRef]
- Musiek, E.S.; Holtzman, D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016, 354, 1004–1008. [Google Scholar] [CrossRef]
- Koh, K.; Evans, J.M.; Hendricks, J.C.; Sehgal, A. A Drosophila model for age-associated changes in sleep: Wake cycles. Proc. Natl. Acad. Sci. USA 2006, 103, 13843–13847. [Google Scholar] [CrossRef] [PubMed]
- Vienne, J.; Spann, R.; Guo, F.; Rosbash, M. Age-Related Reduction of Recovery Sleep and Arousal Threshold in Drosophila. Sleep 2016, 39, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Pedraz, J.M.I.; Chen, L.-Y.; Yin, F.; Cadenas, E.; Reddy, S.; Comai, L. Downregulation of the Werner syndrome protein induces a metabolic shift that compromises redox homeostasis and limits proliferation of cancer cells. Aging Cell 2013, 13, 367–378. [Google Scholar] [CrossRef]
- Massip, L.; Garand, C.; Paquet, E.R.; Cogger, V.C.; O’Reilly, J.N.; Tworek, L.; Hatherell, A.; Taylor, C.G.; Thorin, E.; Zahradka, P.; et al. Vitamin C restores healthy aging in a mouse model for Werner syndrome. FASEB J. 2010, 24, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Labbé, A.; Turaga, R.V.; Paquet, É.R.; Garand, C.; Lebel, M. Expression profiling of mouse embryonic fibroblasts with a deletion in the helicase domain of the Werner Syndrome gene homologue treated with hydrogen peroxide. BMC Genom. 2010, 11, 127. [Google Scholar] [CrossRef]
- Seco-Cervera, M.; Spis, M.; García-Giménez, J.L.; Cabellos, J.S.I.; Velázquez-Ledesma, A.; Esmorís, I.; Bañuls, S.; Machado, G.P.; Pallardó, F.V. Oxidative stress and antioxidant response in fibroblasts from Werner and Atypical Werner Syndromes. Aging 2014, 6, 231–245. [Google Scholar] [CrossRef]
- Pagano, G.; Zatterale, A.; Degan, P.; D’ischia, M.; Kelly, F.J.; Pallardó, F.V.; Kodama, S. Multiple Involvement of Oxidative Stress in Werner Syndrome Phenotype. Biogerontology 2005, 6, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, A.; Garand, C.; Paquet, E.; Mitchell, S.J.; de Cabo, R.; Simard, M.; Lebel, M. Down regulation of miR-124 in both Werner syndrome DNA helicase mutant mice and mutant Caenorhabditis elegans wrn-1 reveals the importance of this microRNA in accelerated aging. Aging 2012, 4, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- von Kobbe, C.; May, A.; Grandori, C.; Bohr, V.A. Werner syndrome cells escape hydrogen peroxide-induced cell proliferation arrest. FASEB J. 2004, 18, 1970–1972. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Chang, W.-C.; Ma, W.-L. Hypothesis: Solid tumours behave as systemic metabolic dictators. J. Cell. Mol. Med. 2016, 20, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Klepsatel, P.; Wildridge, D.; Gáliková, M. Temperature induces changes in Drosophila energy stores. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Harbison, S.T.; Sehgal, A. Energy Stores Are Not Altered by Long-Term Partial Sleep Deprivation in Drosophila melanogaster. PLoS ONE 2009, 4, e6211. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Dauvilliers, Y.; Mongrain, V.; Franken, P.; Tafti, M. Age-related changes in sleep in inbred mice are genotype dependent. Neurobiol. Aging 2012, 33, 195.e13–195.e26. [Google Scholar] [CrossRef]
