MicroRNA-193a-5p Regulates the Synthesis of Polyunsaturated Fatty Acids by Targeting Fatty Acid Desaturase 1 (FADS1) in Bovine Mammary Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Transcriptome Sequencing
2.3. Identifition of Target-Related miRNA and DEG
2.4. Cell Culture and Transfection
2.5. RNA Extraction and qRT-PCR
2.6. Western Bolt
2.7. Luciferase Reporter Assay
2.8. Co-Transfection of MiR-193a-5p Inhibitor and Small RNA
2.9. Triglycerides Content Assay
2.10. Fatty Acid Profiles Assay
2.11. Statistical Analysis
3. Results
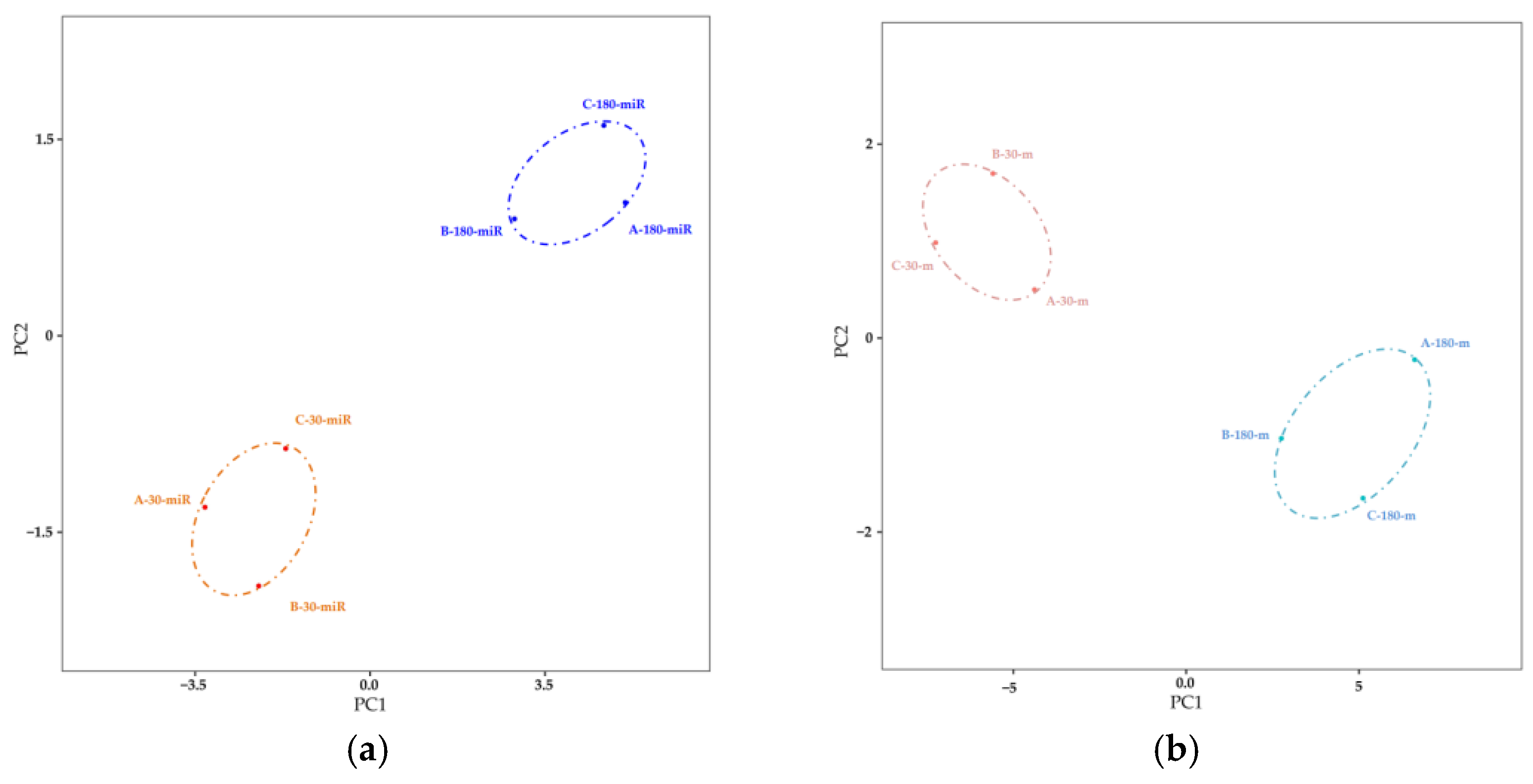
3.1. Analysis of Differentially Expressed miRNAs and mRNA
3.1.1. Identification of Differentially Expressed miRNAs and mRNAs
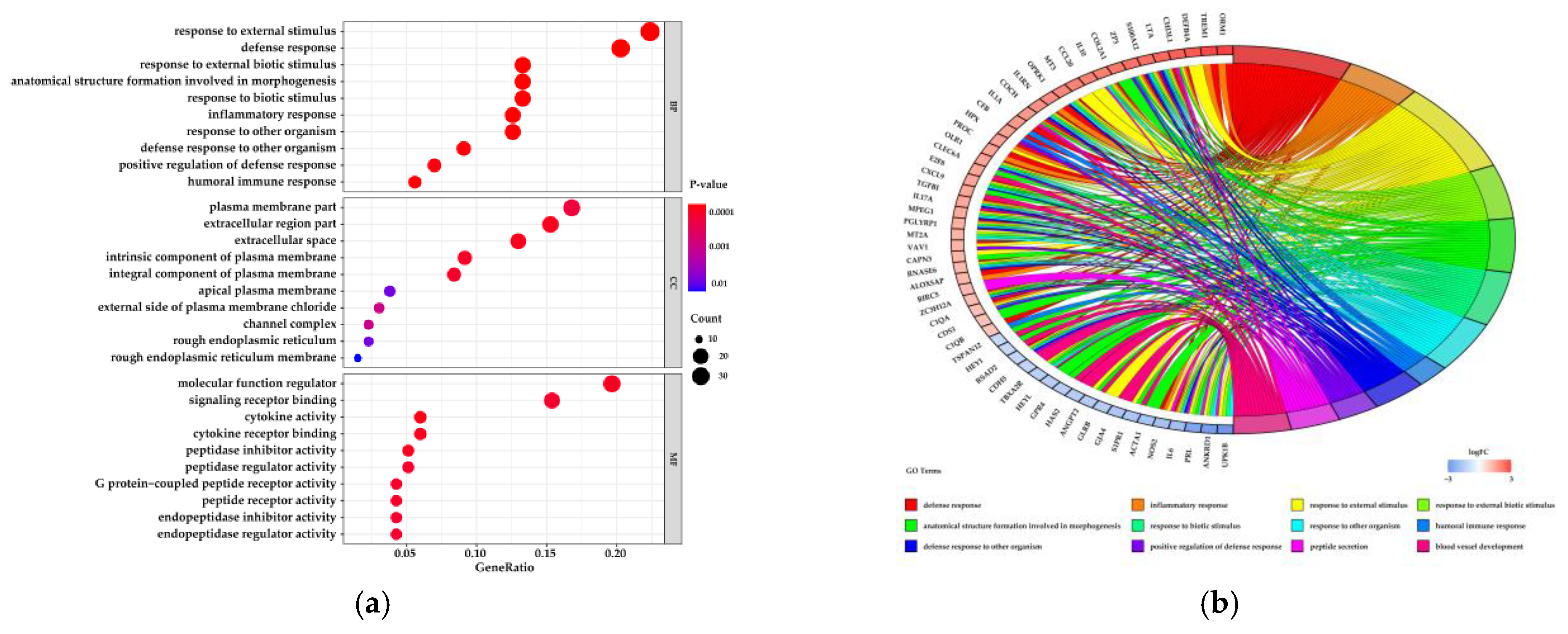
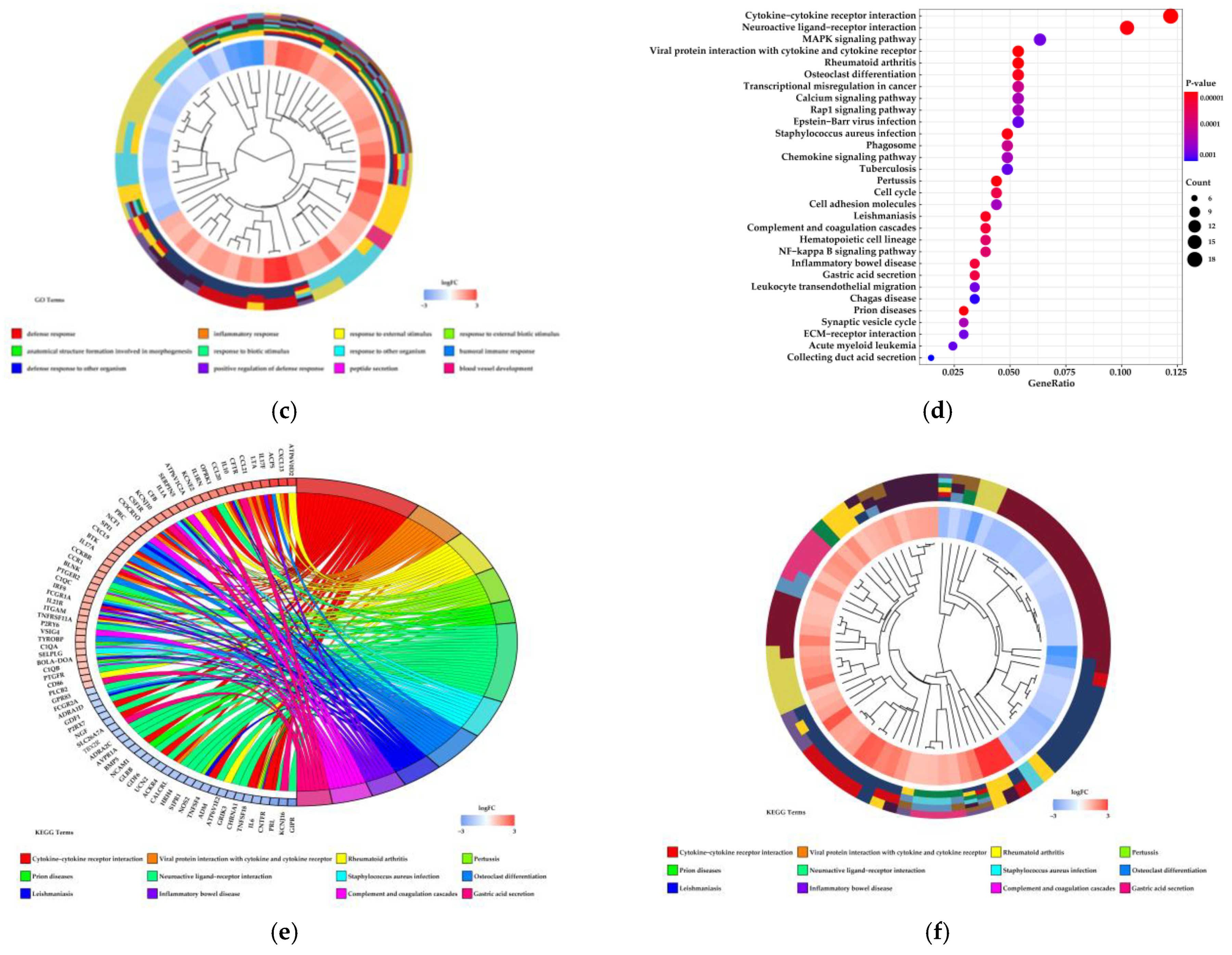
3.1.2. Functional Analysis of Differentially Expressed Genes
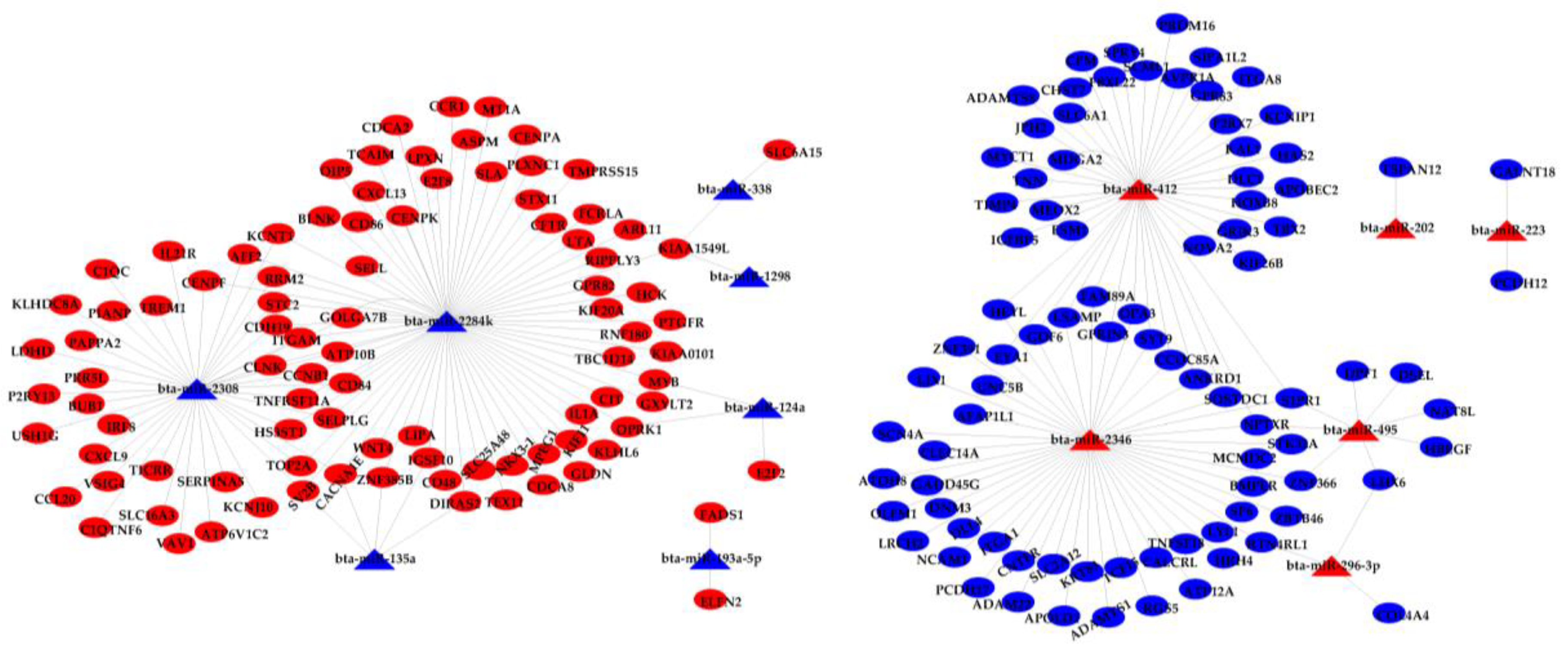
3.2. Integration Analysis of DE miRNAs and DE mRNAs
3.3. MiR-193a-5p Specific Targeting of FADS1
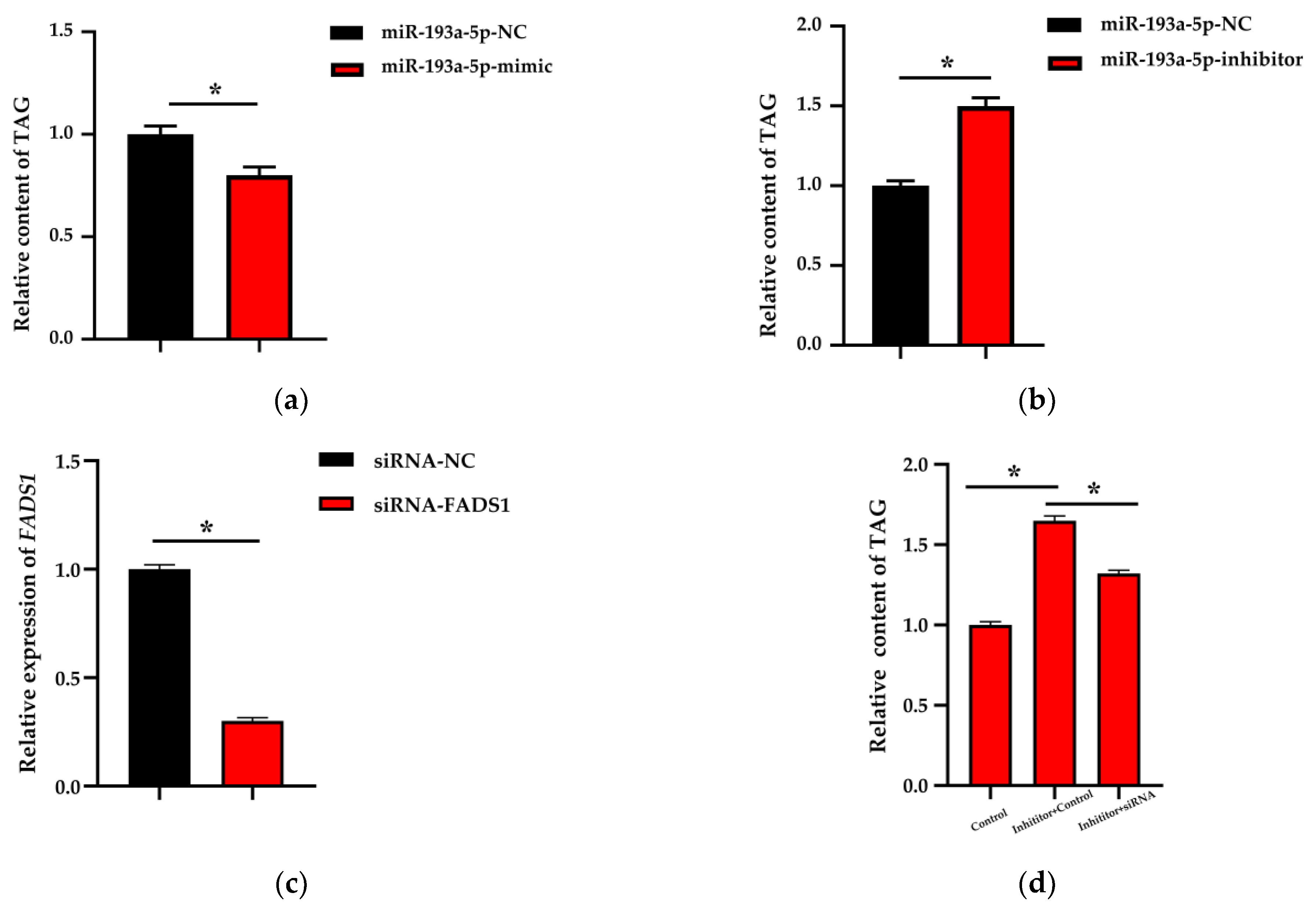
3.4. Triglycerides Content Analysis
3.5. Partial Rescue of the Triglycerides Reduction
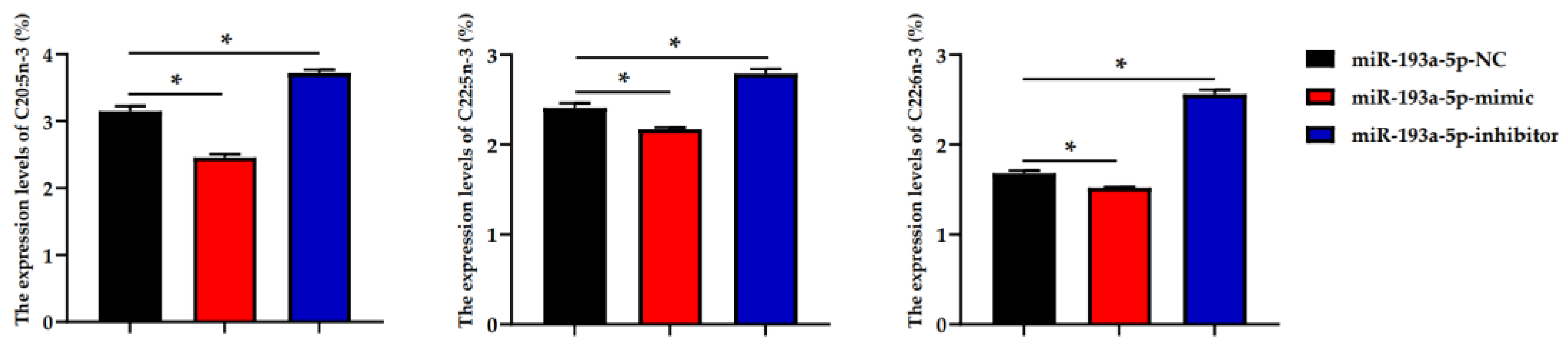
3.6. Fatty Acid Profiles Analysis
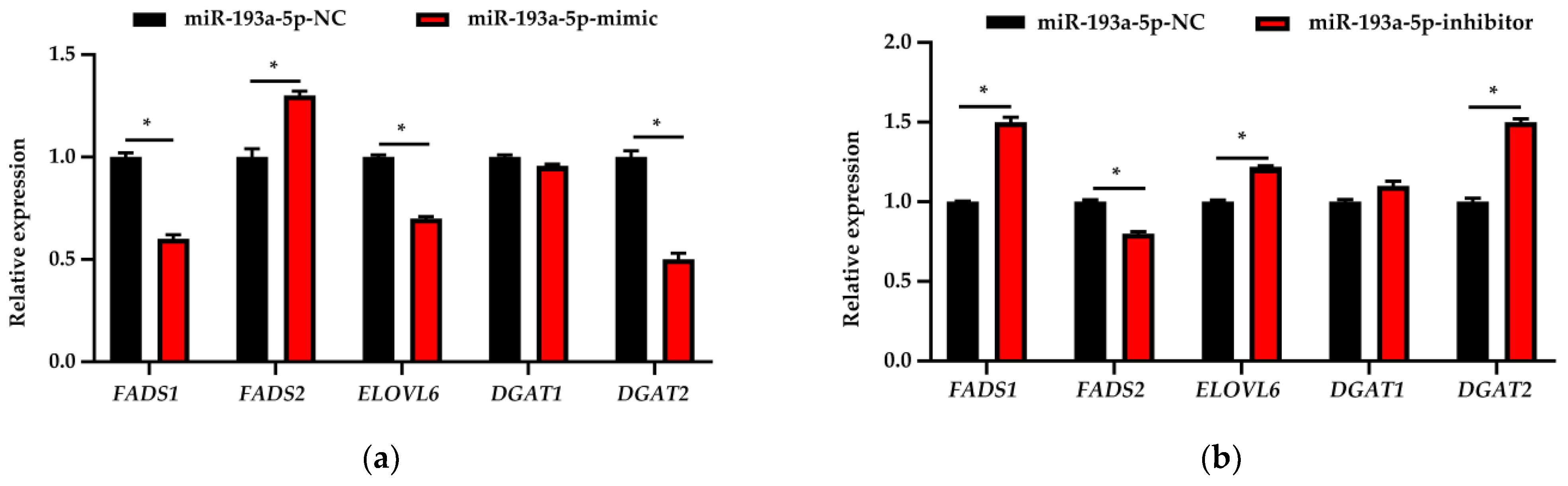
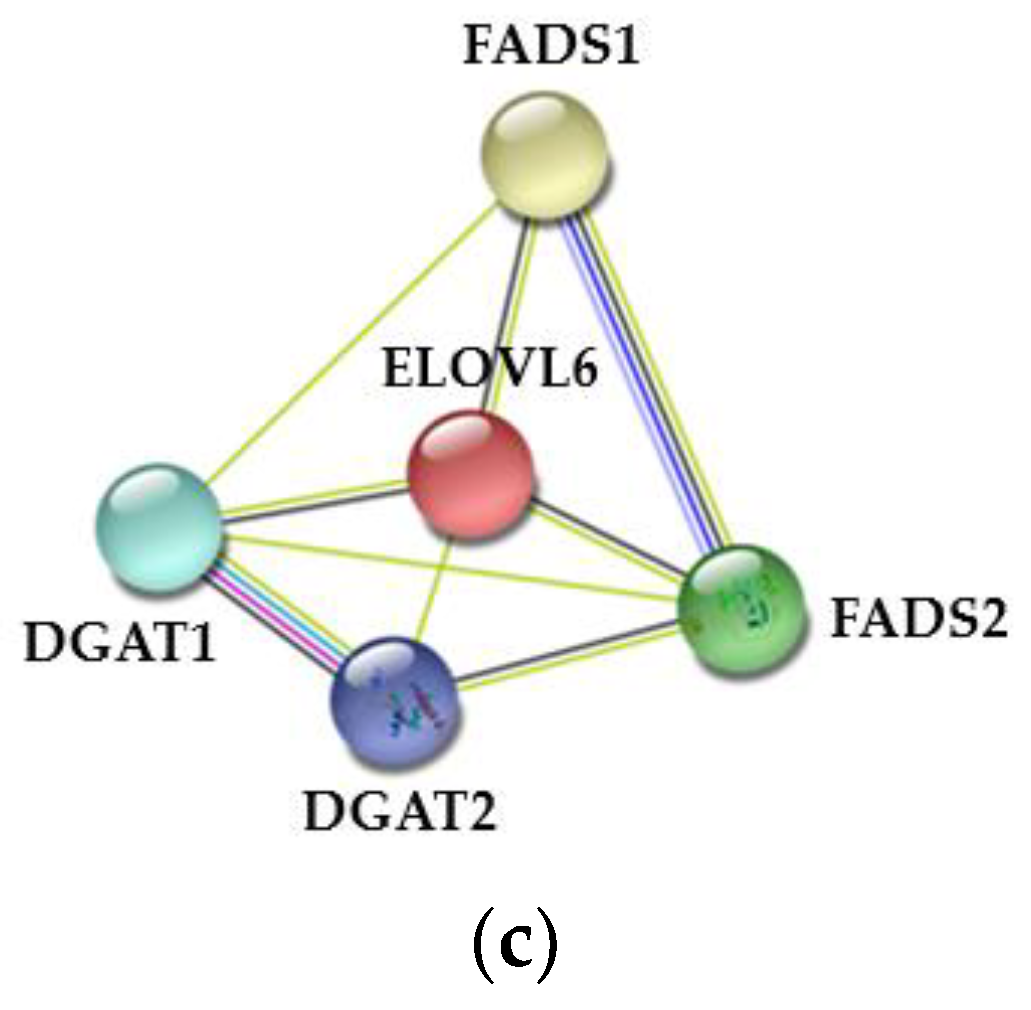
3.7. Expression Analysis of Fatty Acid Metabolism-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knutsen, T.M.; Olsen, H.G.; Tafintseva, V.; Svendsen, M.; Kohler, A.; Kent, M.P.; Lien, S. Unravelling genetic variation underlying de novo-synthesis of bovine milk fatty acids. Sci. Rep. 2018, 8, 2179. [Google Scholar] [CrossRef] [PubMed]
- Pușcaș, A.; Mureșan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Tsiafoulis, C.G.; Papaemmanouil, C.; Alivertis, D.; Tzamaloukas, O.; Miltiadou, D.; Balayssac, S.; Malet-Martino, M.; Gerothanassis, I.P. NMR-Based Μetabolomics of the Lipid Fraction of Organic and Conventional Bovine Milk. Molecules 2019, 24, 1067. [Google Scholar] [CrossRef] [PubMed]
- Michelle, B.; Kristina, P.; Penny, K.E. Saturated Fatty Acids and Cardiovascular Disease: Replacements for Saturated Fat to Reduce Cardiovascular Risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy Fats and Cardiovascular Disease: Do We Really Need to be Concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef]
