1. Introduction
Everyday exposure to different loads is known to affect the human body and cause musculoskeletal diseases [
1]. Bones are a prominent component of this system and are often associated with recurrent injuries and illnesses. From both an economic and health perspective, musculoskeletal problems can impose a persistent burden on individual countries. A fracture of the femur can result in job absenteeism, which then triggers a domino effect, i.e., diminished productivity, a reduced quality of life, significant treatment expenses, and sociopsychological issues. These consequences, among others, have prompted researchers to investigate many domains and to propose multidisciplinary solutions for the development of artificial bone structures that are capable of supporting, assisting, and/or replacing bone tissue [
2,
3,
4].
Bone is a type of connective tissue, which is identifiable by its heterogenous composition, both hierarchy- and component-wise; it comprises both organic (e.g., collagen type I) and inorganic compounds (e.g., hydroxyapatite). Bone stores minerals and provides protection and support to other tissues [
5,
6]. According to the current understanding of bone tissue engineering, bone regeneration requires three main components—cells that can generate a functional matrix; bio-active substances, such as growth factors, which promote specific issues; and scaffolds, which provide mechanical support and cellular adhesion [
7].
Consequently, scaffolding is a fundamental element of bone tissue engineering. The objective of bone tissue scaffolds is to emulate the composition and functionality of the natural bone’s extracellular matrix (ECM), facilitating a three-dimensional (3D) environment that encourages adhesion, proliferation, and differentiation, while possessing the requisite physical properties for bone regeneration [
8]. Desirable scaffold qualities include biodegradability, biocompatibility, bioactivity, osteoconductivity, and osteoinductivity [
4]. Bone scaffolds incorporating biomaterials and additives, including pharmaceuticals [
9,
10,
11], growth factors [
11,
12,
13], and stem cells [
14,
15,
16], have demonstrated efficacy in bone regeneration [
17].
Considering the nature of the body, cells that comprise tissues are subjected to a wide range of stimuli, which, in turn, affects their function and fate [
18]. Consequently, researchers have been focusing their attention on the development of scaffold designs that have the potential to address the temporal properties of native cells. A great number of studies have demonstrated that dynamic physical stimuli, such as those that are electrical, magnetic, acoustic, and mechanical, have the ability to efficiently control a wide variety of cell responses. On the other hand, extensions to in vivo applications, which require stimuli from the outside, have been restricted [
19,
20,
21,
22,
23,
24]. Ultrasonic stimulation is one of the most frequently utilized methods in clinical settings for the sake of both therapeutic [
25] and diagnostic applications [
26], having been used for a number of years [
24]. From a cell behavior perspective, as seen in clinics and the literature, ultrasound stimulation has been used as a tool for supporting and advancing the healing process of defected tissue. In particular, one research paper that focuses on the cell behavior under ultrasound effect can explain the potential outcomes. As pointed out by the referenced article, cells under ultrasound stimulation can experience translation, proliferation, apoptosis, lysis, oscillation, and transient membrane permeation [
27]. Additionally, they have been reported to observe internal and cytoskeletal changes in cells. In light of the previously mentioned clinical uses, ultrasound has been used as a tool for bone tissue healing (in the form of low-intensity pulsed ultrasound) and as a source of mechanical stimulation that can activate the integrin/phosphatidylinositol 3-OH kinase/Akt pathway and upregulate osteogenic proteins by producing prostaglandin E and cyclooxygenase-2 (COX-2) [
28]. Thus, in the presence of ultrasound stimulation, such behaviors from the cell have the potential to be observed.
Researchers have recently begun investigating the possibility of using ultrasound stimulation (US) in conjunction with tissue scaffolds to enhance tissue growth and regeneration. The majority of these studies have focused on the interaction between ultrasound waves and developing tissue, while ignoring the key factor of mechanical stimulation, which results from the waves interacting with the scaffold [
24,
29,
30]. Additionally, a great number of studies have utilized finite element analysis (FEA) to investigate the various effects of ultrasound (such as in the form a tool for investigating material–ultrasound wave interactions and tissue scaffold–ultrasound wave interactions). Loving et al. revealed that the fiber orientation and defect size in bioinspired composites substantially influenced the energy and propagation direction of ultrasonic waves within the composite [
31], while Zhao et al. employed Mia scattering as an analytical tool to evaluate the validity of both experimental and computational research regarding ultrasonic wave propagation in tissue scaffolds [
32]. Thus, it was observed that scattering predominantly occurs at the scaffold surface and that reduced pore size and lower wave signal frequencies facilitate the transmission of wave energy through the scaffold. A subsequent study examined the peak density, which is defined as the number of local peaks and troughs in the frequency power spectrum of the ultrasonic signal following its transmission through a material [
33]. Furthermore, researchers have also utilized ultrasound technology for PLA and PCL scaffolds as an element for stimulation as part of the production process. Khodaei et al. utilized ultrasound vibrations as part of the process of 3D printing in order to inhibit any clogging that occurs due to the presence of nanoparticles. For this research, PLA scaffolds containing different percentages of akerminite were printed via the Fused Filament Fabrication method. The authors reported that ultrasonic-assisted 3D printing is an improved manufacturing process that can overcome the potential limitations resulting from viscosity [
34]. Another approach that used ultrasound as part of its production technique was investigated by Olmo et al., who utilized PLA, PLA:PEG, and PLA with NaCl nanoparticles and ultrasound as part of a molding process in the production of scaffolds [
35]. In summary, the current literature focuses on different materials and techniques, with the purpose of using ultrasound in combination with tissue scaffolds. Nevertheless, one study focuses on bone regeneration via the use of two commonly used biopolymers—polycaprolactone (PCL) and polylactic acid (PLA). Camarero-Espinosa et al. manufactured PLA/PCL blends in different percentages in order to achieve phase segregation, which was the main application for ultrasound technology. They further discovered that in the phase-segregated PCL/PLA blend (50:50), one polymer acts as a damping element (PCL), while the other one acts as a deflecting element (PLA). They named these types of scaffolds Janus scaffolds (named after Janus particles), and experimental results showed that under US simulation, increases in cell proliferation, osteogenic differentiation, and the matrix deposition of bone marrow-derived stromal cells (BMSCs) were achieved [
24]. However, there are a few issues that need to be further investigated. First, the tissue scaffolds that were used during experiments were scaffolds with a uniform porosity and a single layer. As has been established, the biomimicry of bone tissues is complicated due to the nature of bone, and a significant amount of research has been conducted to try and achieve optimal properties (pore size, porosity percentage, geometrical structure, etc.). Another problem that needs to be pointed out is the manufacturing method. The 3D printer that was used for this study deposits pellets by melting them directly onto the surface, and both polymers were purchased in pellet forms. By adapting this manufacturing method into filament-based FDM, 3D printers would improve the availability and accessibility of such a technology. Such potential improvements have led us to conduct the current study. This study aims to address the following research gaps: the limitation of the current literature in relation to ultrasound-responsive scaffold manufacturing methods lacking accessibility; the need for the adaptation and application of ultrasound technology (e.g., low ultrasonic vibrations sourced from an ultrasound generator) with FDM filaments and FDM printing for improved accessibility; the need for research regarding ultrasound technology applications with tissue scaffolds that possess a complex geometry (e.g., TPMS and functionally gradient), i.e., the need to investigate and understand scaffold and ultrasound wave interactions for these scaffolds and what these interactions can mean for bone tissue scaffolds; and, due to the current literature lacking focus on scaffold geometry complexity, the need for further investigation into how ultrasound interacts with such complexity in scaffold layers as well as the geometry of the scaffolds.
In this study, the potential of commercial FDM filaments for ultrasound technology in bone tissue scaffolds was investigated within the scope of the key elements of scaffold composition (i.e., the production of ultrasound-responsive materials), mechanical characteristics (e.g., flexural modulus, hardness, and elastic modulus), and printability (i.e., the potential to be adapted into additive manufacturing). These key elements are based on both the current ultrasound-based studies and the essential characteristics of bone tissue scaffolds. With this aim, by using commercialized PLA FDM filaments and medical-grade PCL, the following aspects were investigated: (1) the adaptability of the suggested technology to FDM printing via the production and analysis of printing conditions; (2) the presence of phase segregation between PCL and PLA polymers, which creates the dynamic nature of a scaffold, via X-ray diffraction (XRD), Raman spectroscopy, and scanning electron microscopy (SEM); (3) the mechanical characteristics of polymer blends compared to commercial PCL and PLA filaments via nanoindentation testing; (4) the flexural modulus of PLAPCL4060 and PLAPCL5050 to determine responsiveness under ultrasound propagation; and (5) the potential of the proposed technology in bone tissue scaffolds via investigating the accelerated degradation of TPMS scaffolds with uniform, x/y, and radial gradients in the presence of ultrasound stimulation.
4. Discussion
In this study, the potential of using commercial filaments for ultrasound technology in bone tissue scaffolds was investigated. For this purpose, experiments were conducted, and the results of these experiments are reported herein.
Even though a combination of commercial filaments of PLA and medical-grade polymers of PCL was used for the production of customized filaments, forward characterizations were all conducted using PCL FDM filaments. Such an approach was chosen due to the limitation of not being able to use medical-grade polymers with FDM printers. However, the results and following statements are believed to hold valid reasoning due to both types being the same polymer in different forms (FDM filament and pellets), as well as our main aim being the adaptation of ultrasound technology using FDM printers for accessibility.