- Kuo, T.-H.; Pike, D.H.; Beizaeipour, Z.; Williams, J.A. Sleep triggered by an immune response in Drosophila is regulated by the circadian clock and requires the NF B Relish. BMC Neurosci. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Hill, V.M.; O’Connor, R.M.; Sissoko, G.B.; Irobunda, I.S.; Leong, S.; Canman, J.C.; Stavropoulos, N.; Shirasu-Hiza, M. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018, 16, e2005206. [Google Scholar] [CrossRef] [PubMed]
- Bushey, D.; Hughes, A.K.; Tononi, G.; Cirelli, C. Sleep, aging, and lifespan in Drosophila. BMC Neurosci. 2010, 11, 56. [Google Scholar] [CrossRef]
- Hill, V.M.; O’Connor, R.M.; Shirasu-Hiza, M. Tired and stressed: Examining the need for sleep. Eur. J. Neurosci. 2020, 51, 494–508. [Google Scholar] [CrossRef]
- Miura, M.; Takahashi, A. Starvation tolerance associated with prolonged sleep bouts upon starvation in a single natural population of Drosophila melanogaster. J. Evol. Biol. 2019, 32, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Lim, A.S.P.; Kowgier, M.; Yu, L.; Buchman, A.S.; Bennett, D.A. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 2013, 36, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- van Alphen, B.; Semenza, E.R.; Yap, M.; van Swinderen, B.; Allada, R. A deep sleep stage in Drosophila with a functional role in waste clearance. Sci Adv. 2021, 7, eabc2999. [Google Scholar] [CrossRef]
- Zimmerman, J.E.; Mackiewicz, M.; Galante, R.J.; Zhang, L.; Cater, J.; Zoh, C.; Rizzo, W.; Pack, A.I. Glycogen in the brain of Drosophila melanogaster: Diurnal rhythm and the effect of rest deprivation. J. Neurochem. 2003, 88, 32–40. [Google Scholar] [CrossRef]
- Yurgel, M.E.; Masek, P.; DiAngelo, J.; Keene, A.C. Genetic dissection of sleep-metabolism interactions in the fruit fly. J. Comp. Physiol. A 2014, 201, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Carhan, A.; Tang, K.; Shirras, C.A.; Shirras, A.; Isaac, R.E. Loss of Angiotensin-converting enzyme-related (ACER) peptidase disrupts night-time sleep in adult Drosophila melanogaster. J. Exp. Biol. 2011, 214, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Thimgan, M.S.; Suzuki, Y.; Seugnet, L.; Gottschalk, L.; Shaw, P.J. The Perilipin Homologue, Lipid Storage Droplet 2, Regulates Sleep Homeostasis and Prevents Learning Impairments Following Sleep Loss. PLoS Biol. 2010, 8, e1000466. [Google Scholar] [CrossRef]
- Rehman, N.; Varghese, J. Larval nutrition influences adult fat stores and starvation resistance in Drosophila. PLoS ONE 2021, 16, e0247175. [Google Scholar] [CrossRef] [PubMed]
- Aguila, J.R.; Suszko, J.; Gibbs, A.G.; Hoshizaki, D.K. The role of larval fat cells in adult Drosophila melanogaster. J. Exp. Biol. 2007, 210, 956–963. [Google Scholar] [CrossRef]
- Labbé, A.; Lafleur, V.N.; Patten, D.A.; Robitaille, G.A.; Garand, C.; Lamalice, L.; Lebel, M.; Richard, D.E. The Werner syndrome gene product (WRN): A repressor of hypoxia-inducible factor-1 activity. Exp. Cell Res. 2012, 318, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hou, Y.; Lautrup, S.; Jensen, M.B.; Yang, B.; Sengupta, T.; Caponio, D.; Khezri, R.; Demarest, T.G.; Aman, Y.; et al. NAD+ augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat. Commun. 2019, 10, 5284. [Google Scholar] [CrossRef] [PubMed]