- Yu, E.; Hu, F.B. Dairy Products, Dairy Fatty Acids, and the Prevention of Cardiometabolic Disease: A Review of Recent Evidence. Curr. Atheroscler. Rep. 2018, 20, 24. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Cao, H.; King, I.B.; Lemaitre, R.N.; Song, X.; Siscovick, D.S.; Hotamisligil, G.S. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: A cohort study. Ann. Intern. Med. 2010, 153, 790–799. [Google Scholar] [CrossRef]
- Duda, M.K.; O’Shea, K.M.; Lei, B.; Barrows, B.R.; Azimzadeh, A.M.; Mcelfresh, T.E.; Hoit, B.D.; Kop, W.J.; Stanley, C.W. Dietary supplementation with ω-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc. Res. 2007, 76, 303. [Google Scholar] [CrossRef]
- Mölenberg, F.J.M.; de Goede, J.; Wanders, A.J.; Zock, P.L.; Kromhout, D.; Geleijnse, J.M. Dietary fatty acid intake after myocardial infarction: A theoretical substitution analysis of the Alpha Omega Cohort. Am. J. Clin. Nutr. 2017, 106, 895–901. [Google Scholar] [CrossRef][Green Version]
- Wang, S.P.; Chen, Y.H.; Li, H. Association between the levels of polyunsaturated fatty acids and blood lipids in healthy individuals. Exp. Ther. Med. 2012, 4, 1107–1111. [Google Scholar] [CrossRef][Green Version]
- Fonollá, J.; López-Huertas, E.; Machado, F.J.; Molina, D.; Alvarez, I.; Mármol, E.; Navas, M.; Palacín, E.; García-Valls, M.J.; Remón, B.; et al. Milk enriched with “healthy fatty acids“ improves cardiovascular risk markers and nutritional status in human volunteers. Nutrition 2009, 25, 408–414. [Google Scholar] [CrossRef]
- Givens, D.I. Session 4: Challenges facing the food industry in innovating for health. Impact on CVD risk of modifying milk fat to decrease intake of SFA and increase intake of cis-MUFA. Proc. Nutr. Soc. 2008, 67, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, J.Q.; Bu, D.P. Ruminal microbe of biohydrogenation of trans-vaccenic acid to stearic acid in vitro. BMC Res. Notes 2012, 15, 97. [Google Scholar] [CrossRef][Green Version]
- Fang, X.; Zhong, G.; Wang, Y.; Lin, Z.; Lin, R.; Yao, T. Low GAS5 expression may predict poor survival and cisplatin resistance in cervical cancer. Cell Death Dis. 2020, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Sun, M.Y.; Song, N.H.; Deng, Z.L.; Xue, C.Y.; Yang, J. Prognostic role of microRNA-205 in multiple human malignant neoplasms: A meta-analysis of 17 studies. BMJ Open 2015, 5, e006244. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, J.; Zhang, T.; Tian, H.; Ma, Y.; Xu, H.; Yao, D.; Loor, J.J. MicroRNA-26a/b and their host genes synergistically regulate triacylglycerol synthesis by targeting the INSIG1 gene. RNA Biol. 2016, 13, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, H.; Luo, J.; Yi, Y.; Yao, D.; Zhang, X.; Ma, G.; Loor, J.J. MiR-145 Regulates Lipogenesis in Goat Mammary Cells Via Targeting INSIG1 and Epigenetic Regulation of Lipid-Related Genes. J. Cell. Physiol. 2017, 232, 1030–1040. [Google Scholar] [CrossRef]
- Lu, X.; Xia, H.; Jiang, J.; Xu, X.; Li, M.; Chen, Z.; Sun, Y.; Zhang, H.; Yang, Z. MicroRNA-212 targets SIRT2 to influence lipogenesis in bovine mammary epithelial cell line. J. Dairy Res. 2020, 87, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, H.; Lu, X.; Xu, C.; Li, M.; Chen, Z.; Yang, Z. MicroRNA-141 participates in milk lipid metabolism by targeting SIRT1 in bovine mammary epithelial cells. Anim. Prod. Sci. 2020, 60, 1877–1884. [Google Scholar] [CrossRef]
- Klaas, I.C.; Enevoldsen, C.; Vaarst, M.; Houe, H. Systematic clinical examinations for identification of latent udder health types in Danish dairy herds. J. Dairy Sci. 2004, 87, 1217–1228. [Google Scholar] [CrossRef]
- Thanh, L.P.; Suksombat, W. Milk Yield, Composition, and Fatty Acid Profile in Dairy Cows Fed a High-concentrate Diet Blended with Oil Mixtures Rich in Polyunsaturated Fatty Acids. Asian-Australas. J. Anim. Sci. 2015, 28, 796–806. [Google Scholar] [CrossRef]
- Boschetti, E.; Bordoni, A.; Meluzzi, A.; Castellini, C.; Dal Bosco, A.; Sirri, F. Fatty acid composition of chicken breast meat is dependent on genotype-related variation of FADS1 and FADS2 gene expression and desaturating activity. Anim. Int. J. Anim. Biosci. 2016, 10, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Innis, S.M. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J. Nutr. 2008, 138, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.T.; Zou, Y.X.; White, R.R.; Liu, J.X.; Liu, H.Y. Transcriptomic profiles of the bovine mammary gland during lactation and the dry period. Funct. Integr. Genom. 