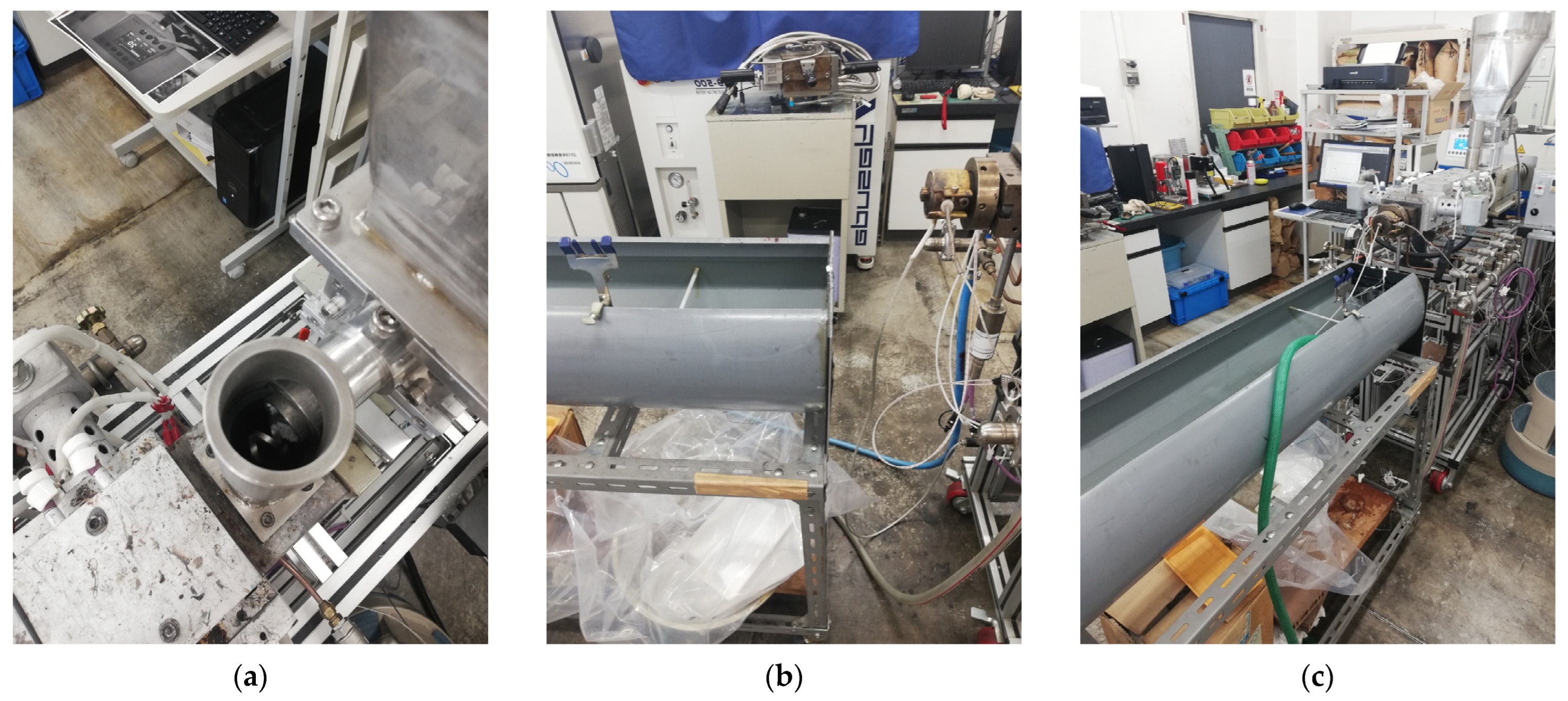
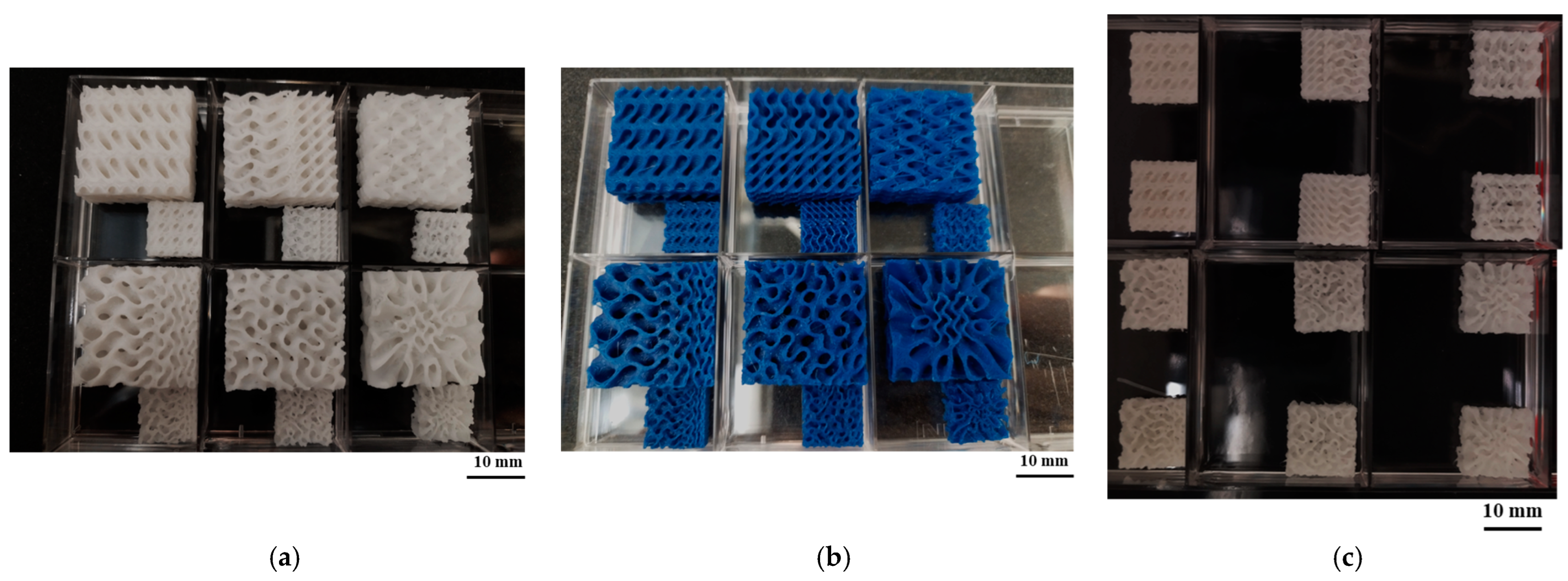
The scaffolds displayed in
Figure 7 are the outcome of an analysis of the printing conditions. As seen in
Figure 7a, the resolution of the printed samples changes for PLA/PCL composite filaments depending on the scale they are printed on. It was found that in comparison to PLA, the FDM filaments of PLAPCL4060 and PLAPCL5050 could not maintain their precision and quality within the overall structure as the complexity increased and dimension size scale decreased. Furthermore, a comparison of two PLAPCL mixture-based constructions revealed variations in quality and precision, as shown in
Figure 7a,c. PLAPCL5050 samples provided continued precision, while PLAPCL4060 scaffolds showed defects in patterns as the structure became complex (
Figure 7a,c). Such an observation can be explained by the higher percentage of PLA in PLAPCL5050 scaffolds. PLA, with its lower flexibility, is easy to adapt for use in various printing methods, whereas PCL has been known to be challenging, especially in FDM printing.
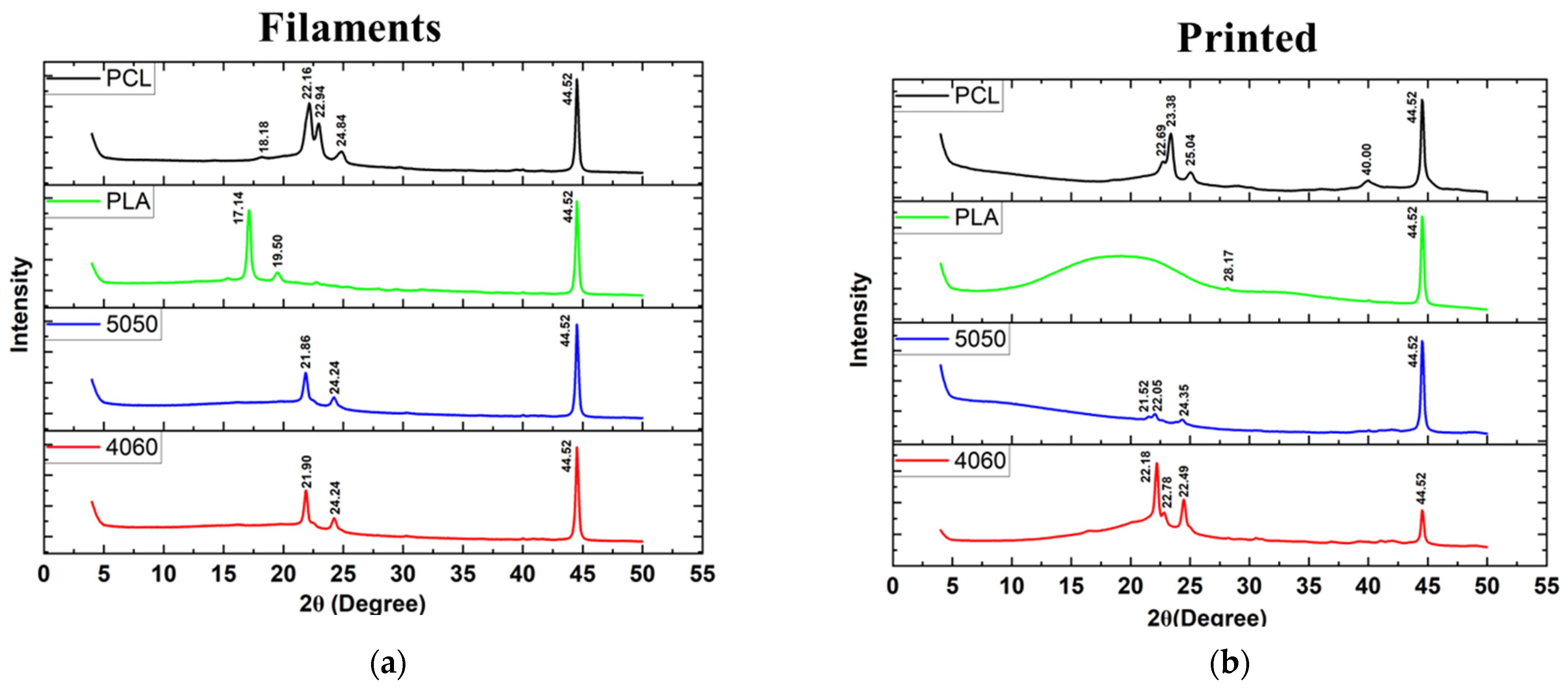
Pure PCL shows peaks at 2θ = 21.5° and 23.85°, corresponding to the crystal planes of (110) and (200), respectively [
49]. However, in this study, XRD spectra (
Figure 8) revealed shifted peaks for PCL FDM filaments and customized filaments containing PCL (PLAPCL4060 and PLAPCL500) for both printed structures and filaments alone. Such phenomena could be explained in several ways. One of the reasons could be the presence of additional compounds in FDM filaments causing the change within the matrix, as seen from Raman spectra identification for certain samples (see
Supplementary Materials, Supplementary Raman) that were different from the samples’ original polymer. For instance, Zhou et al.’s XRD and Raman spectra results for pure PLA display peaks that are different from those in
Figure 8 and
Figure 9 [
50]. These results, as well as those of several other studies, provide evidence that can be used to support the idea that the shifts potentially occur due to the commercial nature of the filaments [
51,
52]. These results can also be explained by focusing on the XRD spectra results for PLAPCL4060 and PLAPCL5050. It is possible that chain confinement during PCL crystal growth or the solid PLA served as a nucleation site for PCL crystal formation during PCL crystallization in a PLA/PCL blend. Sanandaji et al. [
53] assert that substantial chain confinement results in elevated equilibrium melting temperatures, reduced melting temperatures (Tm), and diminished crystallinities. No significant alterations in T
m were noted between PCL and PCL in the various blends (Table 1); however, the normalized crystallinity of PCL in Table 1 diminished as the PCL fraction in the blends decreased. In addition, the T
eq m values for PCL in the blends were marginally elevated compared to those of neat PCL (Table 2). These two discoveries may suggest chain confinement in the blends when PLA constitutes the predominant phase, which would also explain the tighter crystal size distribution for PCL in the blends [
54]. Another reason behind the shifts could be due to the crystallinity changes that occur within the polymer during the extrusion process. The crystallinity of polymers can be adjusted in various ways. One of these ways is to introduce additives to the polymer matrix through physical treatment (e.g., solution mixing, evaporation, and annealing) [
55]. It is possible that during the extrusion process, the additives present in the PLA FDM filament might have caused the change in crystallinity. Another cause of the potential shifts can be explained from the basic description of the occurrence of crystallinity within polymers. In the literature, it is known that upon cooling from melting, solvent evaporation, or mechanical stretching, polymers can crystallize. Therefore, even without the additive, the heat treatment that occurs during extrusion might be one of the reasons that causes the shifts in crystallinity peaks.