- Turaga, R.V.; Paquet, E.R.; Sild, M.; Vignard, J.; Garand, C.; Johnson, F.B.; Masson, J.-Y.; Lebel, M. The Werner syndrome protein affects the expression of genes involved in adipogenesis and inflammation in addition to cell cycle and DNA damage responses. Cell Cycle 2009, 8, 2080–2092. [Google Scholar] [CrossRef] [PubMed]
- Aumailley, L.; Lebel, M. The Impact of Vitamin C on Different System Models of Werner Syndrome. Antioxid. Redox Signal 2021, 34, 856–874. [Google Scholar] [CrossRef]
- Yang, W.; Hekimi, S. A Mitochondrial Superoxide Signal Triggers Increased Longevity in Caenorhabditis elegans. PLoS Biol. 2010, 8, e1000556. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cypser, J.R.; Yashin, A.I.; Johnson, T.E. Multiple mild heat-shocks decrease the Gompertz component of mortality in Caenorhabditis elegans. Exp. Gerontol. 2009, 44, 607–612. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cypser, J.R.; Johnson, T.E. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B109–B114. [Google Scholar] [CrossRef] [PubMed]
- Zada, D.; Sela, Y.; Matosevich, N.; Monsonego, A.; Lerer-Goldshtein, T.; Nir, Y.; Appelbaum, L. Parp1 promotes sleep, which enhances DNA repair in neurons. Mol. Cell 2021. [Google Scholar] [CrossRef]
- Sanz, A.; Stefanatos, R.K. The mitochondrial free radical theory of aging: A critical view. Curr. Aging Sci. 2008, 1, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A. Mitochondrial reactive oxygen species: Do they extend or shorten animal lifespan? Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Scialo’, F.; Sriram, A.; Fernández-Ayala, D.J.M.; Gubina, N.; Lõhmus, M.; Nelson, G.; Logan, A.; Cooper, H.M.; Navas, P.; Enríquez, J.A.; et al. Mitochondrial ROS Produced via Reverse Electron Transport Extend Animal Lifespan. Cell Metab. 2016, 23, 725–734. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Genotype | Larvae | Adult Male | Adult Female |
|---|---|---|---|---|
| Untreated [37] | w1118 | 4.2 ± 0.5 | 1.7 ± 0.3 | 2.6 ± 0.4 |
| WRNexoΔ | 3.5 ± 0.8 | 1.5 ± 0.3 | 2.0 ± 0.5 * | |
| Vitamin C | w1118 | 8.2 ± 1 | 4.0 ± 0.3 | 5.0 ± 0.5 |
| WRNexoΔ | 7.5 ± 0.8 | 2.0 ± 0.3 **** | 3.9 ± 0.5 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epiney, D.G.; Salameh, C.; Cassidy, D.; Zhou, L.T.; Kruithof, J.; Milutinović, R.; Andreani, T.S.; Schirmer, A.E.; Bolterstein, E. Characterization of Stress Responses in a Drosophila Model of Werner Syndrome. Biomolecules 2021, 11, 1868. https://doi.org/10.3390/biom11121868
Epiney DG, Salameh C, Cassidy D, Zhou LT, Kruithof J, Milutinović R, Andreani TS, Schirmer AE, Bolterstein E. Characterization of Stress Responses in a Drosophila Model of Werner Syndrome. Biomolecules. 2021; 11(12):1868. https://doi.org/10.3390/biom11121868
Chicago/Turabian StyleEpiney, Derek G., Charlotte Salameh, Deirdre Cassidy, Luhan T. Zhou, Joshua Kruithof, Rolan Milutinović, Tomas S. Andreani, Aaron E. Schirmer, and Elyse Bolterstein. 2021. "Characterization of Stress Responses in a Drosophila Model of Werner Syndrome" Biomolecules 11, no. 12: 1868. https://doi.org/10.3390/biom11121868
APA StyleEpiney, D. G., Salameh, C., Cassidy, D., Zhou, L. T., Kruithof, J., Milutinović, R., Andreani, T. S., Schirmer, A. E., & Bolterstein, E. (2021). Characterization of Stress Responses in a Drosophila Model of Werner Syndrome. Biomolecules, 11(12), 1868. https://doi.org/10.3390/biom11121868