2018, 18, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Piantoni, P.; Wang, P.; Drackley, J.K.; Hurley, W.L.; Loor, J.J. Expression of metabolic, tissue remodeling, oxidative stress, and inflammatory pathways in mammary tissue during involution in lactating dairy cows. Bioinform. Biol. Insights 2010, 20, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cai, W.; Zhou, C.; Yin, H.; Zhang, Z.; Loor, J.J.; Sun, D.; Zhang, Q.; Liu, J.; Zhang, S. RNA-Seq reveals 10 novel promising candidate genes affecting milk protein concentration in the Chinese Holstein population. Sci. Rep. 2016, 2, 26813. [Google Scholar] [CrossRef] [PubMed]
- Dudhate, A.; Shinde, H.; Tsugama, D.; Liu, S.; Takano, T. Transcriptomic analysis reveals the differentially expressed genes and pathways involved in drought tolerance in pearl millet [Pennisetum glaucum (L.) R. Br]. PLoS ONE 2018, 13, e0195908. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Fan, W.; Zhong, Y.; Qin, M.; Lin, B.; Chen, F.; Yan, H.; Li, W.; Lin, J. Differentially expressed microRNAs in diapausing versus HCl-treated Bombyx embryos. PLoS ONE 2017, 12, e0180085. [Google Scholar] [CrossRef]
- Liu, H.; Seynhaeve, A.L.B.; Brouwer, R.W.W.; van IJcken, W.F.; Yang, L.; Wang, Y.; Chang, Z.; Ten Hagen, T.L.M. CREPT Promotes Melanoma Progression Through Accelerated Proliferation and Enhanced Migration by RhoA-Mediated Actin Filaments and Focal Adhesion Formation. Cancers 2019, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.L.; Yoshida, Y.; Major, I.T.; de Oliveira Ferreira, D.; Weraduwage, S.M.; Froehlich, J.E.; Johnson, B.F.; Kramer, D.M.; Jander, G.; Sharkey, T.D.; et al. Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 2016, 30, 12570. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zheng, X.; Zheng, Y.; Cao, R.; Zhang, M.; Sun, Y.; Wu, J. Construction and analysis of the lncRNA-miRNA-mRNA network based on competitive endogenous RNA reveals functional genes in heart failure. Mol. Med. Rep. 2019, 19, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, P.; Van Vlierberghe, P.; De Weer, A.; Muth, D.; Westermann, F.; Speleman, F.; Vandesompele, J. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009, 10, R64. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, L.; Han, K.; Zhang, X.; Zhang, G.; Dai, G.; Wang, J.; Xie, K. Transcriptome analysis of ovary in relatively greater and lesser egg producing Jinghai Yellow Chicken. Anim. Reprod. Sci. 2019, 2019, 106114. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Pang, S.; Tang, H.; Zhuo, S.; Zang, Y.Q.; Le, Y. Regulation of fasting fuel metabolism by toll-like receptor 4. Diabetes 2010, 59, 3041–3048. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, L.; Zhao, Z.; He, H.; Yang, D.; Feng, X.; Ma, S.; Chen, X.; Zhu, T.; Cao, T.; et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1α upregulation in mice. Cell Res. 2012, 22, 551–564. [Google Scholar] [CrossRef]