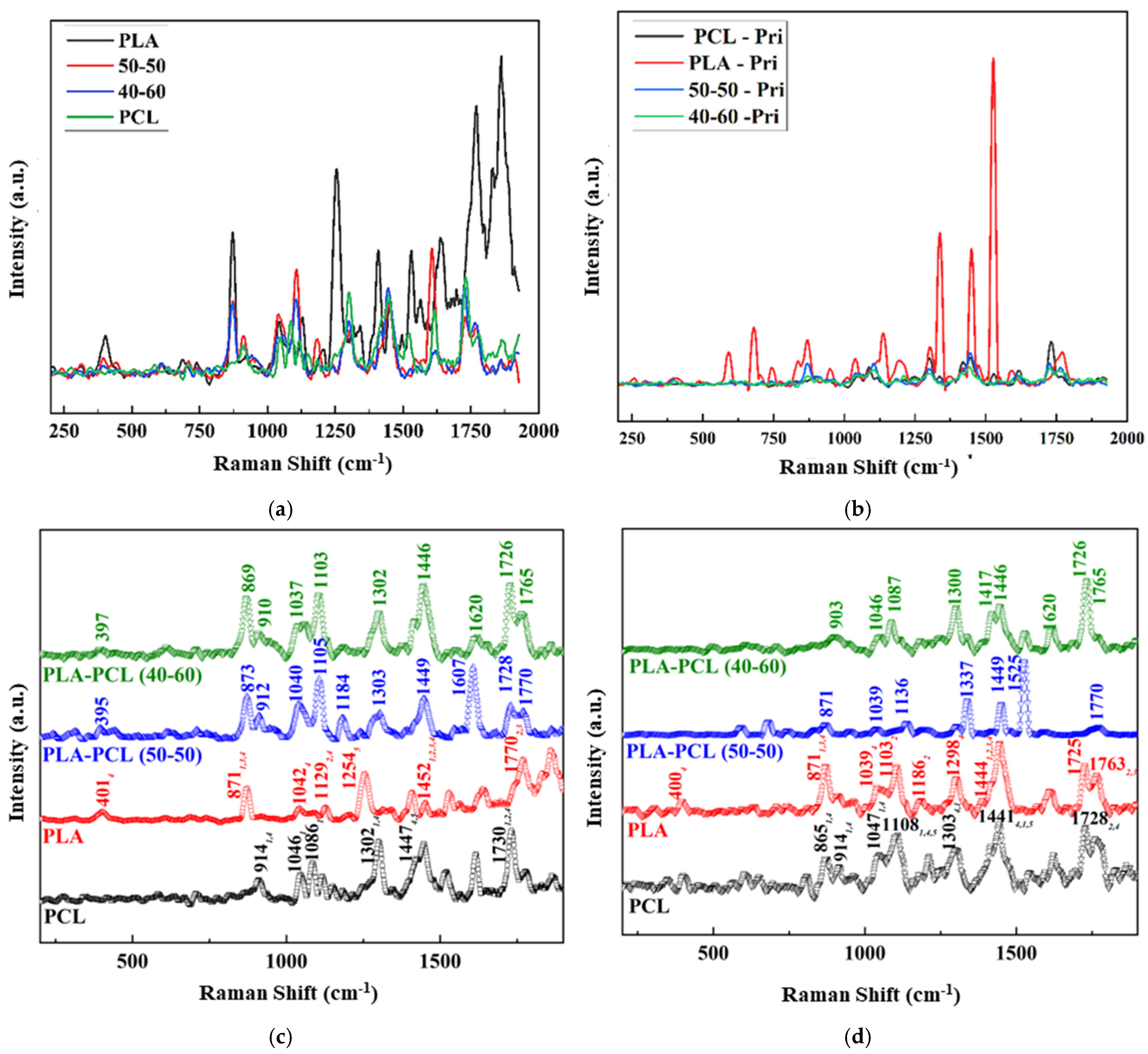
The Raman spectra identification results (
Figure 9) based on the referenced database used in Origin (
Figure 9), as well as the Raman spectra database provided within open-source software (OpenSpecy) (see
Supplementary Materials, Supplementary Raman), have provided information supporting the XRD results and the potential shifts that were observed in the XRD spectra. Our Raman spectra analysis (
Figure 9) showed the occurrence of phenomena similar to those observed in the XRD results; i.e., there were shifts from the referenced Raman shifts for PLAPC4060 and PLAPCL5050, which matched with the reference PLA and PCL Raman shifts. Such changes could be due to the presence of additives that were used during the production of commercial FDM filaments. However, as shown in
Section 2.3.4., for both filament and printed samples, Raman shifts regarding the databases of PLA and PCL matched exactly with the referenced literature. As such, from then on, any deductions were performed while considering the shift changes that were observed. For PLAPCL4060, the presence of PCL and PLA was detected both in the filament and printed samples, while in PLAPCL5050, only PLA peaks were detected. Such outcomes show a divergence from the results provided in the
Supplementary Materials, which were based on the OpenSpecy spectrometer.
The Raman spectrum analysis shown in
Supplementary Raman suggests the presence of PCL in filaments of PCL and PLAPCL4060, as well as in the printed structures of PLAPCL4060, PLAPCL5050, and PCL. However, for PLA and PLAPCL5050 filaments and printed PLA structures, the identification did not show the presence of either PLA or PCL. As mentioned in the previous paragraph, these deviations from the original polymer could be due to additional materials being used during the production of commercial filaments. However, such outcomes do not diminish the potential of commercial filaments for use in ultrasound technology. Moreover, the presence of PCL provides proof of a successful integration between commercial and medical-grade polymers for use in FDM printing.
Based on the XRD and Raman spectra analysis, a further look at the ultrasound responsiveness of these filaments and the implications of these potential causes (e.g., additive, chain confinement, and crystallinity change) should be discussed in detail. The presence of additives as a result of FDM filaments, in relation to ultrasound responsiveness, could be discussed from a polymer matrix perspective. From a basic definition of ultrasound propagation, the physical properties of the medium (acoustic velocity, density, elasticity, and impedance) affect the propagation of ultrasound waves. Thus, in the case of the additive’s presence in a matrix, the density of sections that have additives might potentially differ; the elastic modulus could potentially be affected, as well as the impedance. As the density of the medium increases, the acoustic impedance will increase. Similarly to the difference between a soft tissue like the lung and a hard tissue like bone, the large acoustic difference will create a strong reflection. From an ultrasound responsiveness point of view, one can deduce that a strong reflection would decrease its responsiveness since the reflected wave would not be able to reach deeper layers. Additionally, only a small number would be traveling to deeper layers. However, this issue could be easily resolved by further investigating the correlation between height and penetration depth in order to define a range of dimensions for bone tissue scaffolds. There is a potential for chain confinement in the polymer matrix due to diminished crystallinities; such cases can affect the responsiveness of the matrix. Based on the reference article, the responsiveness of PLA/PCL comes from the PLA acting as a reflecting element (amorphous from melt) and PCL (crystalline from melt) as a damping element within the PLAPCL matrix [
24]. However, in theory, the crystallinity changes could potentially be enhanced (in the case of additional crystallinity formations) or decreased (in case the amorphous nature of PLA is affected). Thus, all of the potential causes of the observed shifts in peaks can potentially affect the ultrasound responsiveness in both a positive and a negative way.
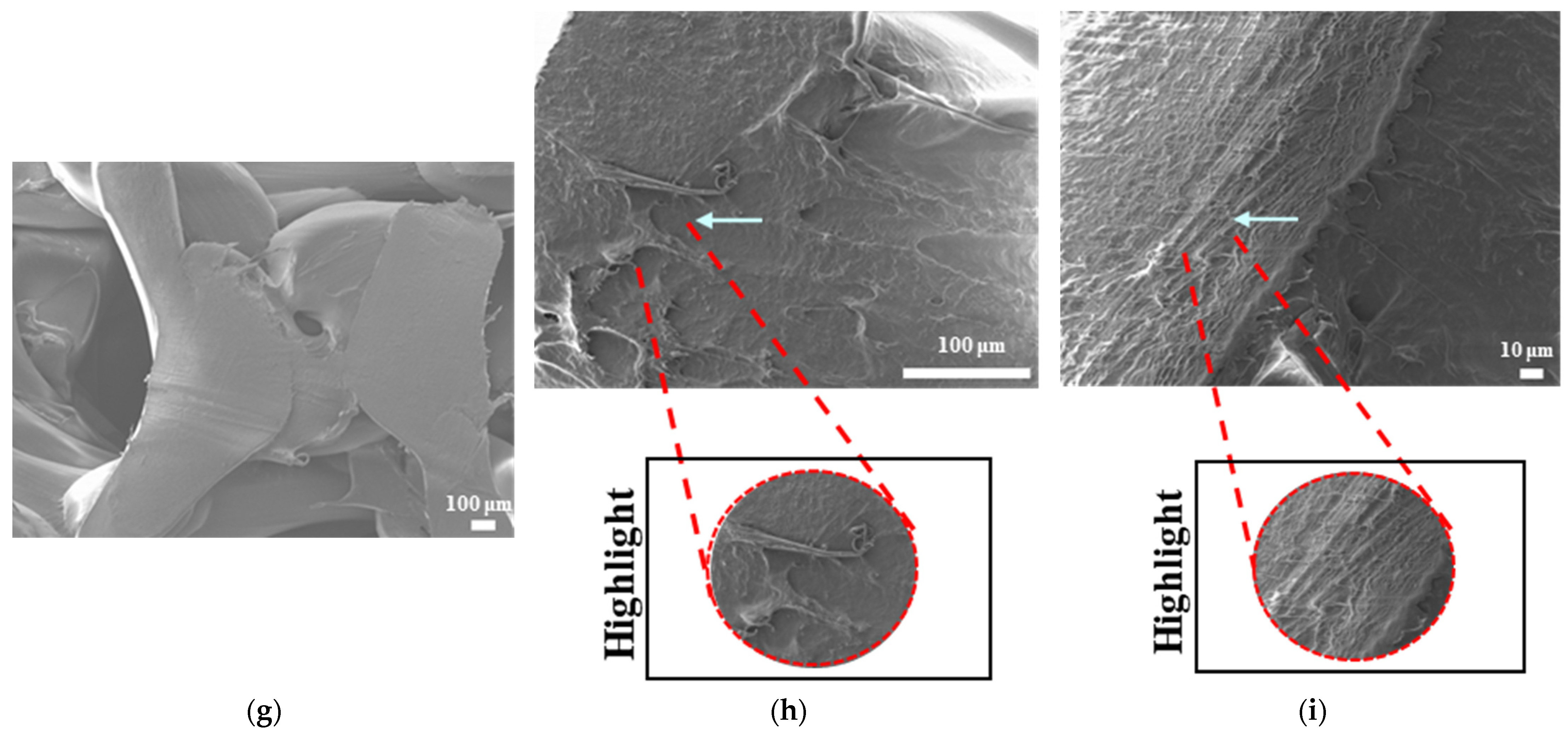
Scanning electron microscopy was primarily used to investigate phase segregation. For such a purpose, the contrast differences mentioned in [
24] and the surface structures were taken into account (for the presence of crystal and amorphous structures). The results from
Figure 10c show a contrast phase difference between lighter gray (PLA, according to the reference article, is observed to be bright) and darker gray (PCL, according to the reference article, is observed to be dark) for PLAPCL4060. For printed PLAPCL4060 (
Figure 11), the same difference can be observed, accompanied by nonhomogeneous surface structures. However, for PLAPCL5050, no such difference in the means of contrast was observed, even though a variance in the surface structures was present. Such results led to the conclusion that a phase segregation for PLAPCL4060 commercial filaments was achieved, differing from the 50:50 PLA:PCL ratio reference articles. In the referenced study, polymers with equal MWs were used, while FDM filaments are reported to be known to potentially have a MW range (PLA: 50,000–140,00 Da, containing the 60,000–80,000 Da); as such, differences in percentages were expected. We would like to mention that even though contrast differences were observed, the limitations of the methods and the results should be discussed. Even though qualitative evidence of the contrast difference was observed, there are still limitations that can potentially arise from using SEM as a method of investigation. As reported in the literature, SEM only allows for a limited amount of thickness to be observed and does not provide a characterization of bulk samples. However, due to the homogeneity of the sample composition and the uniformity between samples prepared for SEM observation, one can take such observations as representative of the bulk structure. Another limitation comes from SEM being based on the main principle of electron microscopy. The reflecting electrons are the ones that create the image, and they can be affected by any height differences that exist on the sample surface. However, the samples used for these experiments (
Figure 11,
Figure 12 and
Figure 13) were all the same scaffold structures that possess the exact same patterns and geometry. Thus, such uniformity prevents any limitation that can affect the outcomes. On the other hand, we also acknowledge the limitation that comes from SEM’s surface-specific nature, which can be summarized as follows. SEM mostly offers details about a sample’s outermost layer. This restriction results from the method’s operation, whereby an electron beam scans the surface, and the image that is produced depends on how the electrons interact with the material. SEM can show the composition and surface morphology in detail, but it cannot reveal the sample’s bulk characteristics or deeper structures. Furthermore, a more detailed investigation with transmission electron microscopy (TEM) can further prove the presence of phase segregation between PLA and PCL. These outcomes and future results (provided from the plans of a detailed investigation with TEM) would further support our main aim of utilizing commercial filaments and FDM technology for ultrasound technology and bone tissue scaffolds.
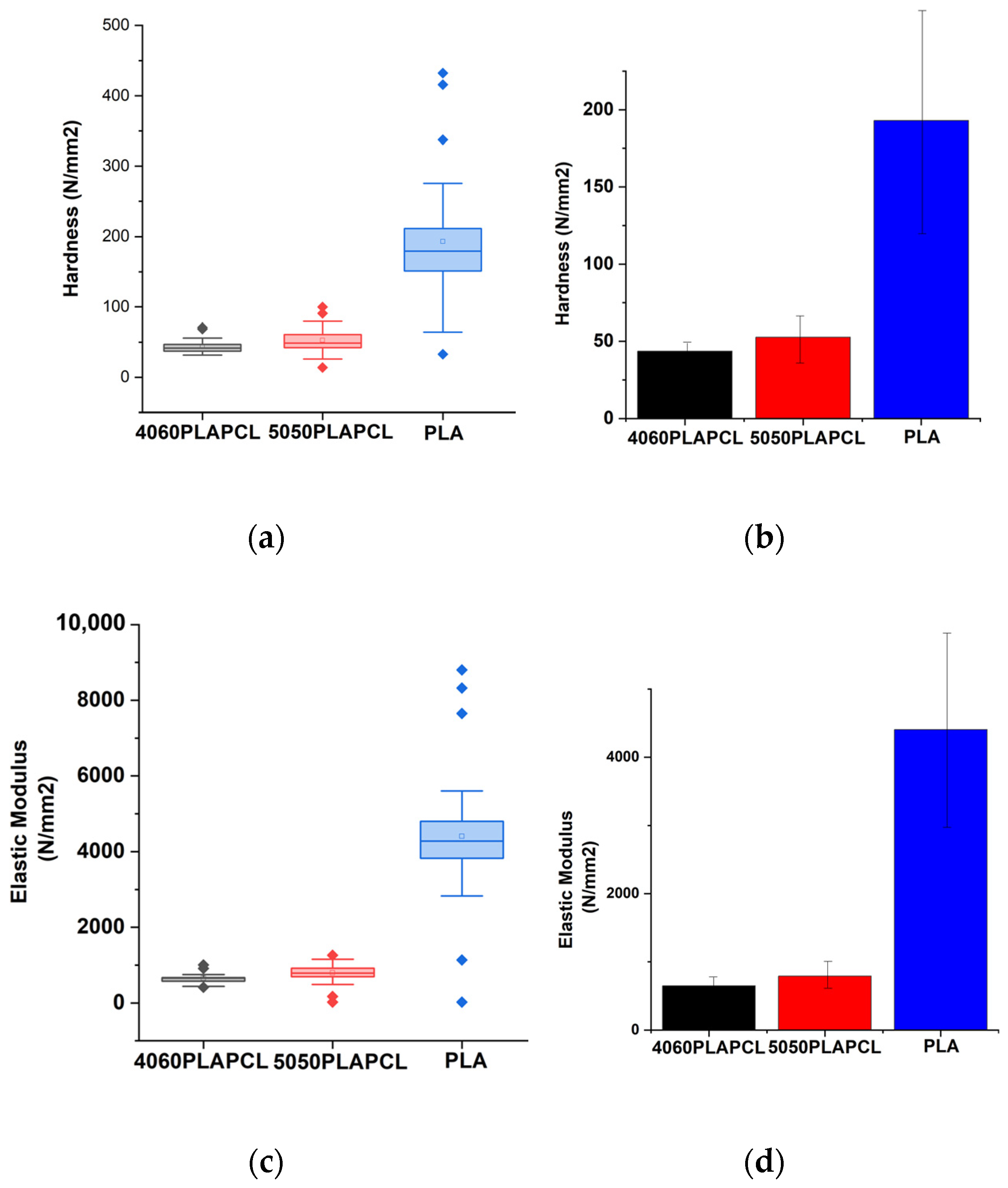
The nanoindentation results presented outcomes that were consistent with the nature of PLA and PCL polymers (i.e., PCL has flexibility), while PLA has tougher characteristics [
56,
57]. As for the statistical analysis (
Figure 14, there were statistically significant differences between PLAPCL5050 and PLA. Additionally, as seen in
Figure 14a–d, as the PLA percentage increased within the filament matrix, both the hardness and elastic modulus increased, while the opposite was observed as the PCL percentage increased. Such outcomes support the nature of PLA and PCL polymers. Furthermore, in terms of the results, Grigora et al.’s study presented a nanoindentation value for PLA filament-based samples of 3945.33 ± 134.74 MPa [
58], while our calculations led to a value of 4406.49 MPa. For PCL, PLAPCL4060, and PLAPCL5050, no research based on FDM filaments was found. As shown in
Table 2 and
Table 3, no significant difference was found between PLAPCL4060 and PLAPCL5050. This result could be due to only a 10% difference being used in the mixtures. Further investigation with a greater range of PLA and PCL percentages would provide information regarding how individual PLA and PCL characteristics affect the mechanical characteristics of FDM filaments. However, these non-significant differences can also be seen as an advantageous property when taking into account that PCL reduces brittleness and PLA increases the elastic modulus. Because of its moderate tensile strength, better elongation at break when compared to pure PLA, and increased toughness from the addition of PCL, this FDM filament is a good option for applications that require flexibility and impact resistance while still retaining some biodegradability. For bone tissue scaffolds, these characteristics are important, since bone tissue is exposed to daily forces that require endurance and long-term stability. Since PLAPCL4060 and PLAPCL5050 show superior mechanical characteristics compared to PLA in terms of elasticity and brittleness, depending on the purpose of the application, either of the FDM filaments can be used. On the other hand, a lower elastic modulus leads to application limitations. This can be improved by introducing an element to the matrix that can support the load distribution and increase the elastic modulus, in a similar manner to how researchers introduce electro-spun fibers to hydrogel matrixes. Furthermore, depending on the scaffold and porosity geometry, the mechanical strength can be adjusted. There are various studies in the literature that focus on PLA-PCL composite filaments and their mechanical characteristics [
59,
60,
61]. One study in particular involves percentages of PCL60PLA40 (wt/wt) and PCL50PLA50 (wt/wt), similarly to our work. Haq et al. investigated the elastic modulus of FDM filaments based on various combinations of PLA-PCL biopolymers using tensile testing [
62]. Based on this study, one can deduce that there was a significant difference between the PCL60PLA40 and PCL50PLA50 samples, which contradicts our mechanical testing. However, in that study, since the researchers used tensile testing and, in our study, nanoindentation was used, one should pay attention to the significant difference between how they are conducted and what they provide in terms of mechanical characterization. Nanoindentation facilitates the precise characterization of the mechanical properties at the nanoscale; the process of applying a tiny, focused force to the surface of a material; and evaluating the resulting indentation depth and load–displacement behavior, which is particularly important for materials that are composed of more than one component. On the other hand, tensile testing is determined by stretching the material under controlled conditions. Furthermore, we would also like to draw attention to printing conditions as one of the potential reasons for the observed non-significant difference. Xiao et al. investigated the influence of specific 3D printing parameters, including printer head temperature, fiber diameter, and degradation, in relation to the mechanical characteristics of FDM-printed polyethylene materials. As a result, they have observed that Young’s modulus and tensile strength decrease as the printer head temperature increases, while smaller-diameter FDM-printed PE fibers that are created at a higher collection speed experience greater stretching, which raises their tensile strength and Young’s modulus values [
63]. Thus, we believe that the FDM printing conditions might be another explanation. As an aside, we would like to point out the differences between each filament and explain the reasoning behind them in order to prevent any further misunderstandings. During nanoindentation testing, there were points that did not have contact with the sample’s surface due to the last layer of the STL-contained structure. As a result, these were not taken into consideration. In addition, if the samples moved during or immediately after testing, they were also not included in order to prevent false results. Also, as seen in
Figure 14, the standard deviation (error bar) for PLA was higher compared to that of PLAPCL4060 and PLAPCL5050. This was observed due to the presence of a very low value observed from one of the indentation points. Since the non-contact point was not an error in this case, the value was still included within the calculations. Despite these problems, the results of the statistical analysis showed a logical correlation between each polymer and polymer percentage. Furthermore, as explained in
Section 3.3.4, due to the limitations of PCL filaments, PCL was not included in our discussion.
Furthermore, when discussing the lack of significant difference between the elastic modulus of PLAPCL4060 and PLAPCL5050, one should also consider its effect on the flexibility properties of scaffolds produced from these polymer blends. As discussed previously, as the PLA percentage increased within the filament matrix, the elastic modulus increased, while the opposite was observed as the PCL percentage increased. Additionally, it is known that the elastic modulus and flexibility of a material have a reverse correlation. Therefore, one can deduce that as the PLA percentage increases, the flexibility of the scaffold under ultrasound stimulation will decrease. However, in our case, since we have not observed a significant difference, both PLAPCL4060 and PLAPCL5050 should be considered as options for ultrasound application.
The flexural modulus results showed consistency with the nanoindentation characterization results. However, the results for the elastic and flexural moduli were different for the samples with the same dimensions, instead matching with the nature of polymeric materials. Additionally, in PLA vs. PLA/PCL, PLA was found to be approximately 3–4 times greater than PLAPCL5050 and PLACPL4060, which is a result similar to that seen in [
24]. This analysis indicates that PLAPCL4060 and PLAPCL5050 have better flexibility compared to PLA, which should be considered when designing a scaffold that requires responsiveness and dynamicity under ultrasound exposure.
With an aim to investigate the potential of commercial FDM filaments for ultrasound technology in bone tissue scaffolds, characterizations using XRD, SEM, Raman spectra, and three-point bending were conducted in relation to scaffold composition, mechanical characteristics, and printability. In light of these characterizations of composite filaments based on PLA and PCL for their potential in ultrasound technology, one can deduce their alignment and importance to bone tissue scaffolds. From a compositional perspective, the biomaterials used in bone tissue scaffolds have been chosen by researchers depending on their purposes, such as for enhanced physical and chemical properties, stimuli responsiveness, or enhanced mechanical properties [
64,
65,
66]. In this study, PLA and PCL biopolymers were used with the purpose of creating a dynamic scaffold that responds to ultrasound stimulation. One of the most promising combinations for tissue engineering is known to be PLA/PCL combinations. The key features of these polymeric compounds are their high purity, suitability for processing, and exceptional mechanical properties, which have enabled the development of scaffolds with a wide range of applications in regenerative medicine. Furthermore, it should be noted that these materials are biodegradable and that their breakdown products can be reabsorbed. As a small organic molecule, lactic acid has a variety of physiological and metabolic routes. These two chemicals improve the biomechanical performance of the constructs, and some studies have found benefits in both mechanical and biological properties [
67]. The XRD and Raman spectroscopy results showed the presence of these two polymers (with shifts from the reference values) in PLAPCL4060 FDM filaments. The potential reasons for the shifts have already been presented and discussed. Furthermore, from a bone tissue scaffold perspective, any limitations could be caused by impurities in the commercial filaments, which are believed to have affected the results obtained. PLA FDM filaments contain a number of additives, e.g., coupling agents, impact modifiers, processing aids, nucleating agent, antioxidants, colorants, and flame delayers. These impurities are used during manufacturing to achieve high-performing PLA FDM filaments; however, these additives can be disadvantageous in bone tissue scaffold scenarios. In contrast, it is known that the presence of some additives can also improve the mechanical properties. However, they can also cause decreased adhesion between printed layers. As a result, one can deduce that the presence of additives can be both advantageous and disadvantageous. Additionally, from a clinical application perspective, despite the abovementioned advantageous properties of PLA/PCL combinations, the presence of additives in FDM filaments can limit their application. In particular, the cytotoxic fumes that are produced during the printing process have been investigated in the literature with various types of cells. However, we would like to point out that these studies focus on the ultrafine and fine particles that are emitted during the manufacturing process [
68,
69] or during the sterilization process [
70]. Additionally, there are studies which use commercial FDM filaments with an aim to assess their biocompatibility. Shilov et al. used 3D-printed PLA, PEEK, and PETG to assess the relation between cell adhesion and printing parameters. As a result of the cytocompatibility test they conducted, no cytotoxic effect was reported [
71]. However, we would like to address the potential cytotoxicity based on the study of Burkhardt et al. In this study, researchers investigated the cytotoxicity of polypropylene (PP) and biocopolyester (BE), which are utilized to fabricate surgical guides using fused filaments. As a result, they came to the conclusion that all materials are biocompatible in vitro in the short term, while in the long term, they expressed potential inflammatory response [
72]. In light of these studies, we acknowledge the potential cytotoxicity risk that can potentially occur in long-term applications as a result of the aforementioned additives. To ensure their adaptation to clinical applications and to investigate the potential cytotoxicity, in vitro tests have been planned for future part of this investigation. Briefly, the in vitro tests can be outlined as follows. By using the MC3T3-E1 Subclone 4 cell line with the minimum essential medium—Alpha Modification (Alpha-MEM)—as a growth medium, supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin solution, experimental optimization will be conducted to assess the cell number and mineralization medium. For this purpose, (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) MTS, ALP, and Alizarin Red staining assays will be conducted. After the optimization studies, cell viability (MTS), differentiation (ALP and Alizarin Red S Staining), and immunocytochemistry with Phalloidin staining will be conducted [
73]. These assays will provide the information required for transitioning from its current lab-scale status to clinical applications, while addressing the potential cytotoxic nature of commercial FDM filament-based scaffolds.