- Zhu, J.J.; Luo, J.; Wang, W.; Yu, K.; Wang, H.B.; Shi, H.B.; Sun, Y.T.; Lin, X.Z.; Li, J. Inhibition of FASN reduces the synthesis of medium-chain fatty acids in goat mammary gland. Anim. Int. J. Anim. Biosci. 2014, 8, 1469–1478. [Google Scholar] [CrossRef]
- Pan, G.; Ameur, A.; Enroth, S.; Bysani, M.; Nord, H.; Cavalli, M.; Essand, M.; Gyllensten, U.; Wadelius, C. PATZ1 down-regulates FADS1 by binding to rs174557 and is opposed by SP1/SREBP1c. Nucleic Acids Res. 2017, 45, 2408–2422. [Google Scholar] [CrossRef] [PubMed]
- Lattka, E.; Illig, T.; Koletzko, B.; Heinrich, J. Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr. Opin. Lipidol. 2010, 21, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.M.; Ma, D.W.; Mutch, D.M. Genetic variation in lipid desaturases and its impact on the development of human disease. Lipids Health Dis. 2010, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, e1332–e1345. [CrossRef]
- Mira, C.; Moya-Albor, E.; Escalante-Ramirez, B.; Olveres, J.; Brieva, J.; Vallejo, E. 3D Hermite Transform Optical Flow Estimation inLeft Ventricle CT Sequences. Sensors 2020, 20, 595. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef]
- Shi, H.B.; Du, Y.; Zhang, C.H.; Sun, C.; He, Y.L.; Wu, Y.H.; Liu, J.X.; Luo, J.; Loor, J.J. Fatty acid elongase 5 (ELOVL5) alters the synthesis of long-chain unsaturated fatty acids in goat mammary epithelial cells. J. Dairy Sci. 2018, 101, 4586–4594. [Google Scholar] [CrossRef]
- Shi, L.; Han, B.; Liu, L.; Lv, X.; Ma, Z.; Li, C.; Xu, L.; Li, Y.; Zhao, F.; Yang, Y.; et al. Determination of Genetic Effects of LIPK and LIPJ Genes on Milk Fatty Acids in Dairy Cattle. Genes 2019, 10, 86. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Jin, X.; Lo, L.; Liu, J. Expression profiles of microRNAs from lactating and non-lactating bovine mammary glands and identification of miRNA related to lactation. BMC Genom. 2012, 27, 731. [Google Scholar] [CrossRef]