From the perspective of mechanical characteristics, in this study, three mechanical characteristics—flexural modulus, hardness, and elastic modulus—were investigated. For a bone tissue scaffold that responds to ultrasound stimulation, the flexural modulus provides important information. Based on the flexural modulus, one can deduce the stiffness and the resistance to bending under load. A lower flexural modulus means that the material is more flexible, correlating with the ability of the scaffold matrix to change its shape under load, which, in this study, results from ultrasound stimulation. In terms of future clinical applications, since there are applications of ultrasound that have already been established and used in clinics, it can be said that the potential transition from research to clinics is highly likely. The elastic modulus and hardness results for PLAPCL4060 and PLAPCL5050 showed no significant differences; therefore, in terms of bone tissue scaffold applications, such a result could potentially open future opportunities based on the aims of the researchers. Additionally, as previously mentioned, the mechanical characteristics can be adjusted depending on the geometrical design and the porosity specifications.
From a printability perspective, in this study, scaffolds with complex geometrical structures were printed on 200% and 125% scales in order to assess the limitations and the printing parameters. Porosity percentage, pore size, pore geometry, and overall scaffold geometry are crucial properties when designing bone tissue scaffolds. Due to bone tissue’s complex geometrical structure and hierarchy, designing and manufacturing bone tissue scaffolds has been part of bone tissue engineering for years. The results for PLAPCL4060 and PLAPCL5050 show that there were indeed some limitations for PLAPCL4060 FDM filaments, since some defects within the scaffold pattern and geometry were observed. This will limit the design range of bone tissue scaffolds, as well as their potential clinical applications; however, the FDM printer quality also plays a role. Therefore, using an FDM printer with higher specifications and precision could potentially be a solution to this problem.
As previously stated, researchers have recently investigated the potential of utilizing ultrasound stimulation (US) in conjunction with tissue scaffolds to enhance tissue growth and regeneration. To further investigate the potential of PLAPCL4060 and PLAPCL5050 for ultrasound technology in bone tissue scaffolds, accelerated degradation experiments for TPMS structures with varying porosity gradients were conducted. We utilized NaOH degradation to understand time- and composition-related degradation mechanics, which are important factors in bone tissue scaffolds in the context of healing processes. For bone tissue scaffolds, as the defective tissue regenerates, the scaffold in question should degrade, allowing new tissue formation to replace the scaffold. Therefore, this section of this study focused on such applications by investigating the weight change and surface morphology. However, we would like to point out that the degradation observed in this study has been in the form of mechanical degradation, rather than polymeric degradation. Furthermore, we have discussed the effect of this mechanical degradation, as well as the patterns that were observed as a result of this. Additionally, when investigating degradation, it was determined that the rate of degradation depends on the initial state of the polymer; thus, parameters such as molecular weight and crystallinity are important. In the literature, our initial investigation of an FDM filament’s molecular weight resulted in observing a wide range, depending on the manufacturer’s intended aim. However, as the investigation continued, as well as a general range of 60,000–80,000 Da MW, we also observed a range of 50,000–140,000 Da MW being discussed in several articles [
74,
75,
76]. This is a common issue when working with commercial FDM filaments. The technical datasheet includes information regarding density and temperature, but lacks information such as molecular weight and crystallinity. However, to improve the accessibility of the FDM filaments, a future study has been planned. In the future study, it is planned to investigate the MW, crystallinity of PLA FDM filaments, and crystallinity of PCL medical-grade pellets in order to better understand the degradation kinematics and also investigate the degradation in the long term in order to achieve polymeric degradation.
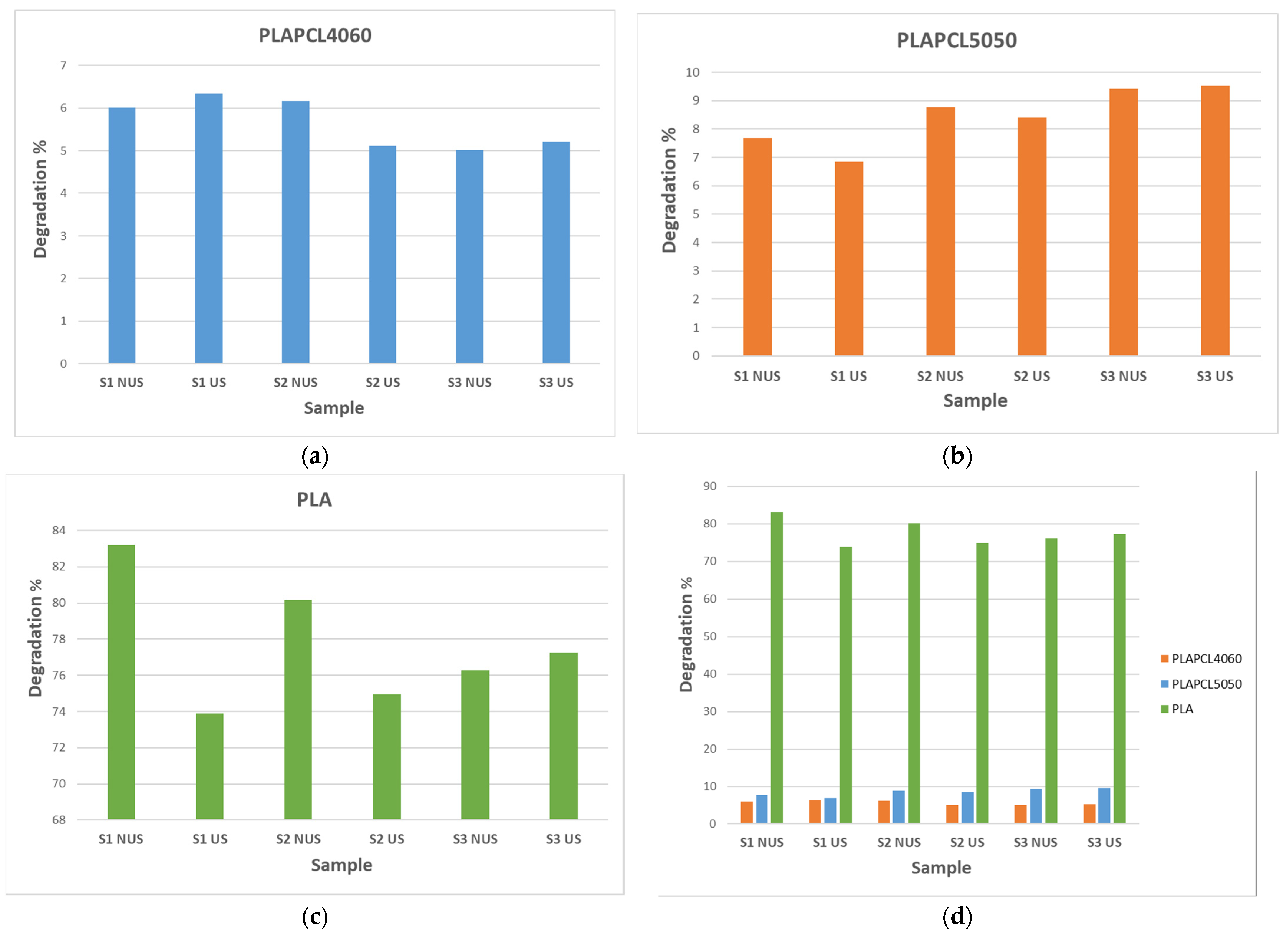
As shown in
Figure 18, despite the small and non-significant differences between PLAPCL4060 and PLAPCL5050, there was a variation between scaffold types and between the FDM filaments that the samples were produced from. Briefly, the weight differences before and after a total of 6 h incubation at 37 °C with 5 M NaOH (+ 2 h RT ultrasound stimulation for US samples) treatment were as follows: (1) PLA scaffolds degraded independently of scaffold type and the presence of ultrasound stimulation. Between scaffold types S1 and S2, the sample degradation percentage was higher for NUS samples; for S3 scaffolds, it was the opposite. (2) PLAPCL4060 S1 scaffolds and PLAPCL5050 S1 scaffolds showed contrasting responses to ultrasound stimulation. From these results, one can deduce that, for uniform gyroid bone tissue scaffolds (S1), ultrasound stimulation can accelerate the degradation process in FDM filaments with ultrasound-responsive characteristics. (3) For S2 and S3 scaffolds, the beforementioned composite FDM filaments showed a similar behavior within the scaffold types. For radial gradient gyroid bone tissue scaffolds (S2), independently from possessing ultrasound stimulation responsiveness, ultrasound stimulation seemed to decelerate the degradation. Such an outcome could be either due to the presence of ultrasound stimulation or due to the radial gradient geometry that the scaffolds possess. For x/y gradient gyroid bone tissue scaffolds, the opposite was observed compared to S2 scaffolds, whereby the presence of ultrasound stimulation accelerated the degradation process. (4) Despite their complete degradation and the potential errors that can occur, when compared to PLAPCL4060 and PLAPCL5050, the results from the PLA scaffold provide the following statements.
Considering the outcomes from FDM filament characterizations, the PLAPCL4060 FDM filament was claimed to possess ultrasound-responsive characteristics; in the context of accelerated degradation, a deduction can be performed for uniform gyroid scaffolds. When PLA S1 scaffolds are compared to PLAPCL4060 and PLAPCL5050 scaffolds, it is seen that US samples accelerated the degradation of the scaffolds produced from ultrasound-responsive FDM filaments, while for the ones that do not have such characteristics, the results were the opposite. Furthermore, when compared to the porosity gradient type and their responsiveness to ultrasound stimulation, it can be deduced that radial gyroid gradients affect the ultrasound wave propagation and degradation kinetics, which could be seen as a limiting factor or a further improvement depending on the aim of the application that one considers. When comparing uniform (PLAPCL4060 US) vs. radial (PLAPCL4060 US) samples, it can be deduced that the pore geometry is one of the parameters that affects the wave prorogation in a tissue scaffold. To further assess pore geometry vs. ultrasound wave propagation, an investigation based on Ferguson et al.’s approach to ultrasonic wave prorogation in randomly layered heterogonies media will be conducted [
77]. Additionally, future studies individually investigating these scaffold types to assess their potential in bone tissue scaffolds, such as their biodegradation in phosphate-buffered solution (PBS) or their bioactivity in stimulated body fluid (SBF), would provide a greater understanding.
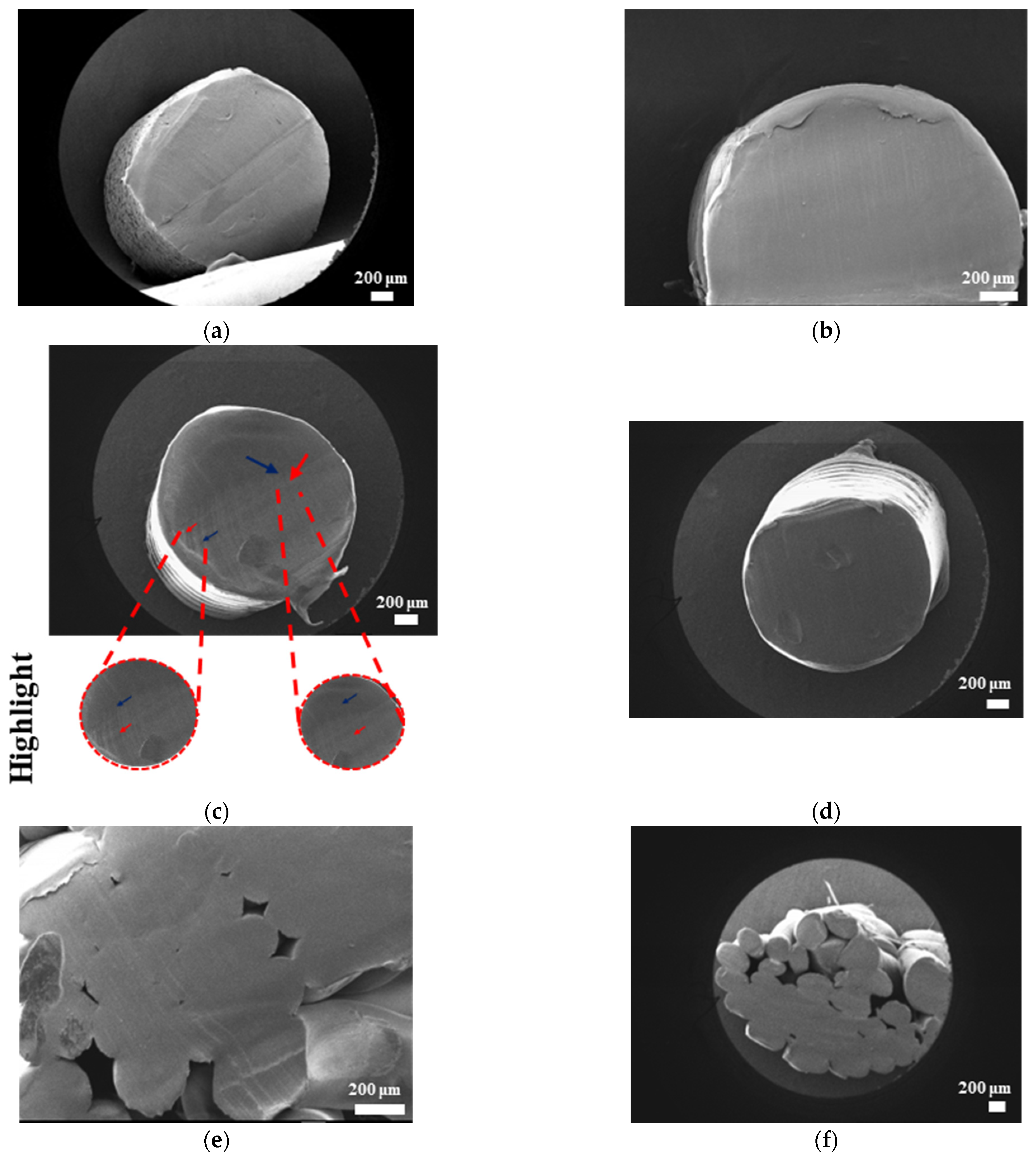
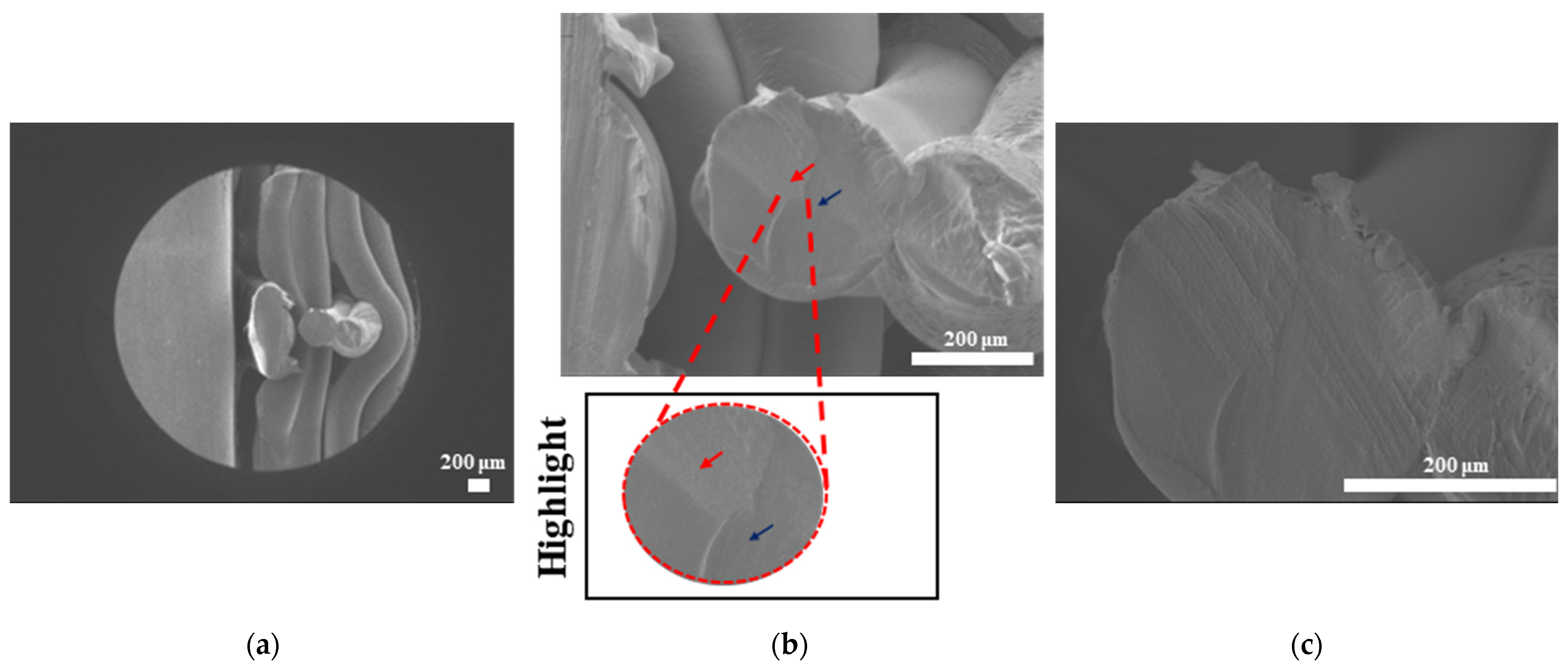
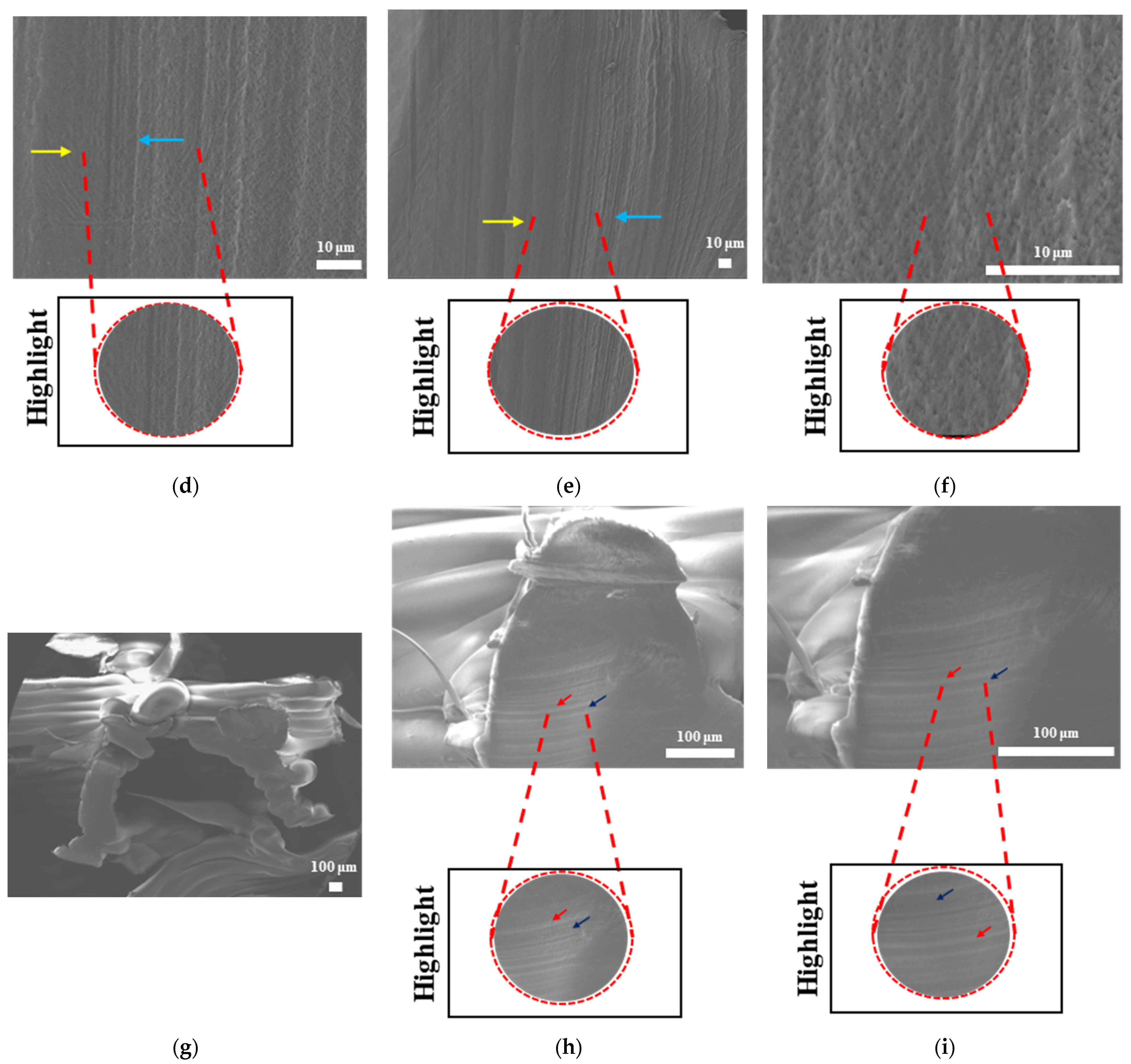
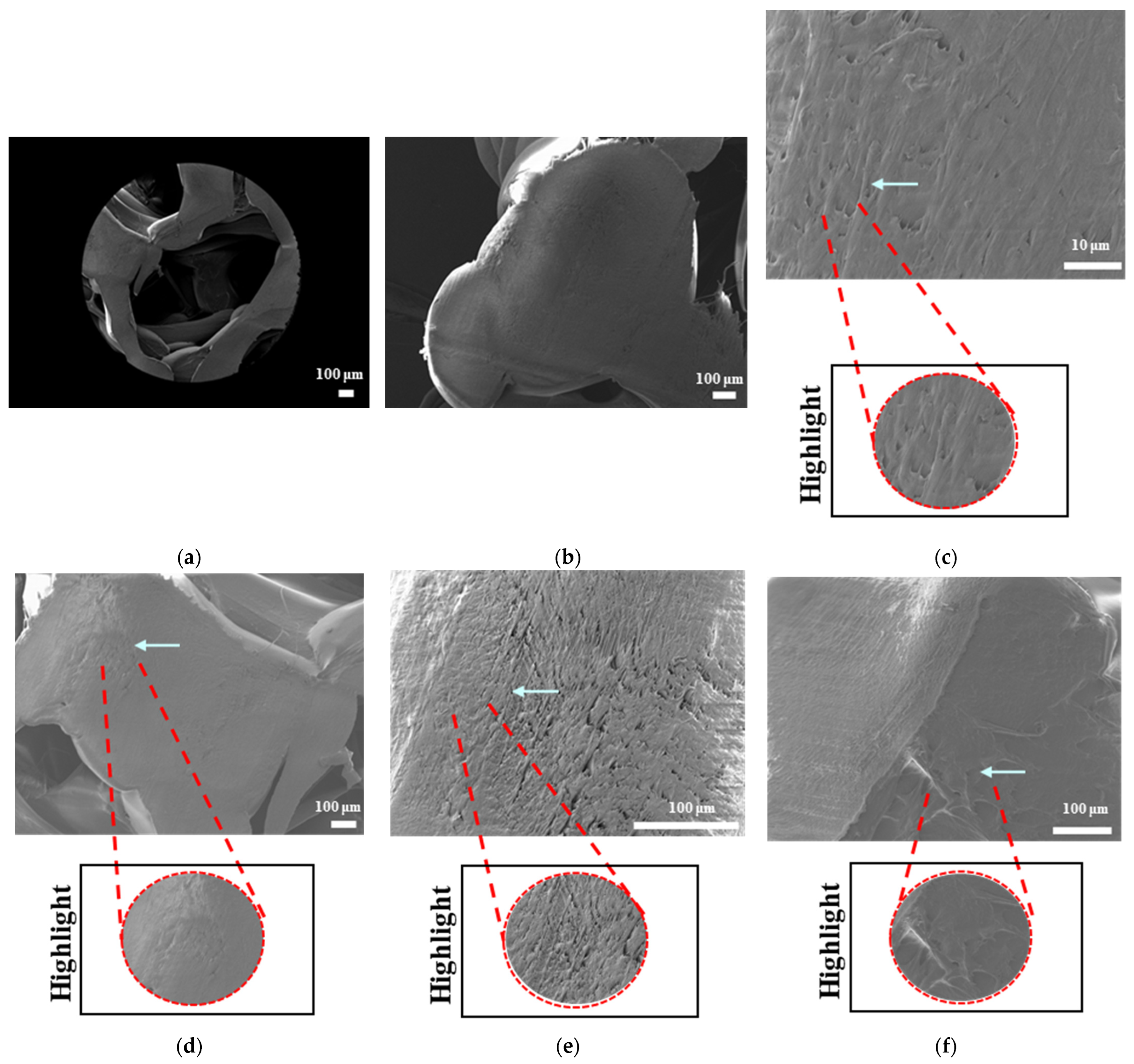
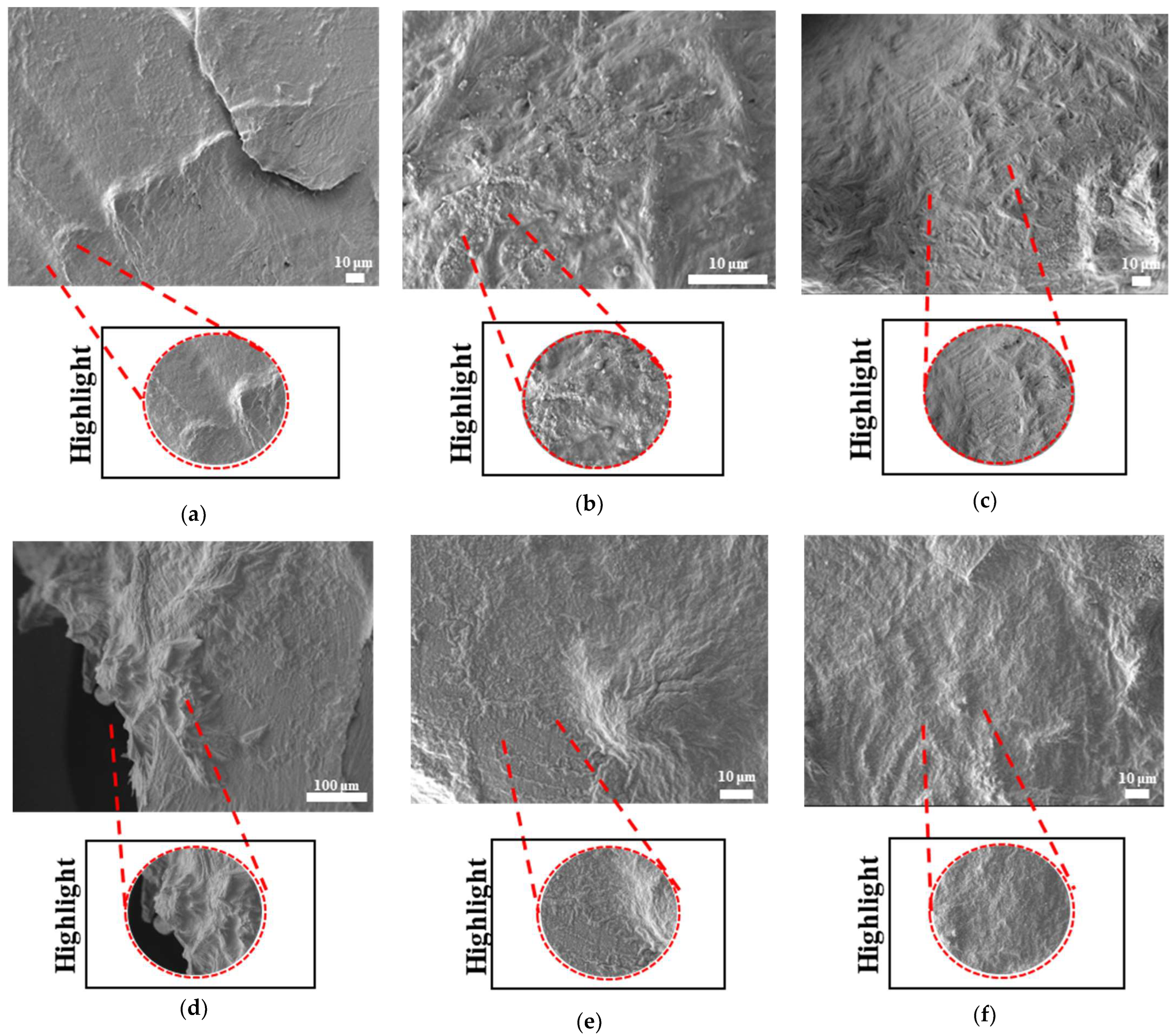
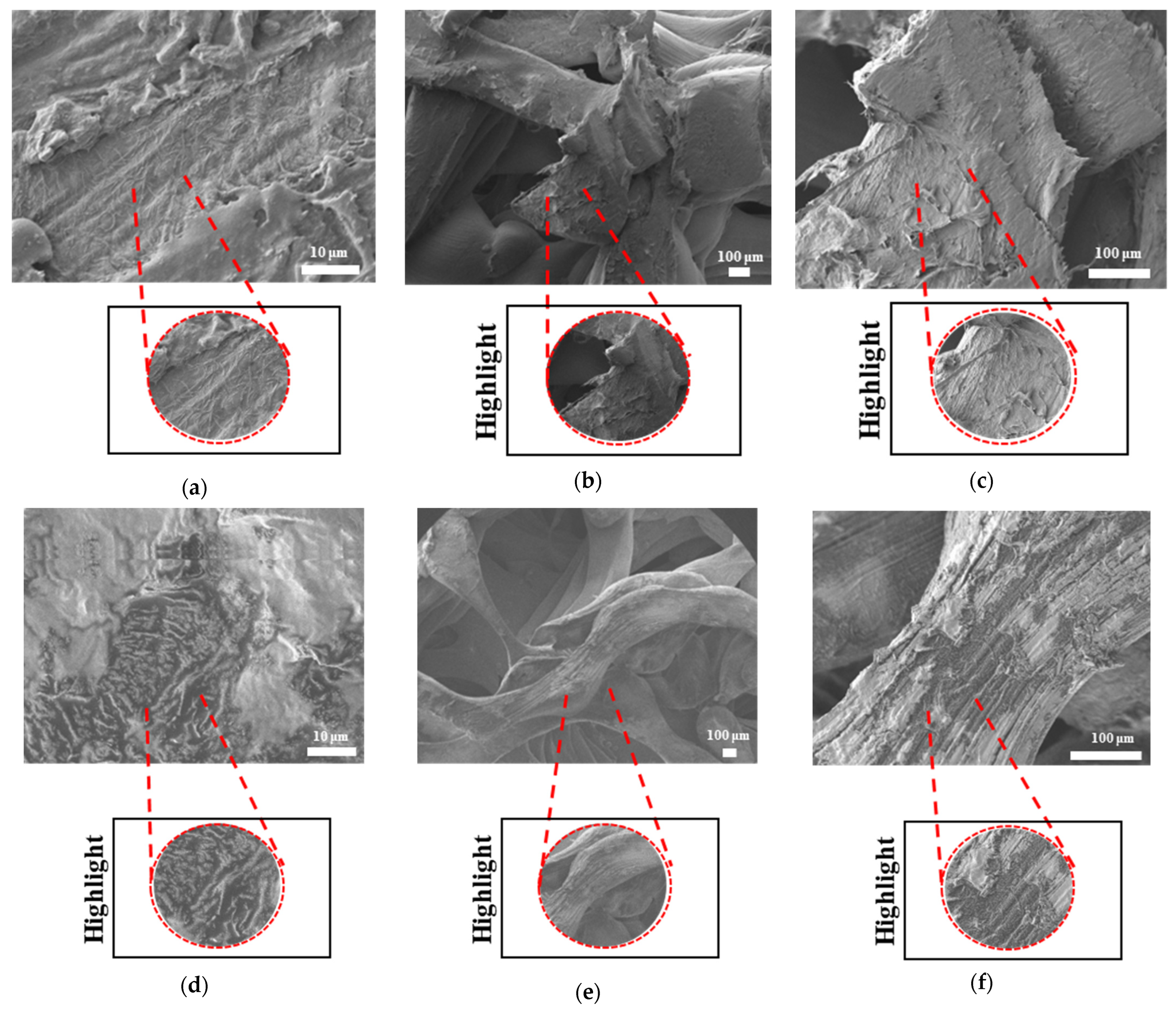
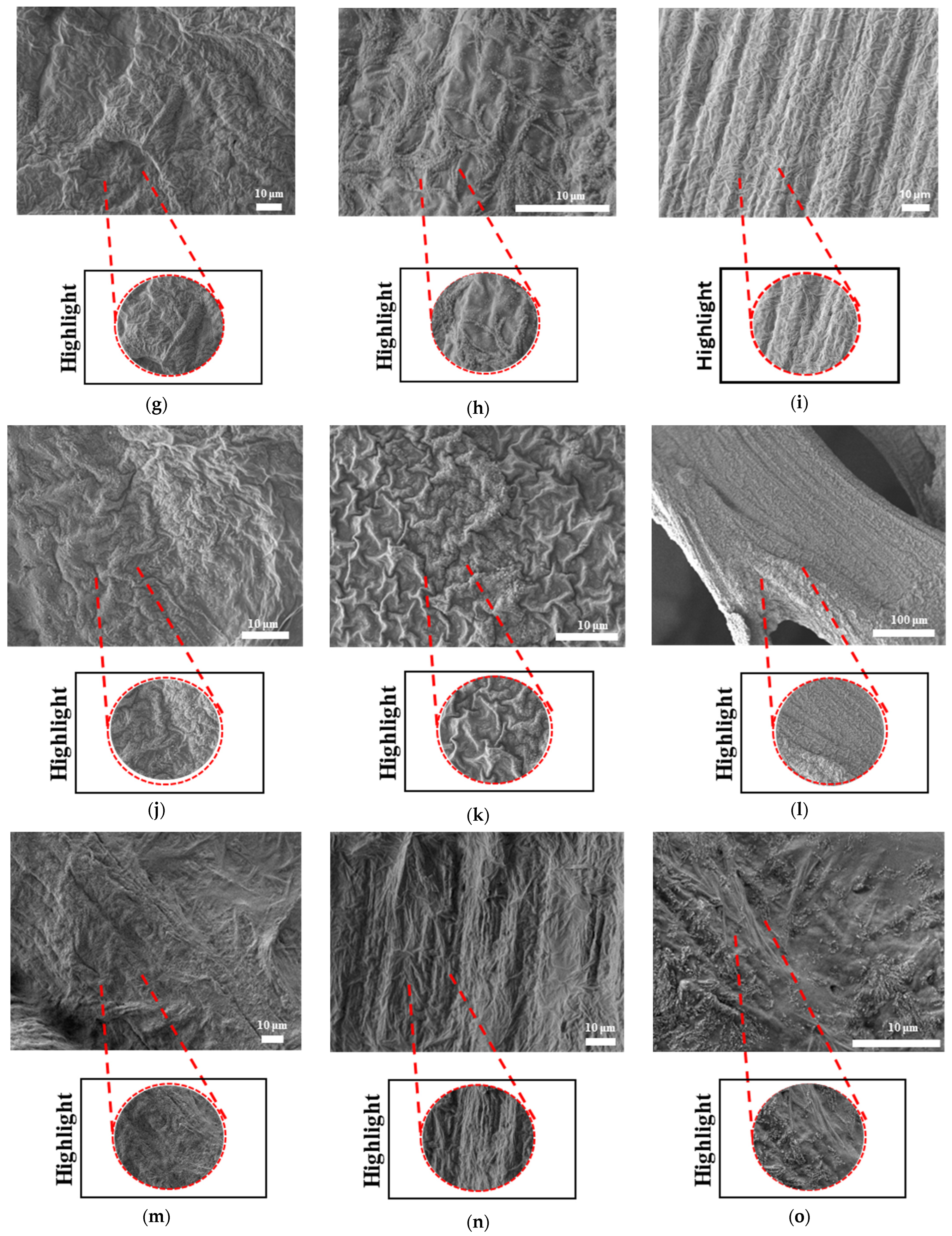
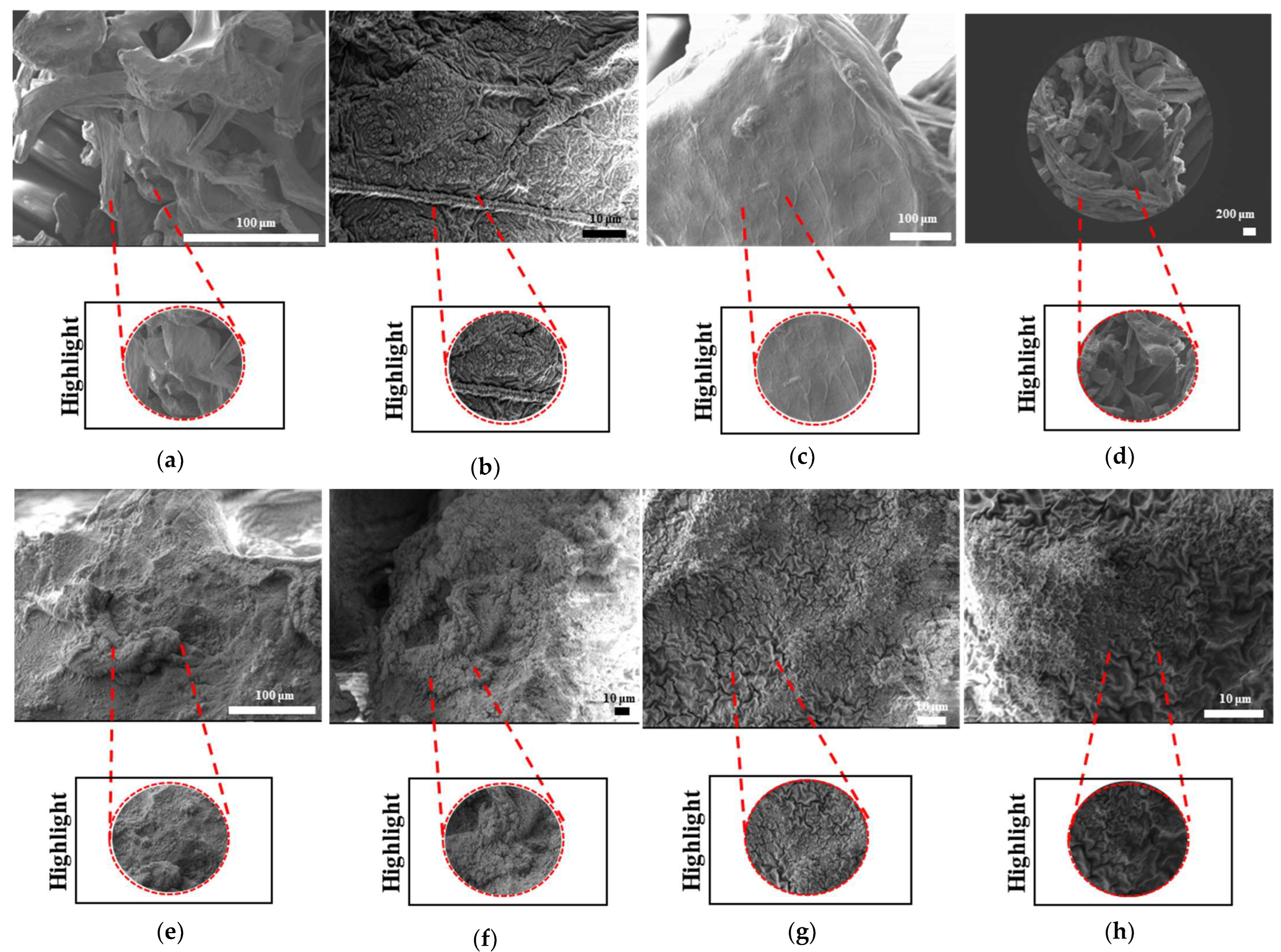
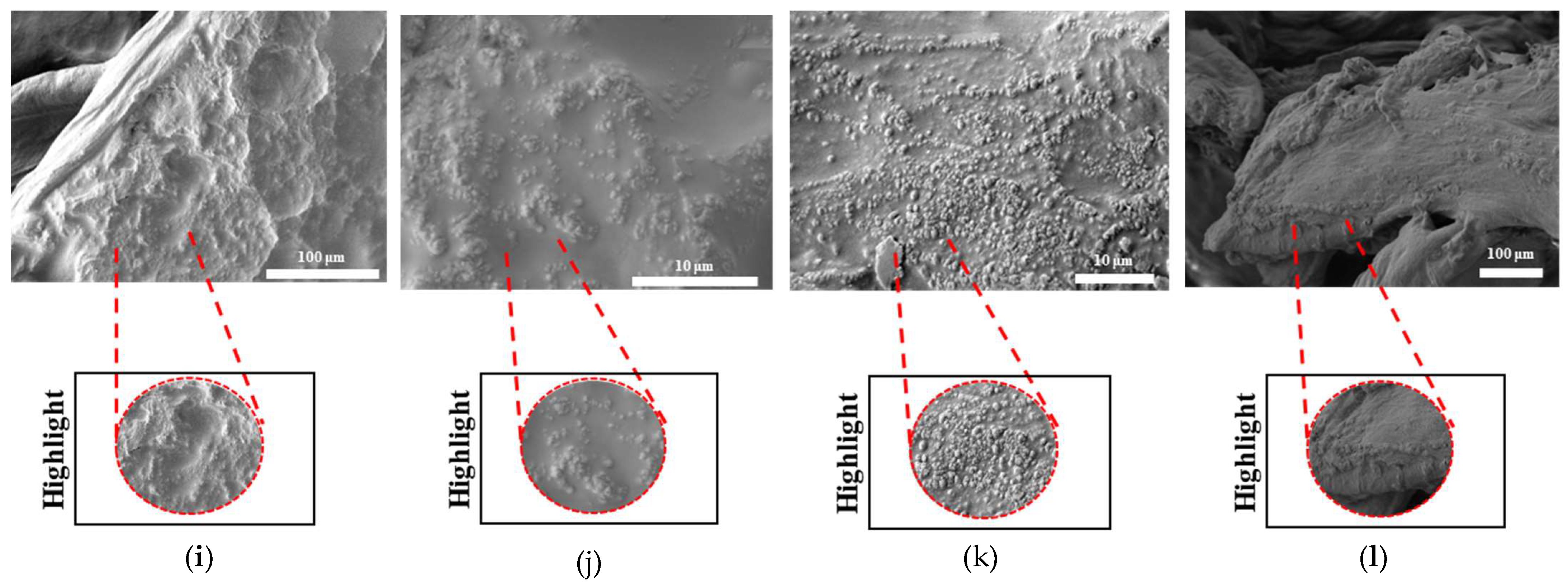
The surface morphologies of uniform TPMS (S1), radial TPMS (S2), and x/y gradient (S3) scaffolds degrading under ultrasound stimulation were analyzed. Overall, a number of degradation patterns were observed and are reported in
Figure 19,
Figure 20 and
Figure 21. Comparing the results with the degradation percentages shown in
Figure 18 for PLAPCL4060 TPMS,
Figure 19 S1 NUS (a–c) and
Figure 19 US (d–f) aligned with the result of
Figure 18a, since the degradation pattern was able to be observed on a larger scale.
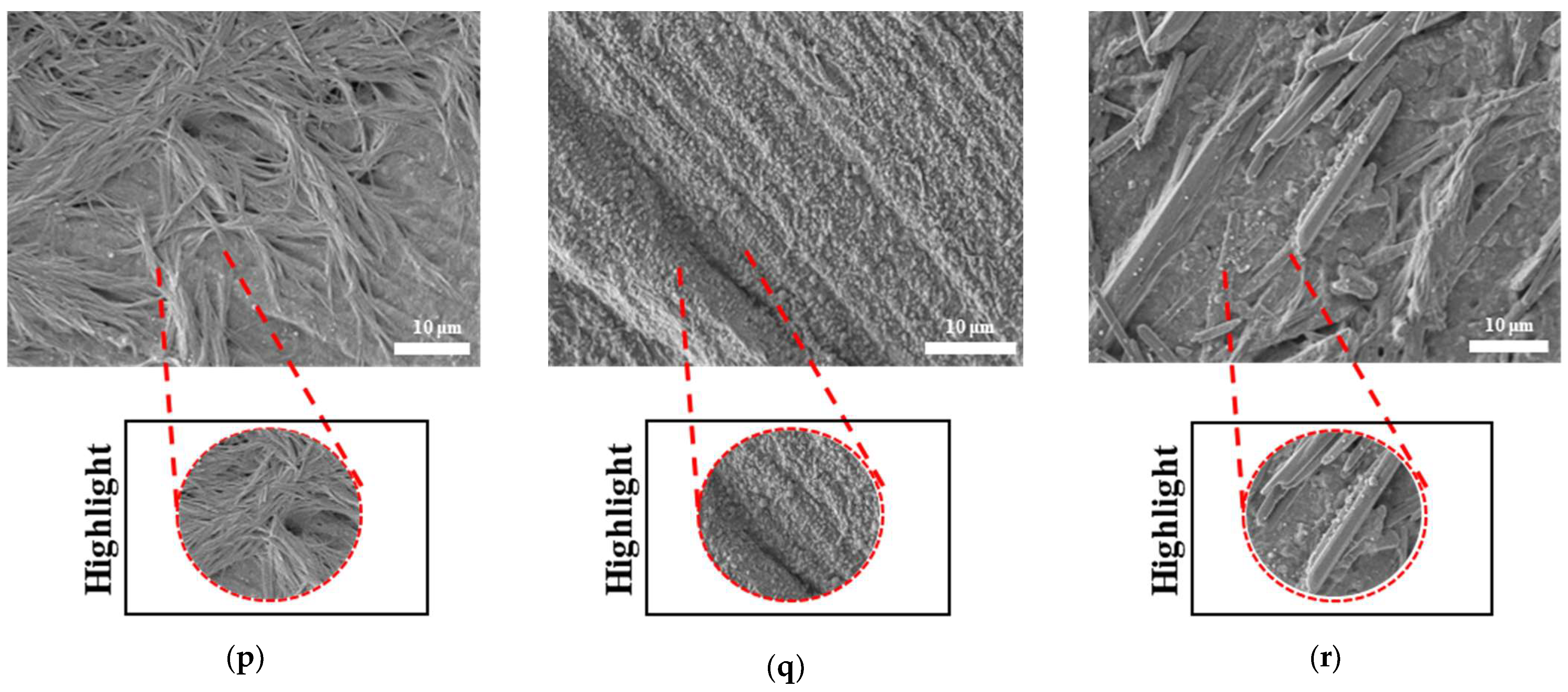
Figure 19 S3 NUS (m–o) and
Figure 19 S3 US (p–t) aligned with the result of