- Davis, B.C.; Kris-Etherton, P.M. Achieving optimal essential fatty acid status in vegetarians: Current knowledge and practical implications. Am. J. Clin. Nutr. 2003, 78, 640s–646s. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.A.; Kantor, E.D.; Lampe, J.W.; Kristal, A.R.; Heckbert, S.R.; White, E. Intake of long-chain ω-3 fatty acids from diet and supplements in relation to mortality. Am. J. Epidemiol. 2014, 179, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Xun, P.; Iribarren, C.; Van Horn, L.; Steffen, L.; Daviglus, M.L.; Siscovick, D.; Liu, K.; He, K. Intake of fish and long-chain omega-3 polyunsaturated fatty acids and incidence of metabolic syndrome among American young adults: A 25-year follow-up study. Eur. J. Nutr. 2016, 55, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, R.; Jiang, F.; Zhang, H.; Zhao, A.; Xu, B.; Jin, L.; Wang, T.; Jia, W.; Jia, W.; et al. FADS1-FADS2 genetic polymorphisms are associated with fatty acid metabolism through changes in DNA methylation and gene expression. Clin. Epigenet. 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. Annu. Rev. Nutr. 2019, 39, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Ching, Y.K.; Chin, Y.S.; Appukutty, M.; Ramanchadran, V.; Yu, C.Y.; Ang, G.Y.; Gan, W.Y.; Chan, Y.M.; Teh, L.K.; Salleh, M.Z. Interaction of Dietary Linoleic Acid and α-Linolenic Acids with rs174547 in FADS1 Gene on Metabolic Syndrome Components among Vegetarians. Nutrients 2019, 11, 1686. [Google Scholar] [CrossRef]
- Joshi, K.; Gadgil, M.; Pandit, A.; Otiv, S.; Kothapalli, K.S.D.; Brenna, J.T. Dietary pattern regulates fatty acid desaturase 1 gene expression in Indian pregnant women to spare overall long chain polyunsaturated fatty acids levels. Mol. Biol. Rep. 2019, 46, 687–693. [Google Scholar] [CrossRef]
- Lankinen, M.; Uusitupa, M.; Schwab, U. Genes and Dietary Fatty Acids in Regulation of Fatty Acid Composition of Plasma and Erythrocyte Membranes. Nutrients 2018, 10, 1785. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Kuba, M.; Koyasu, S.; Yamamoto, Y.; Motomura, K.; Arulmozhiraja, S.; Ohno, H.; Sharma, R.; Shimura, T.; Okajima, Y.; et al. Hepatocyte ELOVL Fatty Acid Elongase 6 Determines Ceramide Acyl-Chain Length and Hepatic Insulin Sensitivity in Mice. Hepatology 2020, 71, 1609–1625. [Google Scholar] [CrossRef]
- Du, J.; Xu, Y.; Zhang, P.; Zhao, X.; Gan, M.; Li, Q.; Ma, J.; Tang, G.; Jiang, Y.; Wang, J.; et al. MicroRNA-125a-5p Affects Adipocytes Proliferation, Differentiation and Fatty Acid Composition of Porcine Intramuscular Fat. Int. J. Mol. Sci. 2018, 19, 501. [Google Scholar] [CrossRef]
- Takamura, T.A.; Tsuchiya, T.; Oda, M.; Watanabe, M.; Saito, R.; Sato-Ishida, R.; Akao, H.; Kawai, Y.; Kitayama, M.; Kajinami, K. Circulating malondialdehyde-modified low-density lipoprotein (MDA-LDL) as a novel predictor of clinical outcome after endovascular therapy in patients with peripheral artery disease (PAD). Atherosclerosis 2017, 192–197. [Google Scholar] [CrossRef]
- Shi, H.; Wang, L.; Luo, J.; Liu, J.; Loor, J.J.; Liu, H. Fatty Acid Elongase 7 (ELOVL7) Plays a Role in the Synthesis of Long-Chain Unsaturated Fatty Acids in Goat Mammary Epithelial Cells. Anim. Open Access J. 2019, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. Thematic review series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.J.; Myers, H.M.; Watkins, S.M.; Brown, B.E.; Feingold, K.R.; Elias, P.M.; Farese, R.V., Jr. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 2004, 279, 11767–11776. [Google Scholar] [CrossRef] [PubMed]
- Cases, S.; Stone, S.J.; Zhou, P.; Yen, E.; Tow, B.; Lardizabal, K.D.; Voelker, T.; Farese, R.V., Jr. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 2001, 276, 38870–38876. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Ou, K.; Wang, T.; Li, Z.; Tian, Y.; Wang, Y.; Kang, X.; Li, H.; Liu, X. Evolution, dynamic expression changes and regulatory characteristics of gene families involved in the glycerophosphate pathway of triglyceride synthesis in chicken (Gallus gallus). Sci. Rep. 2019, 9, 12735. [Google Scholar] [CrossRef]
- Huo Yung Kai, S.; Bongard, V.; Simon, C.; Ruidavets, J.B.; Arveiler, D.; Dallongeville, J.; Wagner, A.; Amouyel, P.; Ferrières, J. Low-fat and high-fat dairy products are differently related to blood lipids and cardiovascular risk score. Eur. J. Opreventive Cardiol. 2014, 21, 1557–1567. [Google Scholar] [CrossRef]
- Turpeinen, O. Effect of cholesterol-lowering diet on mortality from coronary heart disease and other causes. Circulation 1979, 59, 1–7. [Google Scholar] [CrossRef]