Figure 18a. For PLAPCL5050 TPMS scaffolds, the SEM analysis showed that S2 and S3 scaffolds, for both NUS and US treatments, possess string-like degradation patterns, while both had an exception—(k) for the NUS sample and (p) for the US sample. When compared to the degradation percentage results for PLAPCL5050 (
Figure 18b), S2 NUS and S3 US were found to be higher. Even though such different degradation patterns do not exactly reflect the degradation percentage difference between these US and NUS samples, it is still a factor that should be considered as part of the supporting conclusions. Furthermore, despite losing their scaffold shape after degradation, PLA scaffolds still show a different degradation pattern for S2 and S3 scaffolds. To further evaluate these outcomes, a literature review found that there are a number of studies that focused on PLA, PCL, and PLA-PCL. However, these studies did not provide information regarding the ultrasound application or the differences in application time and solution concentration used during the experiments. Therefore, no further comparison was conducted.
Overall, our findings support the potential use of commercial filaments for ultrasound technology in bone tissue scaffolds. Based on our aims, the production of PLAPCL4060 and PLAPCL5050 was successful, and the printing condition analysis showed that these filaments could be used in complex tissue scaffold geometries, even with the limitations that come with FDM printing; the search for phase segregation and the presence of both polymers in printed scaffolds was successful; the nanoindentation and three-point bending testing provided the mechanical characteristics for PLAPCL4060 and PLAPCL5050, and presented FDM filaments that have both flexibility and strength for bone tissue scaffolds; and the accelerated degradation provided insights into the ultrasound stimulations of the FDM filaments, as well as into the presence of porosity gradients and their interaction with ultrasound stimulation.