- Li, M.; Lu, X.; Gao, Q.; Wang, M.; Arbab, A.A.I.; Sun, Y.; Chen, Z.; Zhang, H.; Karrow, N.A.; Yang, Z.; et al. A Functional 3’ UTR Polymorphism of FADS2 Affects Cow Milk Composition through Modifying Mir-744 Binding. Anim. Open Access J. 2019, 9, 1090. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Chen, Z.; Cao, W.T.; Xu, H.F.; Gou, D.M.; Zhu, J.J. MicroRNA-24 can control triacylglycerol synthesis in goat mammary epithelial cells by targeting the fatty acid synthase gene. J. Dairy Sci. 2015, 98, 9001–9014. [Google Scholar] [CrossRef]



| Fatty Acid (%) | Treatment | ||
|---|---|---|---|
| NC | miR-193a-5p-Mimic | miR-193a-5p-Inhibitor | |
| C12:0 | 0.16 ± 0.02 | 0.15 ± 0.02 | 0.15 ± 0.02 |
| C14:0 | 1.51 ± 0.03 c | 1.59 ± 0.05 b | 1.66 ± 0.04 a |
| C16:0 | 21.31 ± 0.26 b | 23.29 ± 0.32 a | 21.33 ± 0.35 b |
| C18:0 | 27.16 ± 0.51 a | 25.20 ± 0.38 b | 27.38 ± 0.52 a |
| C16:1 | 1.58 ± 0.03 | 1.56 ± 0.04 | 1.53 ± 0.02 |
| Cis-9-C18:1 | 21.05 ± 0.46 | 21.01 ± 0.34 | 21.09 ± 0.37 |
| C18:1 | 14.64 ± 0.24 b | 15.25 ± 0.13 a | 13.11 ± 0.17 c |
| C18:2 | 2.26 ± 0.05 | 2.23 ± 0.04 | 2.21 ± 0.03 |
| C20:4n-3 | 3.09 ± 0.07 b | 3.57 ± 0.11 a | 2.47 ± 0.06 c |
| C20:5n-3 | 3.15 ± 0.08 b | 2.46 ± 0.05 c | 3.72 ± 0.05 a |
| C22:5n-3 | 2.41 ± 0.05 b | 2.17 ± 0.02 c | 2.79 ± 0.05 a |
| C22:6n-3 | 1.68 ± 0.03 b | 1.52 ± 0.01 c | 2.56 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Arbab, A.A.I.; Zhang, H.; Yang, Y.; Lu, X.; Han, Z.; Yang, Z. MicroRNA-193a-5p Regulates the Synthesis of Polyunsaturated Fatty Acids by Targeting Fatty Acid Desaturase 1 (FADS1) in Bovine Mammary Epithelial Cells. Biomolecules 2021, 11, 157. https://doi.org/10.3390/biom11020157
Fan Y, Arbab AAI, Zhang H, Yang Y, Lu X, Han Z, Yang Z. MicroRNA-193a-5p Regulates the Synthesis of Polyunsaturated Fatty Acids by Targeting Fatty Acid Desaturase 1 (FADS1) in Bovine Mammary Epithelial Cells. Biomolecules. 2021; 11(2):157. https://doi.org/10.3390/biom11020157
Chicago/Turabian StyleFan, Yongliang, Abdelaziz Adam Idriss Arbab, Huimin Zhang, Yi Yang, Xubin Lu, Ziyin Han, and Zhangping Yang. 2021. "MicroRNA-193a-5p Regulates the Synthesis of Polyunsaturated Fatty Acids by Targeting Fatty Acid Desaturase 1 (FADS1) in Bovine Mammary Epithelial Cells" Biomolecules 11, no. 2: 157. https://doi.org/10.3390/biom11020157
APA StyleFan, Y., Arbab, A. A. I., Zhang, H., Yang, Y., Lu, X., Han, Z., & Yang, Z. (2021). MicroRNA-193a-5p Regulates the Synthesis of Polyunsaturated Fatty Acids by Targeting Fatty Acid Desaturase 1 (FADS1) in Bovine Mammary Epithelial Cells. Biomolecules, 11(2), 157. https://doi.org/10.3390/biom11020157





